Abstract
The aim of the research was to study morphological changes that occur in cortical catecholaminergic forebrain structures of Wistar rats during postnatal development. Rat’s forebrain sections at different stages of postnatal development (postnatal day 7, postnatal day 30, 4–6 months, and 23 months) were studied using immunohistochemistry methods. It has been shown that distinct cortical areas perform a unique distribution of catecholaminergic fibers due to their functional features. Age-related changes in density of the distribution of catecholaminergic fibers were analyzed, and it has been stated that the density of catecholaminergic fibers in the sensorimotor cortex increases with aging. It has been demonstrated that confocal laser scanning microscopy offers a wide variety of opportunities for qualitative and quantitative analysis of immunohistochemical results and can be a useful tool for tyrosine hydroxylase distribution studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cortical catecholaminergic structures of the telencephalon of vertebrates are mainly represented by processes of noradrenergic neurons of group A6 of the locus coeruleus and processes of dopaminergic neurons of group A10 of the ventral tegmental area (VTA) and group A9 of the substantia nigra (SN) (Sukhorukova et al., 2014). Their neurotransmitters, dopamine and norepinephrine, play a key role in the regulation of many physiological (such as locomotor and endocrine) (Barishpolets et al., 2009) and cognitive (in particular, learning and memory) (Cools, 2008) functions of the central nervous system. The development of the catecholaminergic system in rats begins by the end of the second week of embryonic development (Gates et al., 2006; Bissonette and Roesch, 2016), and the final formation of catecholaminergic brain structures occurs at the end of the fourth week of postnatal development (Kalinina et al., 2012). However, it is the first postnatal month that is considered to be the critical period in the development of this mediator system, which is confirmed by the long-term change in the system and its regulated functions in case of external influences and manipulation to the laboratory animals during this period (Bonnin et al., 1996; Kalinina and Dygalo, 2013; Sukhareva et al., 2016).
Along with their formation, catecholaminergic brain neurons begin to express tyrosine hydroxylase (TH), an enzyme that catalyzes the first step in the biosynthesis of both dopamine and norepinephrine (Ugrumov et al., 1989, 2002). As a result, the presence of tyrosine hydroxylase in the cell indicates its ability to synthesize catecholamines, which allows us to consider this enzyme as a marker of catecholaminergic neurons. The levels of tyrosine hydroxylase mRNA, which are low at birth, increase with age in rodents; however, the dynamics of changes in expression, activity of the enzyme, and the nature of its distribution have regional features (Kalinina et al., 2012).
Along with development, aging is an important factor affecting the functioning of the nervous system. This natural process is characterized by a progressive decline in the physiological body functions. Studies have shown that progressive CNS lesions during aging, both at the structural and functional levels, are directly related to neurodegenerative disorders, the development of which is due to changes in the activity of the catecholaminergic system of the brain (Hamezah et al., 2017).
Catecholamines play a special role in the functioning of the limbic system of the brain. One of its key structures is the cingulate cortex, which is located on the medial surface of the brain between the cingulate sulcus and the corpus callosum. The cingulate cortex is a highly functional area of the brain with a special structural organization (Vogt et al., 2004). It is involved in the regulation of many bodily functions, from information processing to complex cognitive and social responses (Rushworth et al., 2011). Another part of the cerebral cortex, closely associated with the limbic system—the insular cortex—is located in rodents on the lateral surface of the hemisphere above the rhinal sulcus. This is a specific site of multimodal integration of the sensory, emotional, and cognitive systems of the CNS associated with the cortex (frontal, parietal, and temporal lobes as well as the limbic cortex), basal ganglia, and other parts of the brain (such as the thalamus) (Gogolla, 2017; Kortz and Lillehei, 2021; Livneh and Andermann, 2021). Like the cingulate cortex, the insular region has its own structural features. Many anatomical and functional properties of this area are considered common in rodents and humans (Gogolla, 2017). These factors provide the insular cortex with close attention in both basic neurobiological and clinical research.
Despite the importance of elucidating the processes that ensure the development of the catecholaminergic system in the designated areas of the brain, the mechanisms underlying these processes are still not entirely clear. It is assumed that a comprehensive characterization of the morphological processes occurring in the catecholaminergic system of the neocortex during development and normal aging can help form an idea of the organization of poorly studied cortical regions.
In this regard, the aim of this research was to study the dynamics of morphological changes in the catecholaminergic structures of the rat cerebral cortex occurring in postnatal ontogenesis using immunohistochemical staining for tyrosine hydroxylase.
MATERIALS AND METHODS
Brain sections of male Wistar rats taken at different periods of postnatal development were used as material for the study: seventh (P7) and 30th (P30) postnatal days, mature (5–6 months), and old (23 months) animals (n = 3 for each stage). All procedures involving animals were carried out in accordance with the guidelines established by the Directive 86/609/EEC on the protection of Animals used for Experimental and other scientific purposes (Strasbourg, 1986), “Regulations of work with the use of experimental animals” (order no. 755 of August 12, 1977, Ministry of Health, USSR) and “Rules of Good Laboratory Practice” (order no. 199n dated April 1, 2016, of the Ministry of Health of Russia). The study was approved by the local ethics committee of the Institute of Experimental Medicine (conclusion no. 1/20 dated February 27, 2020). The material was fixed in zinc-ethanol-formaldehyde (Korzhevskii et al., 2015) and embedded in paraffin according to the standard procedure. Frontal 5 µm thick sections were made and mounted onto “Superfrost Ultra Plus” adhesive slides (Menzel Gläser, Germany). After deparaffinization and rehydration of the preparations, the antigen was thermally unmasked in a modified citrate buffer (S1700, Agilent, United States) for 24 min. Inhibition of endogenous peroxidase was carried out by treating sections with 3% aqueous hydrogen peroxide for 10 min. To detect catecholamiergic structures, rabbit polyclonal antibodies to tyrosine hydroxylase (ab112, Abcam, United Kingdom) diluted 1 : 1000 were used. Goat anti-rabbit antibodies conjugated with horseradish peroxidase from the Mouse and Rabbit Specific HRP/DAB IHC Detection Kit (ab236466, Abcam, United Kingdom) were used as secondary reagents. The reaction product was visualized using the chromogen 3'3-diaminobenzidine from the DAB+ kit (Agilent, United States). Some sections were stained with alum hematoxylin. The resulting preparations were analyzed using a Leica DM750 microscope (Germany) and photographed using an ICC50 camera (Leica, Germany). The ImageJ program (Wayne Rasband (NIH), United States) was used for image analysis.
To obtain preparations for examination on a confocal scanning microscope, sections, after incubation in secondary antibodies and washing in buffer, were treated with a solution of goat anti-horseradish peroxidase antibodies conjugated with Cy3 fluorochrome, to which DNA-binding dye SYTOX Green was added to a final dye concentration of 0.6 µg/mL (Invitrogen, United States). Histological preparations were analyzed and photographed using a Zeiss LSM 800 scanning confocal microscope equipped with an Airyscan system (Carl Zeiss AG, Germany). Plan-Apochromat 20×/0.8 M27 and Plan-Apochromat 63×/1.40 Oil DICM27 objectives (oil immersion) were used. To excite Cy3 fluorescence, a laser with a wavelength of 561 nm was used; for SYTOX Green, a laser with a wavelength of 488 nm was used. The obtained images were analyzed using the Zen-2012 computer program (Zeiss, Germany).
To estimate the density of fiber distribution, the total area occupied by the processes of catecholaminergic cells (in square µm) was measured in a frame of 175 × 198 µm at a magnification of ×40, then standardized to a scale length of 1 mm. Statistical processing was carried out using GraphPad Prism 8 (GraphPad Software, United States). Data were presented as the mean ± error of the mean. Shapiro–Wilk test was used to establish the data distribution. For datasets resembled normal distribution, one-way ANOVA was used to compare data, followed by group comparison using Tukey’s post-hoc test. Nonparametric one-way Kruskal–Wallis analysis of variance and Dunn’s post-hoc test were used for other datasets. The variables were considered to be normally distributed at P > 0.05. Differences were considered significant at P < 0.05.
RESULTS
As a result of the immunohistochemical reaction, processes and bodies of catecholaminergic neurons are revealed on sections of the forebrain of rats.
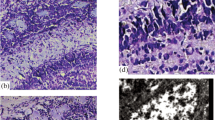
Rare TH-immunoreactive fibers are distributed in the layers of the neocortex on the seventh postnatal day. In the first layer of the cortex, they are represented mainly by processes of neurons cut across (Fig. 1). There are thin fibers with distinct varicosities in the underlying layers. Separate fibers can be traced for a short distance in the neuropil. The density of their distribution is uneven in different layers of the cortex: the highest is in the upper and lower layers, while the lowest is in the middle ones. The sixth layer of the cortex contains a large number of horizontal catecholaminergic fibers. The region of the cingulum and the first layer of the cingulate cortex are filled with large intensely colored processes cut across. As for the differences between different regions of the cortex, the highest density of distribution of TH-immunopositive processes differs in the insular region of the cortex (in comparison with the sensorimotor cortex, Shapiro–Wilk test P < 0.05, Kruskal–Wallis test, post-hoc Dunn test P < 0.05) (Figs. 2, 3).
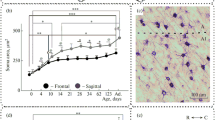
Age-related changes in catecholaminergic fibers in the cingulate cortex. (a) Day 7 of postnatal development; (b) Day 30 of postnatal development; (c) adult animal; (d) old animal; (e) dynamics of changes in the distribution density of TH-positive fibers of the cingulate cortex during postnatal ontogenesis and during aging. Arrows point to catecholaminergic fibers. P7, P30: 7 and 30 days of postnatal development, respectively. P value: ** <0.01; *** <0.001.
Morphological changes occurring with TH-positive fibers in the area of the sensorimotor cortex during postnatal development and aging. (a) Day 7 of postnatal development; (b) Day 30 of postnatal development; (c) adult animal; (d) old animal; (e) dynamics of changes in the distribution density of TH-positive fibers in the sensorimotor area of the cortex. Arrows point to catecholaminergic fibers. P7, P30: 7 and 30 days of postnatal development, respectively. P value: * <0.05; ** <0.01.
TG-positive fibers of the insular cortex during postnatal development and during aging. (a) Day 7 of postnatal development; (b) day 30 of postnatal development; (c) adult animal; (d) old animal; (e) dynamics of changes in the distribution density of TG-positive fibers of the cingulate cortex during postnatal ontogenesis and during aging. Arrows point to catecholaminergic fibers. P7, P30: 7 and 30 days of postnatal development, respectively. P value: * <0.05; *** <0.001.
In addition to processes, single bodies of catecholaminergic neurons can be located in the cortex. These are rather large cells of an oval or stellate shape, from the soma of which one branching process departs. They are most often found in the VI layer of M2 sensorimotor cortex and in the piriform cortex (Fig. 4).
By the 30th postnatal day, the intensity of the reaction slightly increases due to increased branching of TH-positive processes within the cortex. In the first layer of the cingulate cortex, the fibers actively branch (see Fig. 1b); however, their branches do not extend beyond the layer in most cases, which is confirmed by confocal microscopy. This observation is not applicable to vertically directed sparsely branched fibers of layers III–V, which can pass through several layers of the cortex. In the VI layer and cingulum, mainly cross-cut fibers with a high intensity of the immunohistochemical reaction were observed.
Fiber density decreases dramatically in the sensorimotor cortex (Shapiro–Wilk test P > 0.05, ANOVA, post-hoc Tukey test P < 0.05). The intensity of the reaction in this area of the cortex is low and increases in the layer VI adjacent to the white matter due to the presence of a moderate amount of horizontal fibers in it. As in 7-day-old animals, the insular cortex in P30 rats has a apparent immunohistochemical reaction (Fig. 3b). Its first layer is characterized by the presence of transverse processes of catecholaminergic cells. In contrast to the region of the cingulate cortex, there are hardly any long branching fibers in the first layer. The middle layers of the insular cortex are filled with a large number of thin, highly branching fibers. Basically, these branches do not go beyond their layers. The insular and cingulate areas of the cortex are characterized at this time by approximately the same intensity of the layer-by-layer distribution of TH-immunopositive fibers. TH-immunopositive neurons were not observed at this time.
In adult animals, there is an increase in the density of fiber distribution in the neuropil of the insular and cingulate areas of the cortex (as well as in the M2 sensorimotor cortex adjacent to the cingulate cortex). In the rest of the sensorimotor cortex, a very weak reaction was observed in all layers except the sixth. The densest fibers in the cingulate cortex are located in the upper and middle layers, while, vice versa, a large number of strongly branching processes were detected in the middle and lower layers in the insular cortex. At this stage, single catecholaminergic neurons are also detected. They were observed less frequently than in 7‑day-old animals and only in the lower layers of the M2 cortex adjacent to the cingulate cortex.
During the study of bark preparations of old animals, an increase in TH-positive fibers in the sensorimotor cortex was visually noted with a decrease in the overall intensity of staining. With aging, the processes of the sensorimotor and cingulate areas of the cortex begin to branch more intensively (Figs. 1d, 2d). Using morphometric analysis, it was shown that the area occupied by the processes of catecholaminergic neurons increases in the sensorimotor cortex (Shapiro–Wilk test P > 0.05, ANOVA, post-hoc Tukey test, p < 0.05), while it, however, remains approximately the same in the cingulate and insular areas of the cortex in comparison with adult animals (Shapiro–Wilk test P > 0.05, ANOVA, post-hoc Tukey test, p > 0.05). At this time, as in 30-day-old animals, TH-positive cells were not detected in the neocortex, but rare neurons were observed in the corpus callosum pathways adjacent to the cortex (Fig. 4c).
These results were verified using confocal microscopy techniques. To do this, sequential scanning of sections of the sensorimotor cortex along the Z axis was performed with a step of 0.2 μm, which made it possible to obtain three-dimensional reconstructions of the selected areas with a final thickness of 3 μm. It was found that the distribution of TH-immunopositive fibers described at the light level in the region of the sensorimotor cortex is also observed when using high-precision methods of scanning confocal laser microscopy (Fig. 5). As the analysis of optical sections showed, the region of the sensorimotor cortex of old animals is characterized by an increase in the immunohistochemical reaction due to more active branching of catecholaminergic fibers.
DISCUSSION
The study showed the heterogeneity of the distribution of TH-immunopositive fibers and terminals in the telencephalon. The most intensely stained areas correspond to the cortical regions of the limbic system, which are closely associated with mesocortical dopaminergic neurons in the VTA and SN and noradrenergic neurons in the locus coeruleus (Fallon, 1981; Ohara et al., 2003). The lower layers of the cortex and cungulum, in which the pathways pass, are quite intensely stained (Grigoriev et al., 2018). It is worth noting that catecholaminergic fibers are distributed throughout the neocortex of old animals with approximately the same intensity.
It is important to mention that, according to the literature data (Brownstein et al., 1974; Nomura et al., 2014), the rat cerebral cortex receives quite intense noradrenergic innervation. Along with this, the disadvantage of the method used is the inability to determine the exact mediator affiliation of the terminals observed in the cortex. In addition to TH, which is an enzyme that limits the rate of catecholamine synthesis, there are also specific enzymes that catalyze the synthesis of dopamine, norepinephrine, and adrenaline: aromatic amino acid decarboxylase, dopamine β-hydroxylase, and phenylethanolamine-N-methyltransferase, respectively. However, aromatic amino acid decarboxylase also catalyzes the formation of serotonin from 5-hydroxytryptophan, and the presence of adrenergic innervation in the cerebral cortex remains a debatable issue. The presence in the brain of the so-called monoenzymatic neurons containing only one of the enzymes of the dopamine synthesis cascade and participating in the cooperative synthesis of the neurotransmitter provides an additional challenge in assessing individual elements of the catecholaminergic system using the study of tyrosine hydroxylase expression (Ugryumov, 2009). Thus, the use of the TH makes it possible to indirectly analyze both the development of the brain’s catecholaminergic system and the effect of normal aging on it.
Particular interest in the study of the postnatal development of the catecholaminergic system of the cerebral cortex has arisen due to data illustrating a gradual improvement in performance in cognitive tasks mediated by the dopaminergic system during adolescence and until puberty (Reynolds and Flores, 2021). Thus, it was found that dopamine activity in the frontal cortex increases linearly from birth to puberty, presumably during the late formation of catecholaminergic innervation (Berger et al., 1985; Reynolds et al., 2018; Reynolds and Flores, 2021). It is reported that maturation of the dopaminergic innervation of the cortex continues in postnatal development and ends by the 60th postnatal day (Areal and Blakely, 2020). In contrast to the cortex, changes in dopamine activity after the first month of postnatal development and up to adulthood in subcortical structures (namely, in the striatum and olfactory tubercle) are not associated with changes in the density of innervation by catecholaminergic neurons since the mesolimbic and nigrostriatal pathways in rodents are formed by the 20th postnatal day (Voorn et al., 1988; Björklund, 1992); therefore, the density of TH-positive fibers in subcortical structures does not change in the same way after this period as in the cortex. In relation to the noradrenergic system of the brain, a rather rapid gradual maturation of the noradrenergic innervation of the neocortex is noted. At the same time, the activity of the noradrenergic system reaches a level characteristic of mature rats already on the ninth postnatal day (Levitt and Moore, 1979). Other studies using immunohistochemistry methods show that, despite the fact that the morphological pattern of the distribution of noradrenergic fibers in the cortex by the 14th day of postnatal development corresponds to that in mature rats, their innervation density is established only by the end of the third week of postnatal development (Latsari et al., 2002). Using the quantitative analysis performed in the present study, it was shown that the density of TH-positive fibers tends to increase with age in the areas of the cortex related to the limbic system. Thus, our data indirectly illustrate the phenomenon stated above.
In accordance with the previously noted, the sensorimotor cortex is characterized by a different morphological picture: relatively constant values of fiber density are preserved in this area from the first week of postnatal development to adulthood, followed by a sharp increase in these values with aging. Interestingly, in the other studied areas, no visible morphological and morphometric changes are observed during normal aging. At the same time, the overall staining intensity of brain sections of old animals in comparison with mature animals decreases.
It can be assumed that this change may be compensatory and associated with a decrease in the total number of catecholaminergic neurons during aging. One of the processes that develop during aging is an increase in oxidative stress. Oxidative stress appears to have a greater effect on areas related to movement control (Cardozo-Pelaez et al., 1999; Norrara et al., 2018). The neurodegenerative process develops during the autooxidative process with the formation of radicals, which ultimately worsen the biochemical, physiological, and morphological state of tissues (Finkel and Holbrook, 2000; Luo and Roth, 2004). Therefore, it seems logical that compensatory mechanisms arise that prevent the neurodegenerative processes that develop during aging.
Conversely, an increase in the density of TH-positive fibers may be a sign of developing cognitive impairment. For example, an increase in the number of glutamatergic presynapses in the frontal lobes of the cortex positively correlated with cognitive decline in patients with mild cognitive impairment (Bell et al., 2007). While this is an interesting observation, its physiological and behavioral implications for the body are not yet well understood. It is known that catecholamine levels are positively regulated by such a signaling molecule as glial cell line-derived neurotrophic factor (GDNF) (Arenas et al., 1995; Zaman et al., 2003; Grondin et al., 2019). A study by Matsunaga et al. showed that the level of GDNF expression in the frontal cortex increases with aging (Matsunaga et al., 2006), which may promote fiber growth and, thus, may partly explain the observed increase in catecholaminergic process density. It is also possible that this compensatory increase in fiber density is associated with a decrease in the number of catecholamine receptors on target cells during aging (Weiss et al., 1979). It should separately be noted that TH-positive processes in the sensorimotor cortex in adult animals are mainly represented by noradrenergic fibers (Nomura et al., 2014). However, due to the limitations of the method, it is not possible to check whether such a mediator ratio is maintained in aging rats or, conversely, fiber growth is associated with the activity of the dopaminergic system. Thus, this observation needs further research.
An unexpected result that we obtained during the study of sections at the light level led us to take an additional check by analyzing optical sections of the confocal microscope, the thickness of which is set using the software scan settings. Thus, images obtained from a confocal microscope reduce possible errors in the thickness of the produced paraffin sections and minimize the false positive results associated with these errors. Since the results we described at the light level are reproduced using high-precision methods of scanning confocal microscopy, we can really believe that the catecholaminergic fibers of the sensorimotor cortex have their own structural features that manifest themselves in the course of aging.
As for the TH-immunopositive neurons that were observed in the cortex, it is noted that these cells are present in different species (Berger et al., 1985; Satoh and Suzuki, 1990; Weihe et al., 2006), and they are especially numerous in the human neocortex (Benavides-Piccione and DeFelipe, 2007). In rodents, catecholaminergic neurons are found in the deep layers of the cortex at different periods of early postnatal development, including the 60th day, and their number reaches a maximum by the end of the second week and then gradually decreases (Berger et al., 1985; Satoh and Suzuki, 1990). Apparently, the presence of these cells is characteristic of the cortex at any period of postnatal development, and their absence in sections is most likely associated with a low frequency of occurrence in older animals compared to P7. It is assumed that the decrease in the number of TH-immunopositive cells is not due to programmed cell death but due to changes in the amount of the basal level of the enzyme present in these cells. It has also been suggested that at least some tyrosine hydroxylase-producing cortical cells belong to a subpopulation of calretinin-containing GABAergic interneurons (Asmus et al., 2008). Other studies show that tyrosine hydroxylase colocalizes in cortex cells with choline acetyltransferase (ChAT) and vasoactive intestinal peptide (VIP) (Asmus et al., 2011). Such cololazation, however, seems to be not typical of human cerebral cortex neurons (Asmus et al., 2016). Since it is known that ChAT, calretinin, and VIP express interneurons belonging to a large group of cells containing the 5HT3a receptor and those expressing calretinin, but not ChAT and VIP, belong to somatostatin-containing interneurons (Tremblay et al., 2016), it can be assumed that the cells we observe in the cortex belong to the two largest systematic groups of GABAergic interneurons in the rat cerebral cortex.
CONCLUSIONS
In the present study, a comparative characteristic of the catecholaminergic system of three sections of the cerebral cortex was carried out during postnatal development.
Differences between cortical areas belonging to different functional systems were analyzed and it was found that the density of catecholaminergic fibers in the sensorimotor cortex increases with aging.
Our results show that the density of catecholaminergic fibers in the sensorimotor cortex increases with aging. This process involves changes in the levels of catecholamines (dopamine and noradrenaline) in the brain and may influence the behavioral changes associated with cognitive impairment that are observed with aging.
The present study shows that the confocal microscopy method has more opportunities for quantitative analysis of the results of immunocytochemical studies and can be used to analyze the distribution of tyrosine hydroxylase.
REFERENCES
Areal, L.B. and Blakely, R.D., Neurobehavioral changes arising from early life dopamine signaling perturbations, Neurochem. Int., 2020, vol. 137, p. 104747.
Arenas, E., Trupp, M., Åkerud, P., et al., GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo, Neuron, 1995, vol. 15, no. 6, pp. 1465–1473.
Asmus, S.E., Anderson, E.K., Ball, M.W., et al., Neurochemical characterization of tyrosine hydroxylase-immunoreactive interneurons in the developing rat cerebral cortex, Brain Res., 2008, vol. 1222, pp. 95–105.
Asmus, S.E., Cocanougher, B.T., Allen, D.L., et al., Increasing proportions of tyrosine hydroxylase-immunoreactive interneurons colocalize with choline acetyltransferase or vasoactive intestinal peptide in the developing rat cerebral cortex, Brain Res., 2011, vol. 1383, pp. 108–119.
Asmus, S.E., Raghanti, M.A., Beyerle, E.R., et al., Tyrosine hydroxylase-producing neurons in the human cerebral cortex do not colocalize with calcium-binding proteins or the serotonin 3A receptor, J. Chem. Neuroanat., 2016, vol. 78, pp. 1–9.
Barishpolets, V.V., Fedotova, Yu.O., and Sapronov, N.S., Structural and functional organization of the dopaminergic system of the brain, Eksp. Klin. Farmakol., 2009, vol. 72, no. 3, pp. 44–49.
Bell, K.F.S., Bennett, D.A., and Cuello, A.C., Paradoxical upregulation of glutamatergic presynaptic boutons during mild cognitive impairment, J. Neurosci., 2007, vol. 27, no. 40, pp. 10810–10817.
Benavides-Piccione, R. and DeFelipe, J., Distribution of neurons expressing tyrosine hydroxylase in the human cerebral cortex, J. Anat., 2007, vol. 211, no. 2, pp. 212–222.
Berger, B., Verney, C., Febvret, A., et al., Postnatal ontogenesis of the dopaminergic innervation in the rat anterior cingulate cortex (area 24). Immunocytochemical and catecholamine fluorescence histochemical analysis, Brain Res., 1985, vol. 353, no. 1, pp. 31–47.
Berger, B., Verney, C., Gaspar, P., et al., Transient expression of tyrosine hydroxylase immunoreactivity in some neurons of the rat neocortex during postnatal development, Brain Res., 1985, vol. 355, no. 1, pp. 141–144.
Bissonette, G.B. and Roesch, M.R., Development and function of the midbrain dopamine system: what we know and what we need to, Genes Brain Behav., 2016, vol. 15, no. 1, pp. 62–73.
Björklund, A., Handbook of Chemical Neuroanatomy, vol. 21: Dopamine, Amsterdam: Elsevier, 1992.
Bonnin, A., Miguel, R., Castro, J.G., et al., Effects of perinatal exposure to delta 9-tetrahydrocannabinol on the fetal and early postnatal development of tyrosine hydroxylase-containing neurons in rat brain, J. Mol. Neurosci., 1996, vol. 7, no. 4, pp. 291–308.
Brownstein, M., Saavedra, J.M., and Palkovits, M., Norepinephrine and dopamine in the limbic system of the rat, Brain Res., 1974, vol. 79, no. 3, pp. 431–436.
Cardozo-Pelaez, F., Song, S., Parthasarathy, A., et al., Oxidative DNA damage in the aging mouse brain, Mov. Disord., 1999, vol. 14, no. 6, pp. 972–980.
Cools, R., Role of dopamine in the motivational and cognitive control of behavior, Neuroscientist, 2008, vol. 14, no. 4, pp. 381–395.
Fallon, J., Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex, J. Neurosci., 1981, vol. 1, no. 12, pp. 1361–1368.
Finkel, T. and Holbrook, N.J., Oxidants, oxidative stress and the biology of ageing, Nature, 2000, vol. 408, no. 6809, pp. 239–247.
Gates, M.A., Torres, E.M., White, A., et al., Re-examining the ontogeny of substantia nigra dopamine neurons, Eur. J. Neurosci., 2006, vol. 23, no. 5, pp. 1384–1390.
Gogolla, N., The insular cortex, Curr. Biol., 2017, vol. 27, no. 12, pp. R580–R586.
Grigor’ev, I.P., Alekseeva, O.S., Kirik, O.V., et al., Distribution of low molecular weight proteins of neurofilaments in the cingulate cortex of the rat brain, Morfologiya, 2018, vol. 154, no. 5, pp. 7–12.
Grondin, R., Littrell, O.M., Zhang, Z., et al., GDNF revisited: a novel mammalian cell-derived variant form of GDNF increases dopamine turnover and improves brain biodistribution, Neuropharmacology, 2019, vol. 147, pp. 28–36.
Hamezah, H.S., Durani, L.W., Ibrahim, N.F., et al., Volumetric changes in the aging rat brain and its impact on cognitive and locomotor functions, Exp. Gerontol., 2017, vol. 99, pp. 69–79.
Kalinina, T.S. and Dygalo, N.N., Development of the noradrenergic system of the rat brain after prenatalexposure to corticosterone, Biol. Bull. (Moscow), 2013, vol. 40, no. 6, pp. 545–549.
Kalinina, T.S., Shishkina, G.T., and Dygalo, N.N., Induction of tyrosine hydroxylase gene expression by glucocorticoids in the perinatal rat brain is age-dependent, Neurochem. Res., 2012, vol. 37, no. 4, pp. 811–818.
Kortz, M.W. and Lillehei, K.O., Insular Cortex, FL: StatPearls Publishing, 2021.
Korzhevskii, D.E., Sukhorukova, E.G., Kirik, O.V., et al., Immunohistochemical demonstration of specific antigens in the human brain fixed in zinc–ethanol–formaldehyde, Eur. J. Histochem., 2015, vol. 59, no. 3, pp. 5–9.
Latsari, M., Dori, I., Antonopoulos, J., et al., Noradrenergic innervation of the developing and mature visual and motor cortex of the rat brain: a light and electron microscopic immunocytochemical analysis, J. Comp. Neurol., 2002, vol. 445, no. 2, pp. 145–158.
Levitt, P. and Moore, R.Y., Development of the noradrenergic innervation of neocortex, Brain Res., 1979, vol. 162, no. 2, pp. 243–259.
Livneh, Y. and Andermann, M.L., Cellular activity in insular cortex across seconds to hours: sensations and predictions of bodily states, Neuron, 2021, vol. 109, pp. 1–18.
Luo, Y. and Roth, G.S., The roles of dopamine oxidative stress and dopamine receptor signaling in aging and age-related neurodegeneration, Antioxid. Redox Signal., 2004, vol. 2, no. 3, pp. 449–460.
Matsunaga, W., Isobe, K., and Shirokawa, T., Involvement of neurotrophic factors in aging of noradrenergic innervations in hippocampus and frontal cortex, Neurosci. Res., 2006, vol. 54, no. 4, pp. 313–318.
Nomura, S., Bouhadana, M., Morel, C., et al., Noradrenalin and dopamine receptors both control cAMP-PKA signaling throughout the cerebral cortex, Front. Cell. Neurosci., 2014, vol. 8, article ID 247.
Norrara, B., Fiuza, F.P., Arrais, A.C., et al., Pattern of tyrosine hydroxylase expression during aging of mesolimbic pathway of the rat, J. Chem. Neuroanat., 2018, vol. 92, pp. 83–91.
Ohara, P.T., Granato, A., Moallem, T.M., et al., Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat, J. Neurocytol., 2003, vol. 32, no. 2, pp. 131–141.
Reynolds, L.M. and Flores, C., Mesocorticolimbic dopamine pathways across adolescence: diversity in development, Front. Neural Circuits, 2021, vol. 15, pp. 94–111.
Reynolds, L.M., Pokinko, M., Torres-Berrío, A., et al., Dcc receptors drive prefrontal cortex maturation by determining dopamine axon targeting in adolescence, Biol. Psychiatry, 2018, vol. 83, no. 2, pp. 181–192.
Rushworth, M.F.S., Noonan, M.A.P., Boorman, E.D., et al., Frontal cortex and reward-guided learning and decision-making, Neuron, 2011, vol. 70, no. 6, pp. 1054–1069.
Satoh, J. and Suzuki, K., Tyrosine hydroxylase-immunoreactive neurons in the mouse cerebral cortex during the postnatal period, Brain Res. Dev. Brain Res, 1990, vol. 53, no. 1, pp. 1–5.
Sukhareva, E.V., Kalinina, T.S., Bulygina, V.V., et al., Brain tyrosine hydroxylase and its regulation by glucocorticoids, Vavilov. Zh. Genet. Sel., 2016, vol. 20, no. 2, pp. 212–219.
Sukhorukova, E.G., Alekseeva, O.S., and Korzhevskii, D.E., Mammalian brain catecholaminergic neurons and neuromelanin, Zh. Evol. Biokhim. Fiziol., 2014, vol. 50, no. 5, pp. 336–342.
Tremblay, R., Lee, S., and Rudy, B., GABAergic interneurons in the neocortex: from cellular properties to circuits, Neuron, 2016, vol. 91, no. 2, pp. 260–292.
Ugrumov, M.V., Synthesis of monoamines by non-monoaminergic neurons: illusion or reality?, Fiziol. Zh. im. I.M. Sechenova, 2009, vol. 95, no. 3, pp. 273–282.
Ugrumov, M.V., Taxi, J., Tixier-Vidal, A., et al., Ontogenesis of tyrosine hydroxylase-immunopositive structures in the rat hypothalamus. An atlas of neuronal cell bodies, Neuroscience, 1989, vol. 29, no. 1, pp. 135–156.
Ugrumov, M.V., Melnikova, V., Ershov, P., et al., Tyrosine hydroxylase- and/or aromatic l-amino acid decarboxylase-expressing neurons in the rat arcuate nucleus: ontogenesis and functional significance, Psychoneuroendocrinology, 2002, vol. 27, no. 5, pp. 533–548.
Vogt, B.A., Vogt, L., and Farber, N.B., Cingulate cortex and disease models, in The Rat Nervous System, New York: Elsevier, 2004, Chapter 22, pp. 705–727.
Voorn, P., Kalsbeek, A., Jorritsma-Byham, B., et al., The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat, Neuroscience, 1988, vol. 25, no. 3, pp. 857–887.
Weihe, E., Depboylu, C., Schutz, B., et al., Three types of tyrosine hydroxylase-positive CNS neurons distinguished by DOPA decarboxylase and VMAT2 co-expression, Cell. Mol. Neurobiol., 2006, vol. 26, no. 4, pp. 657–676.
Weiss, B., Greenberg, L., and Cantor, E., Age-related alterations in the development of adrenergic denervation supersensitivity, Fed. Proc., 1979, vol. 38, no. 5, pp. 1915–1921.
Zaman, V., Li, Z., Middaugh, L., et al., The noradrenergic system of aged GDNF heterozygous mice, Cell Transplant., 2003, vol. 12, no. 3, pp. 291–303.
Funding
The work was carried out within the framework of the state task of the Federal State Budgetary Scientific Institution “Institute of Experimental Medicine.”
Author information
Authors and Affiliations
Contributions
V.A. Razenkova: taking material, wiring and pouring into paraffin blocks, staining preparations, photographing and analyzing preparations, statistical analysis of the obtained preparations, working with drawings, writing the text of the article. D.E. Korzhevskii: design of the experiment, work with drawings and writing the text of the article.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare they have no conflicts of interest.
Statement on the welfare of animals. During the study, all applicable international, national, and institutional (conclusion no. 1/20 dated February 27, 2020, of the local ethics committee of the IEM) principles of care and use of animals were observed.
Rights and permissions
About this article
Cite this article
Razenkova, V.A., Korzhevskii, D.E. Catecholaminergic Rat’s Forebrain Structures in Early Postnatal Development and Aging. Russ J Dev Biol 53, 208–216 (2022). https://doi.org/10.1134/S1062360422030067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360422030067