Abstract
We carried out a comparative morphometric analysis of neurons in the cat dorsal lateral geniculate nucleus (dLGN) in frontal vs. sagittal slices. Using the SMI-32 antibody to non-phosphorylated domains of heavy-chain neurofilaments, the postnatal dynamic of soma parameters (area, roundness, orientation) of dLGN neurons was studied. Measurements were performed in kittens aged 0, 4, 10, 14, 21, 28, 34, 62, 123 days, and in adult cats. A comparison of data obtained in frontal vs. sagittal slices revealed the following significant differences: (1) the soma area of the immunopositive neurons was smaller in frontal vs. sagittal slices in all age groups, and this difference increased with age; (2) the soma orientation was also different in two cutting planes, and a significant age-related change in the soma orientation occurred only in the sagittal, but not frontal, plane. We assume that the difference in the soma area is due to the spatial arrangement of SMI-32-immunopositive neurons in the dLGN, because of which, in the sagittal plane, in contrast to the frontal, neuronal somas were cut parallel to their long axis. In turn, age-related changes in the soma orientation may reflect an age-related internal rearrangement of the dLGN retinotopic organization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
An analysis of morphological parameters of neurons is one of the basics of the studies of the nervous system [1]. It allows not only fundamental conclusions [2, 3], but also assessment of the effect of pharmacological drugs [4, 5], aftereffects of experimental manipulations [6, 7], and the course of diseases [8–10]; these data are also lay the basis for mathematical modeling [11]. In practice, such cellular parameters as the size [3–6, 8], elongation and orientation of the cell soma [12], arrangement of neuronal processes [2, 13], number and spacing density of neurons [3, 8, 10, 14], as well as their clustering [15–17], are widely used.
Up-to-date experimental techniques make it possible to analyze cellular parameters based on three-dimensional reconstructions of cell populations [13, 18, 19]. However, the making and analysis of two-dimensional preparations does not lose its relevance due to the speed, availability and relative simplicity of the analysis of a large number of cells, which is especially important, for example, in clinical studies [14]. Obviously, due to various reasons, such as peculiarities of the anatomy and morphology of a living object and/or histological technique, certain variations in morphometric values in two-dimensional histological preparations are permissible. To reduce the variability of the studied parameters, it is critical to make a correct choice of the cutting plane and proper positioning of the histological specimen before preparing histological slices, especially for structures with a complex shape [14]. As an example, cell populations of the gray matter of the spinal cord could be presented, where, due to spatial orientation of the cell soma and dendrites, a reliable identification of different types of neurons is possible only through combining different cutting planes [20, 21].
When studying the postnatal development of neuronal populations in the dorsal thalamus, namely, the area of the soma of cells in the dorsal lateral geniculate nucleus (dLGN), immunopositive to non-phosphorylated domains of heavy-chain neurofilaments, unexpected discrepancies were obtained in the data when analyzing slices made in the sagittal and frontal planes. Accordingly, this work aimed to analyze comparatively the age-related dynamics of the morphometric parameters of dLGN neurons in slices made in the frontal and sagittal planes.
MATERIALS AND METHODS
The study was carried out in compliance with the requirements of the Directive 2010/63EU of the European Parliament and of the Council of September 22, 2010 on the protection of animals used for scientific purposes, and with the approval of the Ethics Committee of the Pavlov Institute of Physiology (conclusion No. 28/04 of April 28, 2021). We used 31 cats of both sexes, aged 0, 4, 10, 14, 21, 28, 34, 62 and 123 days (n = 2–4 in each group) and adult animals (n = 3). The protocol of perfusion, sampling and immunohistochemical labeling was described in detail elsewhere [21]. Using SMI-32 antibodies [22], non-phosphorylated domains of heavy-chain neurofilaments were detected by an indirect immunohistochemical method on free-floating slices, 50 µm thick. The slices were digitized using an Olympus CX33 brightfield microscope (Olympus Corporation, Japan) and a Nikon D3400 camera (Nikon Corporation, Japan).
Morphometric analysis of neurons immunopositive to SMI-32 (SMI-32(+)) was carried out using the Cell Annotation Software (CAS) according to the previously described method [23]. Image-based detection of SMI-32(+) cells was performed automatically using a Statistical Dominance Algorithm [24] with a subsequent manual error correction by an operator. To exclude small SMI-32(+) cell fragments from the analysis of morphometric parameters, only cells showing no immunohistochemical reaction in the central part of the soma (which corresponded to the position of the unlabeled cell nucleus) were analyzed [25]. To measure the neuron’s soma parameters, the isolated processes of SMI-32(+) cells were cut off using successive procedures removing and adding the identical number of pixels along the perimeter of the detected objects. The number of pixels was chosen empirically and applied to the entire experimental sample.
The following morphometric parameters were analyzed:
1. cross-sectional area of the SMI-32(+) cell soma (µm2);
2. SMI-32(+) cell soma roundness, using “the proximity of
the soma shape to the round” parameter calculated by the formula:  . The resultant values varied
from 0 to 1, where 0.1—extremely elongated oval and 1—round;
. The resultant values varied
from 0 to 1, where 0.1—extremely elongated oval and 1—round;
3. orientation of the soma’s long axis (to determine this parameter, the best fitting ellipse to the detected cell soma was taken). The angle of the soma’s long axis was calculated relative to the A/A1 interlaminar border, where 0°—the soma is oriented perpendicular to the interlaminar border; negative values—rostral (in sagittal slices) or medial (in frontal slices) obliquity of the soma; positive values—caudal (in sagittal slices) and lateral (in frontal slices) obliquity of the soma.
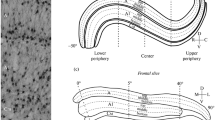
The dLGN has a complex shape, which, in the first approximation, resembles an ellipsoid, the rostral and caudal poles of which are thinned and bent back in opposite directions. As a result, in frontal slices, the shape of the nucleus varies from spherical (at the rostral and caudal poles), to wedge-shaped (in the central part). Accordingly, in sagittal slices, the nucleus is S-shaped (Fig. 1a). The immunopositive reaction was assessed in the two widest dLGN laminae, A and A1. SMI-32(+) cells in the central part of the laminae were analyzed in both cutting planes (gray area in Fig. 1a), where it was possible to draw a straight line along the A/A1 interlaminar border, relative to which cell soma orientation was assessed.
In total, 26711 and 22495 SMI-32(+) cells were analyzed in frontal (Fr.) and sagittal (Sag.) slices, respectively; in individual comparison groups, from 1113 to 4915 SMI-32(+) cells were examined, depending on the age and cutting plane. To compare the samples, we used the Nested ANOVA and post-hoc Tukey’s test (for multiple comparisons) and the Nested t-test (for paired comparisons), developed specially for small samples [26], where N is the number of animals in a certain group, while n is the number of slices per animal. For the majority of animals, by 3 slices were analyzed in each plane; in some animals, only frontal or sagittal slices were used. The data on the dynamics of the SMI-32(+) cell soma area and orientation in sagittal slices were reported previously [27]. The numerical data in the figures are presented as mean ± SD.
Morphometric parameters of the dorsal lateral geniculate nucleus (dLGN). (a) Frontal (left) and sagittal (right) slices of the dLGN; A/A1 are analyzed laminae, the area of soma orientation analysis is marked in gray. (b) Age-related soma dynamics in the area of SMI-32 positive (+) cells. Note that not all statistical differences, but only those between the newborn (0) kittens and adults (Ad.), and between the newborn kittens/adults and significantly different age groups nearest to them are shown. Significant differences between the soma areas in frontal (black) and sagittal (gray) slices within age groups are also shown. (c) An example of SMI-32(+) cells in laminae A/A1 in a sagittal slice. (d) SMI-32(+) cell soma orientation in A/A1 laminae in frontal and sagittal slices; dotted line shows the trend; significant differences between newborns (0) and adults (Ad) are shown. (e) Schematic representation of SMI-32(+) cell soma rotation in the sagittal plane relative to the interlaminar A/A1 border. Common notes: 0–123—age groups (days of life), Ad—adult animals; R, C, M, L, D, V—rostral, caudal, medial, lateral, dorsal, and ventral directions, respectively; data on graphs represent mean values ± SD; * p < 0.05, ** p < 0.01; *** p < 0.001. Pairwise comparisons: post-hoc Tukey’s test or Nested t-test.
RESULTS
Soma area in SMI-32(+) neurons
The first thing that attracts attention when comparing the two cutting planes is a larger soma area of neurons on sagittal vs. frontal slices regardless of the age of animals (Fig. 1b). At the same time, the difference between the soma area in different cutting planes increases from 19 µm2 (15%) in newborn animals to 127 µm2 (41%) in adults (Table 1).
Analysis of the age-related dynamics within the cutting plane shows a significant increase in the soma area with age (Fr.: F (9, 17) = 18.75, p < 0.001; Sag.: F (9, 15) = 19.18, p < 0.001 ). A significant increment in the growth of the soma area, both in frontal and sagittal slices, occurs by day 10 (Fr.: 0D vs. 10D, p < 0.01; Sag.: 0D vs. 10D, p < 0.05). After this age, soma growth slows down; the difference in the soma size loses its statistical significance in frontal slices by day 14 (Fr.: 14D vs. Ad, p > 0.05), and in sagittal slices, a week later, by day 21 (Sag.: 21D vs. Ad, p > 0.05 ) (Fig. 1b).
Soma roundness in SMI-32(+) neurons
When analyzing other morphometric characteristics of the studied cell population, it was noted that the soma of these neurons is, on average, not round but oval in shape (Fig.1c) with a mean roundness value equal to 0.69 ± 0.04, identical both for frontal and sagittal slices (this index is used to denote the soma in Fig. 1e).
Pairwise comparisons of the roundness values in frontal and sagittal slices within age groups showed statistically significant differences only in the group of newborn animals (0D: Fr. vs. Sag.; F (1, 6) = 94.58; p < 0.001), with cells being more rounded in frontal (0.72 ± 0.02) vs. sagittal (0.66 ± 0.03) slices.
Soma orientation in SMI-32(+) neurons
Based on the oval soma shape in dLGN neurons, and the revealed differences in the soma area, it appeared necessary to determine the spatial orientation of the studied cell population. The A/A1 interlaminar border was chosen as the reference plane. It was shown that in frontal slices the soma of SMI-32(+) cells is generally oriented perpendicular to the interlaminar border with a slight medial obliquity (up to –20° in the 62D group), without showing any age-related trend (F (9,18) = 1.170, p > 0.05) (Fig. 1d, black circles). At the same time, in sagittal slices, the soma of SMI-32(+) cells is oriented with a rostral obliquity, which significantly increases with age (F (9, 15) = 6.653, p < 0.001) (Fig. 1d, gray circles) from –24° in newborns to –46° in adult animals (0D vs. Ad, p < 0.01).
DISCUSSION
As a result of our study, it was established that (1) the soma of SMI-32(+) cells has an elongated shape, (2) during postnatal development, the long axis of the soma turns relative to the A/A1 interlaminar border in sagittal, but not frontal, slices, and (3) the soma area is larger in sagittal vs. frontal slices, and this difference increases with age.
Our findings allow the following interpretation (Fig. 1e). The frontal cutting plane, in general, runs perpendicular to the A/A1 interlaminar border, while the soma of SMI-32(+) cells has a certain rostral obliquity relative to this border. To register a maximum soma area, it is necessary to cut along the long axis of the cell. Apparently, this condition is met to a greater extent when cutting in the sagittal, not frontal, plane. As a result, the registered soma area proves to be smaller in frontal vs. sagittal slices. With age, the rostral turn of the soma increases the observed difference in its area in frontal vs. sagittal slices. In addition, the observed turn of the soma can explain the one-week difference in the cessation of SMI-32(+) cell soma growth (two-week in frontal vs. three-week in sagittal slices): in parallel to the permanent growth of the soma of dLGN neurons, their sagittal rotation occurred, reducing the cross-sectional area of the neuronal soma in the frontal plane. Thus, both parameters mutually compensate each other in frontal, but not sagittal, slices. Since the first month of life is of utmost importance for the development of the cat visual system [28], the one-week difference in determining the time limits for the soma growth appears to be considerable.
The soma orientation of SMI-32(+) neurons, as detected in this work, is close to the direction of isometric lines on the retinotopic map in the dLGN [29–31]. In turn, other observations show that soma elongation in dLGN neurons often coincides with the general orientation of their dendritic tree [32, 33]. Despite the fact that antibodies to SMI-32 reveal the soma and only the proximal processes of large dLGN neurons [34, 35], it can be assumed that the dendritic tree of SMI-32(+) cells described in this work, as well as their soma, is generally elongated parallel to isometric lines of retinotopic representation regardless of age. A change in the obliquity angle of the soma in the sagittal plane indicates previously unknown changes in the internal structure of the cat dLGN (specifically, a displacement of isometric lines of retinotopic representation), which arise along with a general increase in its volume [36, 37]. Also, postnatal growth of dLGN neurons is accompanied by an increase in the diameter and a reorganization of their dendritic trees [38–40]. In connection with the above assumptions, morphometric parameters of the dendritic tree, probably likewise the soma size, may differ depending on the analyzed cutting plane.
Thus, the observed difference in the soma size of SMI-32(+) cells in frontal vs. sagittal slices is determined by the rostral obliquity of their soma relative to the A/A1 interlaminar border. With age, the rostral tilt of the soma becomes more pronounced, increasing thereby the registered difference in the soma size in frontal vs. sagittal slices and, probably, reflecting the displacement of the structures responsible for the general architectonics of the dLGN.
These results indicate the importance of considering the peculiarities of the layout of the analyzed cells in the studied structure in investigating the temporal developmental pattern of their morphometric characteristics.
REFERENCES
Oberlaender M (2019) Neuronal Morphology and Its Significance. In: Singer W, Sejnowski TJ, Rakic P (eds) The Neocortex. The MIT Press, Cambridge, Massachusetts, pp 124–138.
Hendry SHC, Jones EG (1983) The organization of pyramidal and non-pyramidal cell dendrites in relation to thalamic afferent terminations in the monkey somatic sensory cortex. J Neurocytol 12:277–298. https://doi.org/10.1007/BF01148465
Herculano-Houzel S, Manger PR, Kaas JH (2014) Brain scaling in mammalian evolution as a consequence of concerted and mosaic changes in numbers of neurons and average neuronal cell size. Front Neuroanat 8:1–28. https://doi.org/10.3389/fnana.2014.00077
Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF (2009) Pharmacological inhibition of mTORCl suppresses anatomical, cellular, and behavioral abnormalities in neural-specific PTEN knock-out mice. J Neurosci 29:1773–1783. https://doi.org/10.1523/JNEUROSCI.5685-08.2009
Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ (1996) Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA 93:11202–11207. https://doi.org/10.1073/pnas.93.20.11202
Loopuijt LD, Villablanca JR, Sharifi P (2001) Soma size of substantia nigra neurons increases after a prenatal neocortical lesion in cats. Dev Brain Res 130:143–147. https://doi.org/10.1016/S0165-3806(01)00223-1
Duffy KR, Slusar JE (2009) Monocular deprivation provokes alteration of the neuronal cytoskeleton in developing cat lateral geniculate nucleus. Vis Neurosci 26:319–28. https://doi.org/10.1017/S0952523809090130
Flood DG, Coleman PD (1988) Neuron numbers and sizes in aging brain: Comparisons of human, monkey, and rodent data. Neurobiol Aging 9:453–463. https://doi.org/10.1016/S0197-4580(88)80098-8
Duyckaerts C, Delatour B, Potier MC (2009) Classification and basic pathology of Alzheimer disease. Acta Neuropathol 118:5–36. https://doi.org/10.1007/s00401-009-0532-1
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HBM, Friedman L, Rajkowska G (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56:640–650. https://doi.org/10.1016/j.biopsych.2004.08.022
Rajković K, Marić DL, Milošević NT, Jeremic S, Arsenijević VA, Rajković N (2016) Mathematical modeling of the neuron morphology using two dimensional images. J Theor Biol 390:80–85. https://doi.org/10.1016/j.jtbi.2015.11.019
FitzGibbon T (2006) Does the development of the perigeniculate nucleus support the notion of a hierarchical progression within the visual pathway? Neuroscience 140:529–546. https://doi.org/10.1016/j.neuroscience.2006.02.038
Acciai L, Soda P, Iannello G (2016) Automated Neuron Tracing Methods: An Updated Account. Neuroinformatics 14:353–367. https://doi.org/10.1007/s12021-016-9310-0
Garman RH, Li AA, Kaufmann W, Auer RN, Bolon B (2016) Recommended Methods for Brain Processing and Quantitative Analysis in Rodent Developmental Neurotoxicity Studies. Toxicol Pathol 44:14–42. https://doi.org/10.1177/0192623315596858
Burnat K, Van Der Gucht E, Waleszczyk WJ, Kossut M, Arckens L (2012) Lack of early pattern stimulation prevents normal development of the alpha (Y) retinal ganglion cell population in the cat. J Comp Neurol 520:2414–2429. https://doi.org/10.1002/cne.23045
Merkulyeva NS, Mikhalkin AAA, Veshchitskii AAA, Merkul’eva NS, Mikhalkin AAA, Veshchitskii AAA (2016) Characteristics of the Distribution of Acetylcholinesterase in the Posterolateral Nucleus of the Thalamus in Cats. Neurosci Behav Physiol 46:507–509. https://doi.org/10.1007/s11055-016-0267-0
Mooser F, Bosking WH, Fitzpatrick D (2004) A morphological basis for orientation tuning in primary visual cortex. Nat Neurosci 7:872–879. https://doi.org/10.1038/nn1287
Yan C, Li A, Zhang B, Ding W, Luo Q, Gong H (2013) Automated and Accurate Detection of Soma Location and Surface Morphology in Large-Scale 3D Neuron Images. PLoS One 8:1–12. https://doi.org/10.1371/journal.pone.0062579
Radojević M, Meijering E (2019) Automated Neuron Reconstruction from 3D Fluorescence Microscopy Images Using Sequential Monte Carlo Estimation. Neuroinformatics 17:423–442. https://doi.org/10.1007/s12021-018-9407-8
Lima D, Coimbra A (1986) A Golgi study of the neuronal population of the marginal zone (lamina I) of the rat spinal cord. J Comp Neurol 244:53–71. https://doi.org/10.1002/cne.902440105
Merkulyeva N, Veshchitskii A, Makarov F, Gerasimenko Y, Musienko P (2016) Distribution of 28 kDa calbindin-immunopositive neurons in the cat spinal cord. Front Neuroanat 9:166. https://doi.org/10.3389/fnana.2015.00166
Sternberger LA, Sternberger NH (1983) Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A 80:6126–6130. https://doi.org/10.1073/pnas.80.19.6126
Nurzynska K, Mikhalkin A, Piorkowski A (2017) CAS: Cell Annotation Software—Research on Neuronal Tissue Has Never Been so Transparent. Neuroinformatics 15:365–382. https://doi.org/10.1007/s12021-017-9340-2
Piorkowski A, Piórkowski A (2016) A statistical dominance algorithm for edge detection and segmentation of medical images. Adv Intell Syst Comput 471:3–14. https://doi.org/10.1007/978-3-319-39796-2_1
Kutcher MR, Duffy KR (2007) Cytoskeleton alteration correlates with gross structural plasticity in the cat lateral geniculate nucleus. Vis Neurosci 24:775–785. https://doi.org/10.1017/S095252380707068X
Quené H, Van Den Bergh H (2004) On multi-level modeling of data from repeated measures designs: A tutorial. Speech Commun 43:103–121. https://doi.org/10.1016/j.specom.2004.02.004
Mikhalkin A, Nikitina N, Merkulyeva N (2020) Heterochrony of postnatal accumulation of nonphosphorylated heavy‐chain neurofilament by neurons of the cat dorsal lateral geniculate nucleus. J Comp Neurol 529(7):1–12. https://doi.org/10.1002/cne.25028
Feller MB, Scanziani M (2005) A precritical period for plasticity in visual cortex. Curr Opin Neurobiol 15:94–100. https://doi.org/10.1016/j.conb.2005.01.012
Eysel UT, Wolfhard U (1983) Morphological fine tuning of retinotopy within the cat lateral geniculate nucleus. Neurosci Lett 39:15–20. https://doi.org/10.1016/0304-3940(83)90158-1
Bishop PO, Kozak W, Levick WR, Vakkur GJ (1962) The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol 163:503–539. https://doi.org/10.1113/jphysiol.1962.sp006991
Sanderson KJ (1971) The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol 143:101–117. https://doi.org/10.1002/cne.901430107
Stanford LR, Friedlander MJ, Sherman SM (1983) Morphological and physiological properties of geniculate W-cells of the cat: a comparison with X- and Y-cells. J Neurophysiol 50:582–608. https://doi.org/10.1152/jn.1983.50.3.582
Friedlander MJ, Lin CS, Stanford LR, Sherman SM (1981) Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol 46:80–129. https://doi.org/10.1152/jn.1981.46.1.80
Bickford ME, Guido W, Godwin DW (1998) Neurofilament proteins in Y-cells of the cat lateral geniculate nucleus: Normal expression and alteration with visual deprivation. J Neurosci 18:6549–6557. https://doi.org/10.1523/JNEUROSCI.18-16-06549.1998
Duffy KR, Crowder NA, LeDue EE (2012) Investigation of cytoskeleton proteins in neurons of the cat lateral geniculate nucleus. J Comp Neurol 520:186–199. https://doi.org/10.1002/cne.22727
Kalil R (1978) Development of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol 182:265–291. https://doi.org/10.1002/cne.901820206
Elgeti H, Elgeti R, Fleischhauer K (1976) Postnatal growth of the dorsal lateral geniculate nucleus of the cat. Anat Embryol (Berl) 149:1–13. https://doi.org/10.1007/BF00315081
Coleman LA, Friedlander MJ (2002) Postnatal dendritic development of Y-like geniculocortical relay neurons. Int J Dev Neurosci 20:137–159. https://doi.org/10.1016/S0736-5748(02)00018-7
Coleman LA, Friedlander MJ (1992) Intracellular injections of permanent tracers in the fixed slice: a comparison of HRP and biocytin. J Neurosci Methods 44:167–177. https://doi.org/10.1016/0165-0270(92)90009-3
Friedlander MJ (1982) Structure of physiologically classified neurones in the kitten dorsal lateral geniculate nucleus. Nature 300:180–183. https://doi.org/10.1038/300180a0
ACKNOWLEDGMENT
The authors are grateful to N.I. Nikitina for her assistance in microscopic studies.
Funding
This work was supported by the Governmental Program 47 “Scientific and Technological Development of the Russian Federation” for 2019–2030, theme 0134-2019-0006 (theoretical part) and the Russian Science Foundation grant No. 21-15-00235 (experimental part).
Author information
Authors and Affiliations
Contributions
A concept and experimental design: A.A.M. and N.S.M.; data collection and processing: A.A.M.; statistical data treatment: A.A.M.; data analysis and interpretation: A.A.M. and N.S.M.; manuscript writing: A.A.M. and N.S.M.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest, both evident and potential, as related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2021, Vol. 57, No. 5, pp. 373–379https://doi.org/10.31857/S0044452921050053.
Rights and permissions
About this article
Cite this article
Mikhalkin, A.A., Merkulyeva, N.S. Peculiarities of Age-Related Dynamics of Neurons in the Cat Lateral Geniculate Nucleus as Revealed in Frontal versus Sagittal Slices. J Evol Biochem Phys 57, 1001–1007 (2021). https://doi.org/10.1134/S0022093021050021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021050021