Abstract
Functional interaction of the gastrointestinal tract (GI) and the central nervous system (CNS) is due to various relationships, which includes autonomic and enteral nervous systems as well as the immune and neuroendocrine systems. The microbiota of the macroorganism plays the central role in this interaction. Microbiota produces hundreds of biologically active substances that have a neurochemical effects through neuroendocrine, immune, and metabolic pathways. The microbiota also synthesizes and releases products (neurotoxins, neurotransmitters, lipopolysaccharides, amyloids, etc.) that can negatively affect the neurochemistry of the CNS, stimulating the development of amyloidosis, synucleinopathies, and tauopathies, thereby promoting the development and/or progression of neurodegenerative diseases. Under the influence of external and internal factors, human microbiota can be changed and the symbionts/pathogens ratio is also changed. The permeability of intestinal and blood-brain barrier varies. Metabolites produced by the altered microflora are able to enter the bloodstream and possibly into the CNS, thereby disrupting its functioning. Infections can play a significant role and even act as a cofactor in the induction of neurodegenerative diseases. Disturbance of the functions of the GI can precede long before the neurodegenerative processes. Early diagnosis, detection, monitoring, and treatment of negative gastrointestinal symptoms, including normalization of the microbiota, can lead to a significant improvement in the quality of life of patients with neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Functional interaction of the gastrointestinal tract (GI) and the central nervous system (CNS) (gut–brain axis) includes the vegetative nervous system and the immune and neuroendocrine systems (Diaz Heijtz et al., 2011; Forsythe et al., 2012; Aziz et al., 2013; Schwartz and Boles, 2013; Holzer and Farzi, 2014; Lyte and Cryan, 2014; Foster et al., 2016) (Fig. 1). The main GI functions are controlled by the enteric nervous system (ENS), which is a part of the peripheral nervous system. The ENS is a specialized autonomous nervous system, which is a part of the metasympathetic nervous system and is virtually independent of the effect of central mechanisms (Hansen, 2003; Furness et al., 2014; Obata and Pachnis, 2016). Internal and external factors can also influence (sometimes significantly) on the ENS function. For example, the ENS activity can depend on ingredients contained in the intestine, such as glucose, short chain fatty acids (butyric, propionic, and acetic acid), as well as depend on pH of the environment. Functional dependence of the ENS can be both long-term and short-term. For example, butyric acid, serotonin, cytokines can sufficiently quickly (within seconds) stimulate the ENS activity. In the long term, active substances can stimulate a change in ENS neurochemical phenotype can affect gene expression changing the appropriate amount of ENS neurons (Soret et al., 2010).
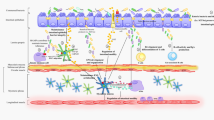
Interaction of microbiota with the brain and other macroorganism organs. The nervous and humoral regulation in the organism is performed by means of neurotransmitters, neuropeptides, and hormones that are simultaneously modulating signals from the brain to the immune system. Besides specific receptors for the immune response, the immune system cells (2) have receptors, particularly, to hormones (1) and neurotransmitters (3); the latter are involved in the regulation of immune reactions affecting the proliferation of lymphocytes and macrophages, modulating the production of lymphokines and cytokines, etc. The intestine has a bidirectional link with CNS using endocrine (1), immune (2), and neural pathways (3) (the gut–brain axis). Since microbiota produces different neurotransmitters and active substances, it is included in the regulation systems of the gut–brain axis (shown by arrows). Microbiota can directly activate ENS (3) using Toll-like receptors in neurons. Pathogenic microorganisms and pathobionts located in places of the mucosal immune system (GI, lungs, urogenital system, nasopharynx, skin) and in other parts of the organism can negatively affect the nerve tissue of the whole organism. Microbiota can stimulate different immune reactions. When an antigen/pathogen invades, the immune reactions are directed to the antigen/pathogen elimination. An increase in the concentration of secretory IgA in the organism, which controls the number of microbiota, is one of such reactions. ENS contains the nerve endings in mucous plexuses that can come into contact with antigen-presenting cells to control the intestine immune responses (2–3). In addition, the release of norepinephrine, acetylcholine, vasoactive peptides, and neuropeptides by the nerve fibers modulate IgA secretion. Stimulating/inhibiting the production of IgA by means of neurohumoral regulation, the organism can control the number of microorganisms on the mucous membranes of the intestine, lungs, etc. The nervous system can also regulate the immune function through the hypothalamic-pituitary-adrenal system stimulating the production of glucocorticoids by kidneys that modulate the organism immune response (4). Microbiota can affect the cardiovascular system (CVS) and, consequently, the blood supply of the brain. For example, microbiota can directly affect the nervus vagus (5) and, through it, the heart activity; can also be involved in the processes of atherogenesis (6). The products of microbiota metabolism, lipopolysaccharides, etc. can stimulate the contraction/relaxation of the blood vessels and heart (6). Microbiota and its metabolites can both stimulate and negatively affect the CVS decreasing the heart work and increasing the probability of an infarction, stroke, and chronic heart failure (CHF). A change in the intestinal microcirculation due to a decreased cardiac output and venous congestion is the important moment in the progression of inflammation in patients with CHF (7). A change in the intestine microcirculation leads to an increase in the permeability of the intestine mucosa with the violation of the epithelium barrier function; this promotes the bacterial colonization and increased LPS penetration in the blood circulation system. The chronic intestine ischemia is usually manifested by prolonged constipation that frequently accompanies Parkinson’s disease. Impaired renal function leads to the accumulation of toxic products of microbiota and contributes to systemic inflammation; this leads to an acceleration of the pathogenesis of CVS diseases and neurodegeneration. Cardiovascular diseases are the main reason for the mortality of patients with chronic renal failure. Microbiota in Fig. 1 includes both microorganisms and their metabolites and the cell fragments, including microorganism RNA and DNA. ENS, enteric nervous system; CVS, cardiovascular system; CHF, chronic heart failure. Mutual regulation of the gut–brain axis: (1) hormonal; (2) immune; (3) nervous; (4) kidney–CNS link; (5) vagus nerve and heart link; (6) microbiota and CVS link; (7) intestine–heart mutual link. Mutual regulatory links (microbiota–kidneys, CVS–kidneys, brain–CVS) are not shown.
No doubt, functional interaction of GI and CNS has a bilateral character and plays an important role in coordination of metabolic and homeostatic functions of the organism as well as in functional violations and chronic diseases, such as diabetes, metabolic syndrome, obesity, a number of autoimmune diseases, stress-induced and psychosomatic disorders, and some neurodegenerative diseases (Backhed et al., 2005; Bravo et al., 2012; Foster et al., 2013; Hornig, 2013; Udit and Gautron, 2013; Dinan and Cryan, 2017).
Numerous evidence of the close interaction of intestinal microbiota and the immune and the central nervous systems of the macroorganism has been shown in the last few years (Rhee et al., 2009; Lyte and Cryan, 2014; Mayer et al., 2014, 2015; Dinan and Cryan, 2017) (Fig. 1). The mechanisms of this interaction are intensively studied both in the norm and in different diseases of the intestine and CNS. Microbiota and the brain can influence each other. The brain effect on microbiota can be manifested in a change of peristalsis and permeability of GI walls as well as in the secretion of active substances. The macroorganism can regulate the hormone release in the intestinal lumen through the regulation of the activity of enterochromaffin cells, thereby affecting both microbiota and the mucosal immune system (mucosa-associated lymphoid tissue, MALT). On the other hand, active substances secreted by enterochromaffin cells can affect the vagus nerve, causing, for example, changes in the intestine peristalsis (up to the vomiting reflex). Such association plays an important role in the pain syndrome, the intestine homeostasis control, and even in the control of the emotional state. The violation of such gut–brain interaction can be observed in different types of GI and CNS, diseases (Rhee et al., 2009; Lyte and Cryan, 2014; Mayer et al., 2015).
The ability of GI bacteria to affect the immune, endocrine, cardiovascular, and nervous systems of the host organism is of special interest (Sobol et al., 2005; Bravo et al., 2012; Sobol et al., 2013; Bhattacharjee and Lukiw, 2013; McVey Neufeld et al., 2013; Heintz and Mair, 2014; Lyte and Cryan, 2014; Sobol, 2014, 2017b; Sobol and Belostotskaya, 2015; Dinan and Cryan, 2017) (Fig. 1). This field is rapidly developing with the emergence of new sequencing and bioinformatic methods and is supplemented by new evidence about the significant role of microbiota and the complex character of relationships of microbial communities between themselves and the host organism (Fig. 1).
The studies conducted on germ-free mice demonstrated that microbiota can affect the passive electric properties of ENS neurons, action potential, and excitability of sensor neurons, thus providing the potential association between GI microbiota and CNS (Foster and McVey Neufeld, 2013; Hornig, 2013; McVey Neufeld et al., 2013) (Fig. 1). In addition, microbiota can activate Toll-like receptors located on ENS neurons (Barajon et al., 2009). Thus, microbiota can send signals to ENS and CNS (Bhattacharjee and Lukiw, 2013; Foster and McVey Neufeld, 2013; Hornig, 2013; McVey Neufeld et al., 2013).
Specialized endocrine glands and the nervous system synthesize a limited number of active substances (hormones and neurotransmitters), while microbiota produces hundreds of biologically active substances (Tkachenko and Uspenskii, 2006; Shenderov, 2011; Sobol, 2014; Clarke et al., 2014; Holzer and Farzi, 2014; Lyte and Cryan, 2014; Wall et al., 2014; Oleskin et al., 2016; Oleskin and Shenderov, 2016; Dinan and Cryan 2017) that influence neurochemical reactions through endocrine, immune, and metabolic pathways (Galland, 2014; Lyte and Cryan, 2014; Oleskin et al., 2016). It should be noted that, besides active substances, the cell fragments, as well as the cell wall of microorganisms, can cause a receptor response of the organism cells (for example, though Toll-like receptors), including neurons (Sobol et al., 2005; Barajon et al., 2009; McVey Neufeld et al., 2013).
The gastrointestinal tract physiology and motility are affected by the signals (molecules) produced directly in GI, as well as in CNS. In turn, neurotransmitters, signaling molecules of the immune system, hormones, and neuropeptides produced in GI can affect the brain (Fig. 1) (Tkachenko and Uspenskii, 2006; Lyte, 2013; Galland, 2014; Selkrig et al., 2014; Wall et al., 2014). The researchers have increasingly begun to identify strong effects of microbiota on the development of CNS (Lyte, 2013; Galland, 2014; Selkrig et al., 2014; Sharon et al., 2016). Certain species of lactobacilli in the intestine, for example, such as Lactobacillus brevis and Bifidobacterium dentium, are able to metabolize glutamate in gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter of human CNS (Barrett et al., 2012). Anxiety and depression, as well as defects in the formation of synapses and cognitive violations, are associated with a dysfunction in the GABA-ergic system of synaptic regulation, including Alzheimer’s disease (Aziz et al., 2013; Hornig, 2013; McVey Neufeld et al., 2013; Mitew et al., 2013; Saulnier et al., 2013). However, the involvement of GABA synthesized by microbiota in neurochemical processes of CNS has been insufficiently studied.
The synthesis of brain-derived neurotrophic factor (BDNF) can be another important example. It is known that BDNF plays an important role in the maintenance of the viability of neurons, contributes to the survival and growth of neurons, as well as synaptogenesis, has a pleiotropic effect on the development of neurons and their differentiation and on the development of synapses and synaptic plasticity, which underlie the development of nervous connections and cognitive functions. The concentration of BDNF is decreased in the brain and in the blood serum in patients with increased anxiety, schizophrenia, and Alzheimer’s disease (Carlino et al., 2013; Lu et al., 2013; Mitew et al., 2013). In the experimental studies conducted on germ-free mice, a decrease in the expression of BDNF in the hippocampus and cerebral cortex of such mice (which was associated with an increase in the anxiety in behavior and the cognitive dysfunction progression) was demonstrated (Bhattacharjee and Lukiw, 2013; Carlino et al., 2013; Foster et al., 2013; Lu et al., 2013).
The intestinal microbiota can produce dopamine and its precursors from the food substrates, and almost half of the dopamine in the organism is developed in GI (Eisenhofer et al., 1997; Wall et al., 2014). It is possible that microbiota can affect the dopamine level in the brain, the amount of which is decreased in Parkinson’s disease. For example, the level of tyrosine in nonmicrobial mice is decreased (Matsumoto et al., 2013). Microbiota also produces acetylcholine, serotonin, noradrenaline, and other biologically active substances (Lyte and Cryan, 2014; Wall et al., 2014).
The fact that some members of the macroorganism microbiota can activate glutamate receptors (that in turn are involved in the regulation of synaptic plasticity and cognitive functions) is another interesting fact (Lakhan et al., 2013). Thus, microbiota for the neuron activation involves the same types of receptors that are involved at natural activation of the nerve cells.
However, besides nutrients and bioactive molecules, microbiota can synthesize and release the products (neurotoxins, neurotransmitters, lipopolysaccharides, amyloids, and etc.) that can negatively influence on the CNS neurochemical reactions stimulating the development of amyloidosis, sinucleinopathy, and tauopathy, thus contributing to the development and/or progression of neurodegenerative diseases (Derkinderen et al., 2011; Ball et al., 2013; Douglas-Escobar et al., 2013; Albenberg and Wu, 2014; Asti and Gioglio, 2014). Lipopolysaccharides and fragments of microorganism cell walls can facilitate the development of amyloidosis. For example, they can initiate the aggregation of beta amyloid (Asti and Gioglio, 2014). Herpes simplex virus (HSV-1) glycoprotein B has a high degree of homology with beta amyloid and can stimulate amyloidosis in vitro (Cribbs et al., 2000). Bacterial amyloids (as well as all functional amyloids) have structural and biochemical properties similar to eukaryotic amyloids and neurodegenerative diseases associated with them. In addition, infectious agents can initiate autoimmune reactions leading to the development of the disease (Carter, 2010), especially in genetically predisposed individuals. Thus, microbiota can stimulate different inflammatory reactions contributing to the development and/or progression of neurodegenerative diseases (Derkinderen et al., 2011; Ball et al., 2013; Douglas-Escobar et al., 2013; Schwartz and Boles, 2013; Albenberg and Wu, 2014; Asti and Gioglio, 2014).
There is an assumption that the intestinal microbiota cyanobacteria can produce an excess of neurotoxic nonprotein amino acid beta-methylamino-L-alanine (BMAA) (Brenner, 2013), increased concentrations of which were detected in the brain of patients with amyotrophic lateral sclerosis and Parkinson’s and Alzheimer’s diseases (Bhattacharjee and Lukiw, 2013). BMAA neurotoxin is the amino acid that is not a part of the proteins; however, it is involved in the processes of incorrect protein folding associated with neurodegenerative diseases (Mulligan and Chakrabartty, 2013). Cyanobacteria have high adaptive properties and are highly adapted to symbiotrophic relationships. The stress, GI diseases, or lack of nutrition can cause the additional BMAA production by cyanobacteria, which can contribute to neurological dysfunction (Bhattacharjee and Lukiw, 2013; Brenner, 2013). Being human microbiota residents, different types of cyanobacteria can generate different neurotoxins, such as neurotoxic alkaloids (anatoxins and saxitoxins) (Brenner, 2013; Voloshko and Pinevich, 2014), that also contribute to the development of human neurological diseases (Bhattacharjee and Lukiw, 2013; Brenner, 2013). These peculiarities of microbiota are especially dangerous for elderly individuals, in which the permeability of both GI and blood-brain barrier is disturbed; as a result, the CNS becomes more vulnerable to the toxic products of human microbiota (Tran and Greenwood-Van Meerveld, 2013). In addition, toxins of cyanobacteria can induce different GI complications in humans, including liver damage, as well as pneumonia, cancer, and different allergic reactions (Voloshko and Pinevich, 2014).
A high level of amyotrophic lateral sclerosis/Parkinson’s disease morbidity (by 50–100 times) was distributed among Chamorro people from the Guam Islands (Cox et al., 2003; Holtcamp, 2012) who used flour from the seeds of neurotoxic sago palm (Cycas micronesica) plants in large amounts. The roots of this plant generate symbiotic relationships with cyanobacteria of the Nostoc genus producing BMAA (Cox et al., 2003; Holtcamp, 2012).
The results of the studies confirm the hypothesis that an increased level of amyotrophic lateral sclerosis morbidity can be caused by the quality of lake water for the drinking water, in which the concentration of cyanobacteria is increased, and by environmental conditions (Holtcamp, 2012; Torbick et al., 2014). BMAA freely penetrates the blood-brain barrier, where it is captured by the proteins and produces a long-lived reservoir, releasing for a long period of time (Holtcamp, 2012; Xie et al., 2013).
Modern studies demonstrate that there are several mechanisms by which BMAA induces the dysfunction and death of motor neurons; however, details are not yet completely understood. BMAA can potentiate the neurotoxic effect of other substances and factors (Lobner et al., 2007). In addition, BMAA can stimulate/activate glutamate receptors: NMDA, calcium-dependent AMPA, kainite and metabotropic receptors, inducing an excessive activation of the glutamatergic system, which leads to excitotoxic death of neurons (Lobner et al., 2007). The glutamate level in patients with amyotrophic lateral sclerosis is higher both in the blood plasma and in the cerebrospinal fluid. Finally, BMAA can induce oxidative stress in neurons (Lobner et al., 2007). The inclusion of BMAA occurs in the L-serine region causing incorrect protein folding, which is observed at such neurodegenerative diseases as Alzheimer’s and Parkinson’s diseases and amyotrophic lateral sclerosis. The studies in vitro demonstrated that the BMAA association with the protein can be suppressed in the presence of the excess of L-serine (Dunlop et al., 2013). Low BMAA concentrations in the culture can selectively kill motoneurons of the spinal cord in mice (Rao et al., 2006). Subcutaneous injections of BMMA to newborn rats caused a progressing neurodegeneration in the hippocampus, including the formation of intracellular neurofibrillary structures, astrogliosis, and activation of microglia (Karlsson et al., 2015). BMAA is also toxic for glia cells (Chiu et al., 2015) and causes damage of motoneurons and astrogliosis in ventral horns of the spinal cord (Yin et al., 2014).
It was demonstrated in the studies on monkeys (Chlorocebus sabaeus) homozygous for the apoE4 gene (apoprotein E is an important biochemical marker of Alzheimer’s disease in humans) that oral administration of BMAA led to histopathologically significant signs of Alzheimer’s disease (formation of amyloid plaques and accumulation of neurofibrillary tangles). And L-serine had a protective effect against neurotoxic effect of BMAA (Cox et al., 2016).
Postmortem studies of human brain tissues in patients with sporadic neurodegenerative diseases, such as amyotrophic lateral sclerosis, Alzheimer’s, Parkinson’s, and Huntington’s diseases, demonstrated the presence of BMMA (Bradley and Mash, 2009; Pablo et al., 2009). Thus, BMAA can play a certain role in the development of neurodegenerative diseases.
At present, the point of view that pathogenic microbes make a potential contribution to the organism aging and, probably, in the development of neurodegenerative diseases (particularly Alzheimer’s disease) is increasingly recognized (Miklossy, 2011; Cho and Blaser, 2012; Heintz and Mair, 2014; Hill et al., 2014; Huang et al., 2014; Mancuso et al., 2014). For example, most changes, such as inflammatory reactions, brain cell atrophy, amyloidosis, cognitive violations, etc., can be a consequence of different microbial infections (see below) in Alzheimer’s disease (Cho and Blaser, 2012; Bhattacharjee and Lukiw, 2013; Foster et al., 2013; Heintz and Mair, 2014; Kim et al., 2013; Hill et al., 2014; Huang et al., 2014; Mancuso et al., 2014).
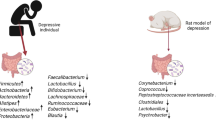
Normally, pathogenic microorganisms and pathobionts of GI are under the control of the macroorganism immune system and symbiotic microbiota. However, an increase in the number of pathogens and pathobionts and/or an increase in their metabolic activity is observed sometimes; it can be associated with a number of the following diseases: diabetes mellitus, metabolic syndrome, obesity, autoimmune diseases, depression, and some stress-induced and neurodegenerative diseases, etc. (Bravo et al., 2012; Bhattacharjee and Lukiw, 2013; Heintz and Mair, 2014; Hill et al., 2014).
ROLE OF THE MICROBIOTA IN THE INFLAMMATORY RESPONSES
Different species of bacteria, such as Bacillus, Pseudomonas, Staphylococcus, Streptomyces, etc., have amyloid structures that activate the organism immune system. Such proteins were found in the blood of patients suffering from Alzheimer’s disease (Hill et al., 2014; Zhao et al., 2015). Amyloids are associated with the cellular structures located on the surface of fungi and bacteria and promote better adhesion to the surfaces. Amyloid proteins (fibers) known as “curli fibrils” can form a special structure (biofilms) in order to resist the macroorganism immune protection (Schwartz and Boles, 2013; Asti and Gioglio, 2014). Biofilms physically bind a large number of bacteria together and represent a matrix of extracellular polymeric amyloids and other complex lipoproteins and lipopolysaccharides in different structural forms (Zhao et al., 2015). Some amyloids can be considered as pathogen-associated molecular patterns (PAMP) and are recognized by the Toll 2-like receptor of the immune system (similarly to Aβ42) (Zhou et al., 2012; Schwartz and Boles, 2013).
Besides amyloids, other molecules of microorganism walls (for example, lipopolysaccharides) also constantly activate the organism immune system. Taking into account these facts, we can say that the macroorganism is under the permanent pressure of the products of microorganisms. Devastating consequences of this pressure increase with age, when the permeability of the blood-brain barrier and GI barrier is violated (Hattori and Taylor, 2009; Tran et al., 2013; Marques et al., 2013).
An increased level of chronic inflammatory reactions is a peculiarity of Alzheimer’s disease. Activated microglia is a strong neuropathological stimulator that leads to a permanent inflammation in the brain (Hill and Lukiw, 2015; Lukiw, 2016; Minter et al., 2016; Varatharaj and Galea, 2017). These progressing proinflammatory and neurodegenerative processes are presumably stimulated by an anomalous immune system response (Lehnardt, 2010; Lukiw, 2016), which can be caused in turn by acute or chronic infection as well as by different products of the host organism microbiota (Zhou et al., 2013; Zhao et al., 2015; Foster et al., 2016).
It is important to understand that most products secreted by microbiota and cell wall components represent a very large class of strong proinflammatory activators of the immune system that can cause the release of proinflammatory cytokines, complement proteins, activate microglia, etc. in the host organism CNS (Zhao et al., 2015). The pathogenic effect of microbiota can increase the permeability of GI (König et al., 2016) and blood-brain barrier (Zhao et al., 2015; Varatharaj and Galea, 2017) that additionally increases amyloidosis and other types of inflammations in CNS. Elevated blood-brain barrier permeability can be associated with the pathogenesis of such neurodegenerative diseases as Alzheimer’s disease, multiple sclerosis (Varatharaj and Galea, 2017), epilepsy, etc.
An increase in the activation of microglia and the production of proinflammatory cytokines changes the nerve cell function and increases their death in the experimental models of Parkinson’s disease and other neurodegenerative diseases (Kannarkat et al., 2013; Sanchez-Guajardo et al., 2013). Moreover, as is known, the inflammatory environment increases the aggregation of alpha-synuclein, which can additionally activate microglia, thus contributing to significant aggregation of alpha-synuclein and the disease progression (Gao et al., 2017). Therefore, a decrease the inflammatory reaction (Valera and Masliah, 2016) and dysbiotic disorders of intestinal microbiota can be an additional therapeutic method to facilitate the symptoms of Parkinson’s disease.
Microglia and astrocytes are the main cells responsible for the innate immunity in CNS to combat the penetration and spread of microorganisms and infectious agents. Therefore, Toll-like receptors, whose expression can be regulated by infection, inflammatory reactions, and other stimuli, are expressed in these cells (Bowman et al., 2003; McKimmie et al., 2005; Arroyo et al., 2011; Carty and Bowie, 2011).
The microbial products, such as amyloid, bacterial lipoproteins, lipopolysaccharide, peptidoglycan, and genetic material of microorganisms (DNA and RNA), activate Toll-like receptors of microglia that subsequently induce the release of proinflammatory cytokines and chemokines having a direct effect on CNS homeostasis and neuropathology (Kielian, 2006; Rivest, 2009; Arroyo et al., 2011; Yu and Ye, 2015). A complex of Toll-like receptors 1 and 2 can recognize the amyloids of biofilms produced by the Firmicutes, Bacteroidetes, and Proteobacteria (Bhattacharjee and Lukiw, 2013; Asti and Gioglio, 2014). Participating in neuroinflammatory reactions, Toll-like receptors play a significant role in neuropathology. In Alzheimer’s disease, Toll-like receptors are involved in beta amyloid recognition and removal. Different types of Toll-like receptors can both promote and inhibit the development of the disease (Arroyo et al., 2011).
When activated, microglia are involved in inflammatory reactions and cytokines with chemokines release, as well as nitrogen superoxide and oxide and other active substances that serve for the subsequent involvement of the immune system or beginning of reparation processes (Glass et al., 2010). This microglia reaction is short-lived and ends during the infectious agent inactivation or tissue repair process. However, a chronic inflammation, which increases pathological changes in CNS, can cause permanent activation of microglia due to the constant stimulation or violation of the mechanisms for resolving inflammation (Glass et al., 2010), since many proinflammatory agents are neurotoxic (Hanisch, 2002; Glass et al., 2010). It is important to note that the activation of Toll-like receptors at different stages of the disease can lead to different results. The activation of receptors at early stages of the disease can decrease the accumulation of beta amyloid. However, the activation of Toll-like receptors with the progression of the disease will most likely contribute to the chronic neuroinflammation and neurotoxicity (Arroyo et al., 2011).
INFECTIONS AND NEURODEGENERATION
Infectious diseases affecting the central nervous system can play a significant role in the induction of neurodegenerative diseases (Bowery et al., 1992; Mattson, 2004; Zambrano et al., 2008; Miklossy, 2011; Hill et al., 2014). Specific bacterial ligands, as well as bacterial and viral DNA and RNA, are able to increase the expression of proinflammatory molecules that activate innate and adaptive components of the immune system. Permanent infection leads to chronic inflammation, neuron destruction, and Aβ deposition. It was demonstrated that Aβ is a pore-forming (Di Scala et al., 2016) antimicrobial neurotoxin/peptide (Soscia et al., 2010), which can be generated in response to the microbial invasion (Soscia et al., 2010; Miklossy, 2011). Lipopolysaccharides stimulate beta amyloidosis (Dasari et al., 2011; Asti and Gioglio, 2014), which can indicate the involvement of microbiota in Alzheimer’s disease pathogenesis (Dasari et al., 2011; Asti and Gioglio, 2014). It should be noted that long-term consequences of different infections on the macroorganism depend on the immune status, accompanying cardiovascular and other diseases, pharmacological treatment, and on the organism genetics. For example, mutations in the genes of presenelins and polypoproteins E increase the risk of Alzheimer’s disease (Licastro et al., 2007; Prusiner, 2013).
An increase in the amount of fungal and yeast proteins, as well as their fragments, was demonstrated in the blood of patients with Alzheimer’s disease; this can indicate that chronic fungal infections can be associated with an increased risk of Alzheimer’s disease (Prusiner, 2013; Alonso et al., 2014; Asti and Gioglio, 2014; Heintz and Mair, 2014). The following examples can demonstrate a possible association of pathogenic microorganisms with CNS diseases.
Herpes. A significant amount of data indicates that neurotrophic herpes-1 virus (HSV-1), which is able to persist for a long time in nervous tissues, can be associated with Alzheimer’s disease (Letenneur et al., 2008; Álvarez et al., 2012; Ball et al., 2013; Agostini et al., 2014; Hill and Lukiw, 2014; Mancuso et al., 2014). However, specific mechanisms of this association are only beginning to be studied (Hill and Lukiw, 2014; Hill et al., 2014). HSV-1 infects CNS and, as well as cytomegalovirus, causes a latent infection, which is repeatedly reactivated (Letenneur et al., 2008; Álvarez et al., 2012). The activation of endogenous herpes virus and other neurotropic viruses, as well as prions, is closely related to neurological stresses stimulating amyloidosis, inflammatory neurodegeneration, and progression of cognitive impairments, which can contribute in its turn to predisposition and/or early development of schizophrenia and Alzheimer’s disease (Hill et al., 2009; Ball et al., 2013; Manuelidis 2013). It was found that HSV-1 stimulates the accumulation of beta amyloid associated with Alzheimer’s disease (Wozniak et al., 2011; Santana et al., 2012). Tau pathology and neurodegeneration can be directly related to HSV-1 infection. The anomalous dynamics of microtubules, tau hyperphosphorylation, and significant damage of neurites (finally leading to apoptosis) was observed in the culture of mouse nervous cells infected with HSV-1 (Zambrano et al., 2008). Such tauopathy was also observed in the culture of human neuroblastoma cells infected with HSV-1 (Álvarez et al., 2012). The accumulation of Aβ and phosphorylation of the tau protein is induced in neurons after the herpes virus (HSV-1) infection both in vitro and in vivo (Letenneur et al., 2008; Miklossy, 2011; Wozniak et al., 2011; Santana et al., 2012).
Prions. The prion disease can occur in the case of direct infection and hereditary transmission as well as sporadically. The prion diseases are finally fatal neurological disorders very similar to Alzheimer’s disease (Manuelidis, 2013; Prusiner, 2013). As well as beta amyloid, prions cause the synaptic failure and amyloidogenesis (Manuelidis, 2013; Prusiner, 2013; Chen et al., 2014; Hernandez-Rapp et al., 2014). The proteins similar to mammalian prions were found in the Saccharomyces cerevisiae yeasts (Allen et al., 2005) as well as in some Podospora anserina fungi. The transmission of such mammalian proteins as prions needs a very thorough check, especially in the light of the fact that the Saccharomyces cerevisiae is actively used in the food industry.
Chlamydophila pneumoniae. Intracellular and extracellular Chlamydophila pneumoniae antigens were observed in patients with Alzheimer’s disease in the temporal and frontal lobes of the brain. Chlamydia, amyloid deposits, and neurofibrillary tangles were present in the same brain regions in close contact with each other (Hammond et al., 2010). Beta amyloid deposits were observed in mice after the C. pneumoniae inhalation (Miklossy, 2011). It should be noted that pneumonia is a frequent complication in Alzheimer’s disease (Manabe et al., 2016) and significantly increases the mortality in such patients (Foley et al., 2015).
Toxoplasma gondii is an intracellular parasite that can cause encephalitis and neurological dysfunction causing chronic inflammatory reactions in the brain and central nervous system. The olfactory dysfunction observed in Alzheimer’s disease, multiple sclerosis, and schizophrenia is frequently associated with a significant increase in the level of immunoglobulin G (antibodies) to the Toxoplasma gondii (Prandota, 2014).
Hepatitis C can significantly increase the risk of Alzheimer’s disease, especially in elderly persons (Chiu et al., 2013).
HIV. Human immunodeficiency virus (HIV), which causes acquired immunodeficiency syndrome (AIDS), affects the human immune system and, first of all, the mucosal immune system (Sobol, 2017a). A progressing increase in the permeability of GI and blood brain barrier begins in HIV patients (Sobol, 2017a). The chronic inflammation, not treatable with antiviral drugs, is a consequence of these processes (Sobol, 2017a). Opportunistic infections occurring in host after weakening immune system can affect different organs. Antiretroviral therapy started in time allows to suppress a significant progression of opportunistic infections.
CNS is a potential HIV reservoir even in patients receiving antiviral therapy. HIV-1 rapidly spreads in the brain tissue during the infection. Different types of macrophages, monocytes, and long-lived brain cells, such as microglia and astrocytes, are the target of HIV infection in the brain and central nervous system (Churchill et al., 2009; Smith et al., 2012). Astrocytes that provide the latency of HIV infection are apparently not susceptible to antiviral treatment (Brew et al., 2015). HIV replication continues in CNS despite of receiving efficient antiretroviral therapy and is independent of the virus replication in the blood; this assumes autonomous virus evolution in CNS (Harrington et al., 2009). Antiretroviral therapy is not very efficient against HIV-induced inflammation in CNS (Suh et al., 2014). The HIV-1 Tat (viral protein) promotes the release of glutamate, inflammatory cytokines, chemokines, and active oxygen forms from glial cells and neurons and it also affects the cell responses caused by NMDA in the hippocampus neurons (Krogh et al., 2015). The factors released from infected glial cells can directly or indirectly cause CNS impairment. The death of neurons can be also caused by glutamate excitotoxicity and by the factors inducing apoptosis (Kramer-Hämmerle et al., 2005). Many of the regulatory functions of astrocytes (synaptogenesis and neurogenesis, regulation of neurotransmitters) are violated during HIV infection (Brew et al., 2015). Viral DNA in astrocytes can be associated with the pathogenesis of HIV-associated dementia (Churchill et al., 2009; Brew et al., 2015; Dickens et al., 2017). HIV infects macrophages, including microglia and astrocytes, and is usually located in these cells in a latent state. In most cases, astrocytes produce no infectious virions, but nonstructural regulatory Nef, Rev, and Tat proteins, which are toxic to neurons in culture (Sabatier et al., 1991). Evidence of progressive brain impairment by the Tat protein during its continuous expression were recently presented (Dickens et al., 2017). Even insignificant Tat expression in the brain can lead to the expression of proinflammatory cytokines, chronic glia activation, and structural brain damages, including synaptic impairment and a decrease in the volume of the brain tissue (Dickens et al., 2017). These results demonstrate that the modern scheme of the treatment with antiretroviral drugs is probably insufficient for the brain’s protection against the negative effect of nonstructural HIV proteins that continue to be expressed in the brain tissue, despite the virus suppression in the blood (Dickens et al., 2017).
HIV-1 can cause severe neurological diseases, including neurodegeneration (Heaton et al., 2010). HIV-infected patients have an increased risk of the development of Alzheimer’s (Xu and Ikezu, 2009) and Parkinson’s (Moulignier et al., 2015) diseases. Amyloidosis and HIV-infection can promote the development of each other (González-Scarano and Martín-García, 2005; Widera et al., 2014). Histopathological studies of the brain tissue samples of HIV-infected patients demonstrated the atrophy of neurites and the loss of neurons in those brain regions in which neuropathology was observed in Alzheimer’s disease (Borjabad and Volsky, 2012; Widera et al., 2014).
Helicobacter pylori. The Helicobacter pylori, a causative agent of a gastric ulcer, can be also associated with Alzheimer’s disease (Kountouras et al., 2006, 2010), since an increased number of specific IgG/IgA antibodies to the Helicobacter pylori was found in the blood serum of patients (Malaguarnera et al., 2004; Kountouras et al., 2010). Intraperitoneal injection of the H. pylori filtrate induced cognitive impairments in rats, and the production of dendritic spines in the hippocampus was decreased (Wang et al., 2014). The H. pylori filtrate injection significantly increased the amount of beta amyloid (Aβ42) both in the hippocampus and in the cerebral cortex, unlike the injections of a filtrate of another species of intestinal bacteria (Escherichia coli) that did not have any effect on the cognitive functions in rats and on the production of beta amyloid (Wang et al., 2014). Medical therapy against the H. pylori has a beneficial effect on patients with Alzheimer’s disease with the H. pylori infection (Malaguarnera et al., 2004). Despite the fact that all these studies are based on clinical observations, there are still no direct evidences of the H. pylori involvement in the pathogenesis of Alzheimer’s disease.
Parkinson’s disease can also be associated with the Helicobacter pylori infection. The prevalence of this infection is high among patients with Parkinson’s disease and can cause motor disorders preventing the absorption of levodopa (the main drug for the treatment of Parkinson’s disease) (Holmqvist et al., 2014). In addition, small intestinal bacterial overgrowth (SIBO) in the small intestine can be associated with Parkinson’s disease; almost a quarter of patients with Parkinson’s disease have SIBO (Parashar and Udayabanu, 2017). In turn, normalization of the composition of microbiota can lead to the improvement of motor functions in Parkinson’s disease (Sampson et al., 2016).
Spirochaetales. The chronic infection caused by spirochete can cause slowly progressing dementia, cortical atrophy, and amyloid deposition. There is a significant association between Alzheimer’s disease and different spirochete species that cause syphilis, Lyme disease, etc. (Miklossy, 2011).
GI AND BLOOD BRAIN BARRIER DYSFUNCTION
Human CNS is constantly exposed to the effect of its own microbiota as well as microorganisms of the environment, including both symbiotic microorganisms and pathobionts with pathogens. These can be different types of bacteria, viruses, and fungi as well as prion proteins. All this microbial load is probably involved in the pathogenesis of Alzheimer’s disease (Hill et al., 2014), gradually affecting both natural barriers (GI and blood-brain) and the cells of the immune and nervous systems. It should be noted that the permeability of both GI and blood-brain barrier increases with age (Brenner, 2013; Montagne et al., 2015; Welling et al., 2015). As a result, CNS is at a greater risk of exposure to neurotoxins and toxic metabolites produced by GI microbiota (Brenner, 2013; Welling et al., 2015). The translocation of toxic microbiota products through weakened protective barriers (blood-brain and GI mucosa) can be critical in neurodegenerative diseases (Montagne et al., 2015; van de Haar et al., 2016; Varatharaj and Galea, 2017). It should be noted that nonpathogenic GI microbiota can decrease the blood-brain barrier permeability contributing to its integrity (Braniste et al., 2014).
PARKINSON’S DISEASE AND MICROBIOTA
It is known that inflammatory reactions in the intestine (Devos et al., 2013) and abnormal functions of GI, such as constipation, dysphagia, nausea, gastroparesis, etc. (that frequently precede long before the onset of motor symptoms of neurodegenerative disease) are observed in patients with Parkinson’s disease (Braak et al., 2003; Kim and Sung, 2015). There is a hypothesis of Braak, according to which anomalous accumulation of alpha-synuclein (a marker protein synthesized during Parkinson’s disease) is initiated in the intestine and probably further spreads through the nervus vagus to the brain (Del Tredici and Braak, 2008). This assumption is confirmed by pathophysiological data (alpha-synuclein inclusions are already observed at an early stage in ENS as well as in glossopharyngeal and vagus nerves (Braak et al., 2003)). It was demonstrated that the injection of alpha-synuclein isolated from the brain tissues of patients with Parkinson’s disease directly to the intestine of healthy rodents is sufficient for the development of synucleinopathy in the vagus nerve and brain stem (Holmqvist et al., 2014). And alpha-synuclein moves through rapid and slow axonal transport (Holmqvist et al., 2014). It was recently demonstrated in germ-free genetically modified mice with artificially reproduced Parkinson’s disease (with an increased alpha-synuclein expression) that microbiota can stimulate synucleinopathy, neuroinflammation, and typical motor dysfunction, which was studied during different motor tests (Sampson et al., 2016). Microbial metabolites (short chain fatty acids) that can be involved in the development of Parkinson’s disease were also identified (Sampson et al., 2016). Moreover, the transfer of microbiota taken from individuals with Parkinson’s disease caused a significant motor dysfunction in germ-free genetically modified mice (Sampson et al., 2016). It was suggested that intestinal microbiota can play a significant role in the pathogenesis of Parkinson’s disease (Sampson et al., 2016).
Besides microorganisms themselves, the products of their metabolism, as well as the cell fragments, can have a negative effect on CNS. For example, epoxomycin, a natural inhibitor of proteasomes (cellular complexes involved in the degradation of ubiquitinated proteins), is a toxin produced by actinomycetes. Sixfold epoxomycin injection during 2 weeks to rats resulted in subsequent development of symptoms typical for Parkinson’s disease (tremor, bradykenisia, etc.). Dopaminergic neurodegeneration in the substantia nigra pars compacta region with the apoptosis of neurons and neuroinflammation was observed. Intracytoplasmic inclusions of α-synuclein/ubiquitin resembling Lewy bodies were present in some remaining neurons in the neurodegeneration region (McNaught et al., 2004). Thus, epoxomycin is a dopaminergic neurotoxin, which can cause neurodegeneration similar to Parkinson’s disease.
Lipopolysaccharides (LPS), endotoxins from gram-negative bacteria, can also be involved in the pathology of Parkinson’s disease (Liu and Bing, 2011). LPS penetrate into the blood, since the permeability of GI is increased in patients with Parkinson’s disease. Penetrating into the brain, LPS then cause inflammatory processes in glial cells, which release neurotoxic factors in its turn stimulating microgliosis and other neurodegenerative processes (Liu and Bing, 2011).
There are epidemiological evidences that pesticides provoke the development of Parkinson’s disease (Ritz et al., 2016), and some pesticides can affect the composition of microbiota (Gao et al., 2017). Thus, under the influence of external/internal factors, human microbiota can undergo changes; the ratio of symbionts/pathogens changes. The permeability of intestinal and blood-brain barriers changes. Metabolites released by changed microbiota can enter the blood circulation and probably CNS, thus violating its functioning.
AUTISM AND MICROBIOTA
Recently, the existence of the association between autism and microbiota was suggested (Frye et al., 2015). The amount of neurotoxin-producing bacteria can probably be increased in autism. Children with autism symptoms also have increased concentration of short chain fatty acids in the intestine (especially propionate). This peculiarity is probably caused by anomalously high Clostridia activity and by the presence of the Sutterella wadsworthensis bacteria, the presence of which is typical for individuals with autism symptoms (Frye et al., 2015; Oleskin and Shenderov, 2016). Intracerebroventricular (in the brain ventricles) propionate injection in adult rats led to behavioral, biochemical, electrophysiological, and neuropathological effects similar to those that were observed in autism (MacFabe et al., 2007, 2011). Propionate can also negatively affect the bioenergetics of mitochondria (Frye et al., 2015). Metabolites and antigens of gut microbiota can negatively affect the mitochondrial function (Frye et al., 2015). In turn, mitochondrial dysfunction can negatively affect the function of GI (Frye et al., 2015).
MULTIPLE SCLEROSIS
Physicians and researchers from Harvard (United States) found that a change in the composition of microbiota is observed in patients with multiple sclerosis as compared with healthy individuals; in turn, this correlates with a change in the activity of the genes playing a certain role in immune response (Jangi et al., 2016).
In another study, the authors analyzed microbiota in the brain tissues of patients with multiple sclerosis obtained during autopsy. The results were compared with the brain tissue samples of individuals without multiple sclerosis (Branton et al., 2016). 16S rRNA sequencing demonstrated that proteobacteria (gram-negative) are a dominant type of bacteria in white matter of the brain in patients with multiple sclerosis, and they dominate with the disease progression. An increase in the expression of inflammatory immune response genes was also observed (Branton et al., 2016). In addition, it was found that peptidoglycan (an important component of the bacterial cell wall) can be associated with inflammatory demyelination. The authors also conclude that dysbiosis can underlie the multiple sclerosis disease (Branton et al., 2016).
With 16S rRNA sequencing, it was found that the percentage of Methanobrevibacter (the main methane-forming Archaea in human intestine) and Akkermansia was increased, while the Butyricimonas content was decreased in patients with multiple sclerosis (Jangi et al., 2016). All these changes in the composition of microbiota correlate with changes in the expression of the genes involved in the maturation of the immune system cells. When analyzing exhaled air, the methane level was higher in patients with multiple sclerosis than in healthy individuals due to an increase in the amount of Methanobrevibacter (Jangi et al., 2016). Bacteria identified in this study (that are in an increased number in patients with multiple sclerosis) cause different inflammatory reactions and can be associated with autoimmune disorders. For example, the Methanobrevibacter is involved in the intestine inflammatory reactions and in asthma (Verma et al., 2010).
Researchers from the Max Planck Institute of Neurobiology (Germany) also believe that intestinal microbiota plays a large role in the development of multiple sclerosis. Using the multiple sclerosis model in mice (transgenic SJL/J mice), it was demonstrated that there was no development of the disease in such mice grown in a nonmicrobial environment, while the introduction of commensal microbiota in the intestine led to a spontaneous development of autoimmune encephalomyelitis (Berer et al., 2011). The indigenous (native) microbiota stimulated the activity of T- and B-lymphocytes that started to attack myelin sheaths of the brain, leading as a result to autoimmune destruction of myelin sheath of neurons. It was suggested that similar processes can occur in individuals without the appropriate genetic predisposition to multiple sclerosis (Berer et al., 2011).
These studies allow us to understand better how intestinal microbiota can influence on the immune system and, through it, on physiological processes occurring in CNS. At the moment, it is unclear whether a change in the composition of microbiota is a consequence of multiple sclerosis disease or a change in the composition of microbiota stimulates the development of multiple sclerosis. However, it can be assumed that targeted normalization of intestinal microbiota can contribute to a decrease in the consequences of multiple sclerosis and, probably, to its treatment.
POTENTIAL OF PROBIOTIC MICROORGANISMS IN NEURODEGENERATION
At present, the use of probiotics and nutritional products for prevention and possible treatment of CNS diseases that can normalize neuroendocrine, neuroimmune, and humoral mechanisms (Bhattacharjee and Lukiw, 2013) are studied. The effect of probiotic microorganisms is very diverse and can manifest itself in the following, probiotics: (1) positively modulate the immunity in infectious diseases; (2) are involved in the synthesis of a number of vital vitamins, especially vitamins of the groups B and K; (3) synthesize important metabolites for the host organism; (4) are involved in the fermentation of complex carbohydrates; (5) compete and exhibit antagonistic activity relative to pathogenic microorganisms in the gastro-intestinal tract; (6) can neutralize carcinogens in GI; (7) administration of probiotics and the products of their metabolism improves the state and barrier function of GI. Probiotics can be successfully used in neurogastroenterology (Saulnier et al., 2013). There are data on the effect of probiotics and different food ingredients on the development of multiple sclerosis disease (von Geldern and Mowry, 2012), on the cognitive processes (Camfield et al., 2011), and on mental disorders, including anxiety, autism, depression, and schizophrenia (Bravo et al., 2011; Bhattacharjee and Lukiw, 2013; Douglas-Escobar et al., 2013). However, administration of probiotics did not affect stress-induced inflammatory reactions and cognitive functions in recent experiments, in which healthy male volunteers participated (Kelly et al., 2017).
To increase the therapeutic effect of probiotic products, we created a new fermented probiotic product (PP) with an increased content of metabolites of probiotic bacteria (Sobol, C.V. and Sobol, Yu.Ts., 2005; Sobol, 2017b). Stimulating the mechanisms of intracellular signaling in different cells, PP increases store-operated calcium entry and has the ability to release intracellular calcium even in conditions of the depletion of intracellular calcium stores by high thapsigargin concentrations (Sobol et al., 2013; Sobol, 2017b). PP significantly stimulated capacitative calcium entry in the PC-12 cells with Swedish double mutation (K670M/N671L), with increased Аβ production. Са2+ entry in nerve cells, observed during the PP application (Sobol and Belostotskaya, 2015), occurred not only through potential-dependent channels, through which Ca2+ enters when microglia are activated by prions and beta-amyloid (Silei et al., 1999). Moreover, we observed no excitotoxicity phenomenon when the spinal cord nervous tissue is stimulated by PP, probably, due to the occurrence of neuron desensitization mechanisms (unpublished).
It is known that a violation in Са2+ signaling is observed in the nerve cells in Alzheimer’s disease, and changes in Ca2+ homeostasis in the cell occur much earlier than histological changes associated with Alzheimer’s disease appear (Bojarski et al., 2008). The store dependent calcium entry in neurons is decreased (Putney, 2000; Laferla, 2002; Gibson and Thakkar, 2017); it is apparently required to maintain the synaptic transmission stability (Zhang et al., 2016). An increase in the concentration of Са2+ ions in endoplasmic reticulum of nerve cells, caused by mutations in presenilin proteins and/or pathological changes associated with the cell aging, is observed (Laferla, 2002; Gibson and Thakkar, 2017). These changes in calcium signaling occur at the initial stages of the disease and can be involved in the processes of violation/loss of synaptic connections and probably violations in the brain neuroplasticity. With disease progression, beta amyloid oligomers also cause intracellular calcium dysregulation, [Ca2+]i (Yu et al., 2009), which leads to mitochondrial dysfunction (cytochrome C release, swelling of mitochondria) and finally to apoptosis (Kim et al., 2002). Dysregulation of [Ca2+]i and pore formation (Di Scala et al., 2016) can underlie the mechanism of beta amyloid neurotoxicity (Mattson et al., 1992; Laferla, 2002). Since an increase in the store dependent Са2+ significantly decreases the production of beta amyloid (Dreses-Werringloer et al., 2008), it is possible that the use of PP can lead to a decrease in the production of amyloid and normalization of calcium homeostasis.
PP also stimulates basal respiration and causes mild uncoupling of the electron transport and oxidative phosphorylation in the rat heart mitochondria (Sobol et al., 2013), thus decreasing the level of active oxygen forms; this leads to a decrease in the oxidative stress accompanying many neurodegenerative diseases.
The growth of neuritis is a critical event in the development of neurons, formation and remodeling of synapses, in reactions to the damage and regenerations. Changes in the hippocampus neurogenesis can be considered as an integral part of Alzheimer’s disease (Maruszak et al., 2014; Oh et al., 2015). It was previously demonstrated that PP stimulates neuritogenesis and induces the differentiation of pheochromocytoma cells (PC-12) in neuron-like cells (Sobol et al., 2005). It cannot be ruled out that PP can have a beneficial effect on neurogenesis.
PP stimulates the large intestine peristalsis (Sobol, 2017b), a violation of which is observed in old age and in Parkinson’s disease, long before the onset of motor symptoms of neurodegenerative disease (Kim and Sung, 2015). PP also has an antigenotoxic effect (Sobol, 2015). Therefore, the prophylactic rectal route of PP administration in the large intestine to improve the intestine peristalsis and to decrease the toxic effect of the stool is indicated both for elderly individuals and for patients with neurodegenerative diseases (especially Parkinson’s disease).
As mentioned above, pneumonia is a frequent complication in Alzheimer’s disease (Manabe et al., 2016) and significantly increases the mortality of such patients (Foley et al., 2015). It should be noted that total pathogenic load on the organism increases with age, the immune protection decreases; this increases the risk of the development of neurodegenerative diseases. PP has a significant antimicrobial effect and is efficient against a wide range of pathogens (Sobol, 2017b). Simultaneously with the suppression of pathogenic microorganisms, PP stimulates the immunity and the growth of symbiotic microbiota (Sobol, 2017a, 2017b). Thus, PP has a complex effect on the organism, and it can be considered as the food with a potential to prevent and facilitate symptoms of neurodegenerative and accompanying diseases.
CONCLUSIONS
Involvement of microbiota in the intensity of inflammatory processes, in incorrect protein folding, amyloidosis, synucleinopathy, and accompanying neurodegenerative diseases is the subject of further studies. It becomes clear that the processes of the development of neurodegenerative diseases are complex, since the immune system, contact with pathogenic and symbiontic microorganisms in pre- and postnatal periods, genetic peculiarities of an individual, different stresses, the quality of internal and external environment (the presence of herbicides, xenobiotics, antibiotics, etc.), pharmacological treatment, and accompanying diseases are involved in these processes. The impairment of the GI functions can precede long before neurodegenerative processes and be caused by the effect of the central and/or enteric nervous system as well as by the side effect of pharmacological drugs. Early diagnosis, detection, control, and treatment of negative GI symptoms, including the normalization of microbiota, can lead to a significant improvement in the quality of life of patients with neurodegenerative diseases.
REFERENCES
Agostini, S., Clerici, M., and Mancuso, R., How plausible is a link between HSV-1 and AD?, Expert Rev. Anti. Infect. Ther., 2014, vol. 12, pp. 275–278. doi 10.1586/ 14787210.2014.887442
Albenberg, L.G. and Wu, G.D., Diet and the intestinal microbiome: associations, functions, and implications for health and disease, Gastroenterology, 2014, vol. 146, no. 6, pp. 1564–1572. doi 10.1053/j.gastro.2014.01.058
Allen, K.D., Wegrzyn, R.D., Chernova, T.A., et al., Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+], Genetics, 2005, vol. 169, no. 3, pp. 1227–1242.
Alonso, R., Pisa, D., Marina, A.I., et al., Fungal infection in patients with Alzheimer’s disease, J. Alzheimers Dis., 2014, vol. 41, pp. 301–311. doi 10.3233/JAD-132681
Álvarez, G., Aldudo, J., Alonso, M., et al., Herpes simplex virus type 1 induces nuclear accumulation of hyperphosphorylated tau in neuronal cells, J. Neurosci. Res., 2012, vol. 90, no. 5, pp. 1020–1029.
Arroyo, D.S., Soria, J.A., Gaviglio, E.A., et al., Toll-like receptors are key players in neurodegeneration, Int. Immunopharmacol., 2011, vol. 11, no. 10, pp. 1415–1421. doi 10.1016/j.intimp.2011.05.006
Asti, A. and Gioglio, L., Can a bacterial endotoxin be a key factor in the kinetics of amyloid fibril formation?, J. Alzheimer’s Dis., 2014, vol. 39, no. 1, pp. 169–179. doi 10.3233/JAD-131394
Aziz, Q., Doré, J., Emmanuel, A., Guarner, F., and Quigley, E.M., Gut microbiota and gastrointestinal health: current concepts and future directions, Neurogastroenterol. Motil., 2013, vol. 25, pp. 4–15. doi 10.1111/nmo.12046
Backhed, F., Ley, R.E., Sonnenburg, J.L., et al., Host–acterial mutualism in the human intestine, Science, 2005, vol. 307, pp. 1915–1920. doi 10.1126/science.1104816
Ball, M.J., Lukiw, W.J., Kammerman, E.M., and Hill, J.M., Intracerebral propagation of Alzheimer’s disease: strengthening evidence of a herpes simplex virus etiology, Alzheimer’s Dement., 2013, vol. 9, pp. 169–175. doi 10.1016/j.jalz.2012.07.005
Barajon, I., Serrao, G., Arnaboldi, F., et al., Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia, J. Histochem. Cytochem., 2009, vol. 57, no. 11, pp. 1013–1023. doi 10.1369/jhc.2009.953539
Barrett, E., Ross, R.P., O’Toole, P.W., et al., γ-Aminobutyric acid production by culturable bacteria from the human intestine, J. Appl. Microbiol., 2012, vol. 113, pp. 411–417. doi 10.1111/j.1365-2672.2012.05344.x
Berer, K., Mues, M., Koutrolos, M., et al., Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination, Nature, 2011, vol. 479, no. 7374, pp. 538–541. doi 10.1038/nature10554
Bhattacharjee, S. and Lukiw, W.J., Alzheimer’s disease and the microbiome, Front. Cell Neurosci., 2013, vol. 7, p. 153. doi 10.3389/fncel.2013.00153
Bojarski, L., Herms, J., and Kuznicki, J., Calcium dysregulation in Alzheimer’s disease, Neurochem. Int., 2008, vol. 52, nos. 4–5, pp. 621–633.
Borjabad, A. and Volsky, D.J., Common transcriptional signatures in brain from patients with HIV-associated neurocognitive disorders, Alzheimer’s, and multiple sclerosis, J. Neuroimmune Pharmacol., 2012, vol. 7, pp. 914–926. doi 10.1007/s11481-012-9409-5
Bowery, N.G., Bagetta, G., and Nistico, G., Intrahippocampal tetanus toxin produces generalized convulsions and neurodegeneration in rats: antagonism by NMDA receptor blockers, Epilepsy Res. Suppl., 1992, vol. 9, pp. 249–256.
Bowma, C.C., Rasley, A., Tranguch, S.L., and Marriott, I., Cultured astrocytes express toll-like receptors for bacterial products, Glia, 2003, vol. 43, pp. 281–291.
Braak, H., Rüb, U., Gai, W.P., and Del Tredici, K., Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen, J. Neural Transm. (Vienna), 2003, vol. 110, no. 5, pp. 517–536.
Bradley, W.G. and Mash, D.C., Beyond Guam: the cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases, Amyotroph. Lateral Scler., 2009, vol. 10, suppl. 2, pp. 7–20. doi 10.3109/ 17482960903286009
Braniste, V., Al-Asmakh, M., Kowal, C., et al., The gut microbiota influences blood-brain barrier permeability in mice, Sci. Transl. Med., 2014, vol. 6, p. 263ra158.
Branton, W.G., Lu, J.Q., Surette, M.G., et al., Brain microbiota disruption within inflammatory demyelinating lesions in multiple sclerosis, Sci. Rep., 2016, vol. 6, p. 37344. doi 10.1038/srep37344
Bravo, J.A., Forsythe, P., Chew, M.V., et al., Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve, Proc. Natl. Acad. Sci. U. S. A., 2011, vol. 108, no. 38, pp. 16050–16055. doi 10.1073/pnas.1102999108
Bravo, J.A., Julio-Pieper, M., Forsythe, P., et al., Communication between gastrointestinal bacteria and the nervous system, Curr. Opin. Pharmacol., 2012, vol. 12, no. 6, pp. 667–672. doi 10.1016/j.coph.2012.09.010
Brenner, S.R., Blue-green algae or cyanobacteria in the intestinal microflora may produce neurotoxins such as Beta-N-Methylamino-L-Alanine (BMAA) which may be related to development of amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinsons-Dementia-Complex in humans and Equine Motor Neuron Disease in horses, Med. Hypotheses, 2013, vol. 80, pp. 103–108. doi 10.1016/j.mehy.2012.10.010
Brew, B.J., Robertson, K., and Wright, E.J., HIV eradication symposium: will the brain be left behind?, J. Neurovirol., 2015, vol. 21, no. 3, pp. 322–334. http://dx.doi.org/ 10.1007/s13365-015-0322-6.
Camfield, D.A., Owen, L., Scholey, A.B., et al., Dairy constituents and neurocognitive health in ageing, Br. J. Nutr., 2011, vol. 106, pp. 159–174. doi 10.1017/ S0007114511000158
Carlino, D., DeVanna, M., and Tongiorgi, E., Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunction?, Neuroscientist, 2013, vol. 19, pp. 345–353. doi 10.1177/1073858412469444
Carter, C.J., Alzheimer’s disease: a pathogenetic autoimmune disorder caused by herpes simplex in a gene-dependent manner, Int. J. Alzheimers Dis., 2010, p. 140539. doi 10.4061/2010/140539
Carty, M. and Bowie, A.G., Evaluating the role of toll-like receptors in diseases of the central nervous system, Biochem. Pharmacol., 2011, vol. 81, no. 7, pp. 825–837. doi 10.1016/j.bcp.2011.01.003
Chen, B., Soto, C., and Morales, R., Peripherally administrated prions reach the brain at sub-infectious quantities, FEBS Lett., 2014, vol. 588, pp. 795–800. doi 10.1016/j.febslet.2014.01.038
Chiu, A.S., Gehringer, M.M., and Braidy, N., Gliotoxicity of the cyanotoxin, β-methyl-amino-L-alanine (BMAA), Sci. Rep., 2013, vol. 3, p. 1482. doi 10.1038/srep01482
Chiu, W.C., Tsan, Y.T., Tsai, S.L., et al., Hepatitis C viral infection and the risk of dementia, Eur. J. Neurol., 2013, vol. 21, no. 8, p. 1068-e59. doi 10.1111/ene.12317
Cho, I. and Blaser, M.J., The human microbiome: at the interface of health and disease, Nat. Rev. Genet., 2012, vol. 13, pp. 260–270. doi 10.1038/nrg3182
Churchill, M.J., Wesselingh, S.L., and Cowley, D., Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia, Ann. Neurol., 2009, vol. 66, no. 2, pp. 253–258. http://dx.doi.org/ 10.1002/ana.21697.
Clarke, G., Stilling, R.M., Kennedy, P.J., et al., Gut microbiota: the neglected endocrine organ, Mol. Endocrinol., 2014, vol. 28, pp. 1221–1238.
Cox, P.A., Banack, S.A., and Murch, S.J., Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam, Proc. Natl. Acad. Sci. U. S. A., 2003, vol. 100, no. 23, pp. 13380–13383.
Cox, P.A., Davis, D.A., Mash, D.C., et al., Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain, Proc. Biol. Sci., 2016, vol. 283, no. 1823, p. 20152397. doi 10.1098/rspb.2015.2397
Cribbs, D.H., Azizeh, B.Y., Cotman, C.W., and Laferla, F.M., Fibril formation and neurotoxicity by a herpes simplex virus glycoprotein B fragment with homology to the Alzheimer’s Aβ peptide, Biochemistry, 2000, vol. 39, pp. 5988–5994.
Dasari, M., Espargaro, A., Sabate, R., et al., Bacterial inclusion bodies of Alzheimer’s disease β-amyloid peptides can be employed to study native-like aggregation intermediate states, Chembiochem, 2011, vol. 12, pp. 407–423.
Derkinderen, P., Rouaud, T., Lebouvier, T., et al., Parkinson disease: the enteric nervous system spills its guts, Neurology, 2011, vol. 77, pp. 1761–1767. doi 10.1212/ WNL.0b013e318236ef60
Devos, D., Lebouvier, T., Lardeux, B., et al., Colonic inflammation in Parkinson’s disease, Neurobiol. Dis., 2013, vol. 50, pp. 42–48.
Diaz Heijtz, R., Wang, S., Anuar, F., et al., Normal gut microbiota modulates brain development and behavior, Proc. Natl. Acad. Sci. U. S. A., 2011, vol. 108, pp. 3047–3052. doi 10.1073/pnas.1010529108
Dickens, A.M., Yoo, S.W., Chin, A.C., et al., Chronic low-level expression of hiv-1 tat promotes a neurodegenerative phenotype with aging, Sci. Rep., 2017, vol. 7, p. 7748. doi 10.1038/s41598-017-07570-5
Dinan, T.G. and Cryan, J.F., Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration, J. Physiol., 2017, vol. 595, no. 2, pp. 489–503. doi 10.1113/JP273106
Douglas-Escobar, M., Elliott, E., and Neu, J., Effect of intestinal microbial ecology on the developing brain, JAMA Pediatr., 2013, vol. 167, pp. 374–379. doi 10.1001/jamapediatrics.2013.497
Dreses-Werringloer, U., Lambert, J.C., Vingtdeux, V., et al., A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk, Cell, 2008, vol. 133, no. 7, pp. 1149–1161.
Dunlop, R.A., Cox, P.A., Banack, S.A., and Rodgers, J.K., The non-protein amino acid BMAA is misincorporated into human proteins in place of L-serine causing protein misfolding and aggregation, PLoS One, 2013, vol. 8, no. 9. e75376. doi 10.1371/journal.pone.0075376
Eisenhofer, G., Aneman, A., Friberg, P., et al., Substantial production of dopamine in the human gastrointestinal tract, J. Clin. Endocrinol. Metab., 1997, vol. 82, pp. 3864–3871.
Foley, N.C., Affoo, R.H., and Martin, R.E., A systematic review and meta-analysis examining pneumonia-associated mortality in dementia, Dement. Geriatr. Cogn. Disord., 2015, vol. 39, nos. 1–2, pp. 52–67. doi 10.1159/000367783
Forsythe, P., Kunze, W.A., and Bienenstock, J., On communication between gut microbes and the brain, Curr. Opin. Gastroenterol., 2012, vol. 28, pp. 557–562. doi 10.1097/MOG.0b013e3283572ffa
Foster, J.A., Lyte, M., Meyer, E., and Cryan, J.F., Gut microbiota and brain function: an evolving field in neuroscience, Int. J. Neuropsychopharmacol., 2016, vol. 19. yv114. doi 10.1093/ijnp/pyv114
Frye, R.E., Rose, S., Slattery, J., and MacFabe, D.F., Gastrointestinal dysfunction in autism spectrum disorder: the role of the mitochondria and the enteric microbiome, Microb. Ecol. Health Dis., 2015, vol. 26, p. 27458.
Furness, J.B., Callaghan, B.P., Rivera, L.R., and Cho, H.J., The enteric nervous system and gastrointestinal innervation: integrated local and central control, Adv. Exp. Med. Biol., 2014, vol. 817, pp. 39–71. doi 10.1007/978-1-4939-0897-4_3
Galland, L., The gut microbiome and the brain, J. Med. Food, 2014, vol. 17, pp. 1261–1272.
Gao, B., Bian, X., Mahbub, R., and Lu, K., Sex-specific effects of organophosphate diazinon on the gut microbiome and its metabolic functions, Environ. Health Perspect., 2017, vol. 125, no. 2, pp. 198–206. doi 10.1289/ EHP202
von Geldern, G. and Mowry, E.M., The influence of nutritional factors on the prognosis of multiple sclerosis, Nat. Rev. Neurol., 2012, vol. 8, pp. 678–689. doi 10.1038/nrneurol.2012.194
Gibson, G.E. and Thakkar, A., Interactions of mitochondria/metabolism and calcium regulation in Alzheimer’s disease: a calcinist point of view, Neurochem. Res., 2017. doi 10.1007/s11064-017-2182-3
Glass, C.K., Saijo, K., Winner, B., et al., Mechanisms underlying inflammation in neurodegeneration, Cell, 2010, vol. 140, no. 6, pp. 918–934. doi 10.1016/ j.cell.2010.02.016
González-Scarano, F. and Martín-García, J., The neuropathogenesis of AIDS, Nat. Rev. Immunol., 2005, vol. 5, no. 1, pp. 69–81. http://dx.doi.org/10.1038/nri1527.
van de Haar, H.J., Burgmans, S., Jansen, J.F., et al., Blood-brain barrier leakage in patients with early Alzheimer disease, Radiology, 2016, vol. 281, no. 2, pp. 527–535. doi 10.1148/radiol.2016152244
Hammond, C.J., Hallock, L.R., Howanski, R.J., et al., Immunohistological detection of Chlamydia pneumoniae in Alzheimer’s disease, BMC Neurosci., 2010, vol. 11, p. 121. doi 10.1186/1471-2202-11-121
Hanisch, U.K., Microglia as a source and target of cytokines, Glia, 2002, vol. 40, no. 2, pp. 140–155.
Hansen, M.B., The enteric nervous system I: organisation and classification, Pharmacol. Toxicol., 2003, vol. 92, no. 3, pp. 105–113. PMID 12753424
Harrington, P.R., Schnell, G., Letendre, S.L., et al., Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course, AIDS, 2009, vol. 23, no. 8, pp. 907–915. http://dx.doi.org/10.1097/QAD.0b013e3283299129.
Hattori, M. and Taylor, T.D., The human intestinal microbiome: a new frontier of human biology, DNA Res., 2009, vol. 16, pp. 1–12.
Heaton, R.K., Clifford, D.B., Franklin, D.R., Jr., et al., HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study, Neurology, 2010, vol. 75, no. 23, pp. 2087–2096. http://dx.doi.org/10.1212/WNL.0b013e318200d727.
Heintz, C. and Mair, W., You are what you host: microbiome modulation of the aging process, Cell, 2014, vol. 156, pp. 408–411. doi 10.1016/j.cell.2014.01.025
Hernandez-Rapp, J., Martin-Lannerée, S., Hirsch, T.Z., et al., Hijacking PrP(c)-dependent signal transduction: when prions impair Aβ clearance, Front. Aging Neurosci., 2014, vol. 6, p. 25. doi 10.3389/fnagi.2014.00025
Hill, J.M. and Lukiw, W.J., Comparing miRNAs and viroids; highly conserved molecular mechanisms for the transmission of genetic information, Front. Cell Neurosci., 2014, vol. 8, p. 45. doi 10.3389/fncel.2014.00045
Hill, J.M. and Lukiw, W.J., Microbial-generated amyloids and Alzheimer’s disease (AD), Front. Aging Neurosci., 2015, vol. 7, p. 9. doi 10.3389/fnagi.2015.00009
Hill, J.M., Zhao, Y., Clement, C., et al., HSV-1 infection of human brain cells induces miRNA-146a and Alzheimer-type inflammatory signaling, Neuroreport, 2009, vol. 20, pp. 1500–1505. doi 10.1097/WNR.0b013e3283329c05
Hill, J.M., Clement, C., and Pogue, A.I., Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD), Front. Aging Neurosci., 2014, vol. 6, p. 127. doi 10.3389/fnagi.2014.00127
Holmqvist, S., Chutna, O., Bousset, L., et al., Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats, Acta Neuropathol., 2014, vol. 128, no. 6, pp. 805–820. doi 10.1007/s00401-014-1343-6
Holtcamp, W., The emerging science of BMAA: do cyanobacteria contribute to neurodegenerative disease?, Environ. Health. Perspect., 2012, vol. 120, no. 3, pp. A110–A116. doi 10.1289/ehp.120-a110
Holzer, P. and Farzi, A., Neuropeptides and the microbiota–gut–brain axis, in Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease, Lyte, M. and Cryan, J.F., Eds., Adv. Exp. Med. Biol., New York: Springer, 2014, vol. 817, pp. 195–219.
Hornig, M., The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness, Curr. Opin. Rheumatol., 2013, vol. 25, pp. 488–795. doi 10.1097/ BOR.0b013e32836208de
Huang, W.S., Yang, T.Y., Shen, W.C., et al., Association between Helicobacter pylori infection and dementia, J. Clin. Neurosci., 2014, vol. 21, no. 8, pp. 1355–1358. doi 10.1016/j.jocn.2013.11.018
Jangi, S., Gandhi, R., Cox, L.M., et al., Alterations of the human gut microbiome in multiple sclerosis, Nat. Commun., 2016, vol. 7, p. 12015. doi 10.1038/ncomms12015
Kannarkat, G.T., Boss, J.M., and Tansey, M.G., The role of innate and adaptive immunity in Parkinson’s disease, J. Parkinsons Dis., 2013, vol. 3, pp. 493–514.
Karlsson, O., Berg, A.L., Hanrieder, J., et al., Intracellular fibril formation, calcification, and enrichment of chaperones, cytoskeletal, and intermediate filament proteins in the adult hippocampus CA1 following neonatal exposure to the nonprotein amino acid BMAA, Arch. Toxicol., 2015, vol. 89, no. 3, pp. 423–436. doi 10.1007/ s00204-014-1262-2
Kelly, J.R., Allen, A.P., Temko, A., et al., Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects, Brain Behav. Immun., 2017, vol. 61, pp. 50–59. doi 10.1016/j.bbi.2016.11.018
Kielian, T., Toll-like receptors in central nervous system glial inflammation and homeostasis, J. Neurosci. Res., 2006, vol. 83, no. 5, pp. 711–730.
Kim, J.S. and Sung, H.Y., Gastrointestinal autonomic dysfunction in patients with Parkinson’s disease, J. Mov. Disord., 2015, vol. 8, no. 2, pp. 76–82. doi 10.14802/jmd.15008
Kim, H.S., Lee, J.H., Lee, J.P., et al., Amyloid beta peptide induces cytochrome c release from isolated mitochondria, Neuroreport, 2002, vol. 13, pp. 1989–1993.
Kim, B.S., Jeon, Y.S., and Chun, J., Current status and future promise of the human microbiome, Pediatr. Gastroenterol. Hepatol. Nutr., 2013, vol. 16, pp. 71–79. doi 10.5223/pghn.2013.16.2.71
König, J., Wells, J., Cani, P.D., et al., Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol., 2016, vol. 7, no. 10. e196. doi 10.1038/ctg.2016.54
Kountouras, J., Tsolaki, M., Gavalas, E., et al., Relationship between Helicobacter pylori infection and Alzheimer disease, Neurology, 2006, vol. 66, pp. 938–940.
Kountouras, J., Boziki, M., Gavalas, E., et al., Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients, Cogn. Behav. Neurol., 2010, vol. 23, no. 3, pp. 199–204. doi 10.1097/WNN.0b013e3181df3034
Kramer-Hämmerle, S., Rothenaigner, I., Wolff, H., et al., Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus, Virus Res., 2005, vol. 111, no. 2, pp. 194–213. http://dx.doi.org/ 10.1016/j.virusres. 2005.04.009. PMID: 15885841.
Krogh, K.A., Lyddon, E., and Thayer, S.A., HIV-1 Tat activates a RhoA signaling pathway to reduce NMDA evoked calcium responses in hippocampal neurons via an actin-dependent mechanism, J. Neurochem., 2015, vol. 132, no. 3, pp. 354–366. http://dx.doi.org/10.1111/jnc.12936.
Laferla, F.M., Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease, Nat. Rev. Neurosci., 2002, vol. 3, pp. 862–872.
Lakhan, S.E., Caro, M., and Hadzimichalis, N., NMDA receptor activity in neuropsychiatric disorders, Front. Psychiatry, 2013, vol. 4, pp. 52–55. doi 10.3389/ fpsyt.2013.00052
Lehnardt, S., Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury, Glia, 2010, vol. 58, no. 3, pp. 253–263. doi 10.1002/glia.20928
Letenneur, L., Peres, K., Fleury, H., et al., Seropositivity to herpes simplex virus antibodies and risk of Alzheimer’s disease: a population-based cohort study, PLoS One, 2008, vol. 3. e3637
Licastro, F., Porcellini, E., Caruso, C., et al., Genetic risk profiles for Alzheimer’s disease: integration of APOE genotype and variants that up-regulate inflammation, Neurobiol. Aging, 2007, vol. 28, pp. 1637–1643.
Liu, M. and Bing, G., Lipopolysaccharide animal models for Parkinson’s disease, Parkinsons Dis., 2011, vol. 2011, p. 327089. doi 10.4061/2011/327089
Lobner, D., Piana, P.M., Salous, A.K., and Peoples, R.W., Beta-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms, Neurobiol Dis., 2007, vol. 25, no. 2, pp. 360–366.
Lu, B., Nagappan, G., Guan, X., et al., BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases, Nat. Rev. Neurosci., 2013, vol. 14, pp. 401–416. doi 10.1038/nrn3505
Lukiw, W.J., Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s disease, Front. Microbiol., 2016, vol. 7, p. 1544. eCollection 2016.
Lyte, M., Microbial endocrinology in the microbiome–gut–rain axis: how bacterial production and utilization of neurochemicals influence behavior, PLoS Pathog., 2013, vol. 9, no. 11. e1003726. doi 10.1371/journal.ppat.1003726
Lyte, M. and Cryan, J.F., Microbial Endocrinology: The Microbiota–Gut–Brain Axis in Health and Disease, Adv. Exp. Med. Biol., New York: Springer, 2014, vol. 817.
MacFabe, D.F., Cain, D.P., Rodriguez-Capote, K., et al., Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders, Behav. Brain Res., 2007, vol. 176, pp. 47–54. doi 10.1016/j.bbr.2010.10.005
MacFabe, D.F., Cain, N.E., Boon, F., et al., Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder, Behav. Brain Res., 2011, vol. 217, no. 1, pp. 149–169. doi 10.1016/j.bbr.2006.07.025
Malaguarnera, M., Bella, R., Alagona, G., et al., Helicobacter pylori and Alzheimer’s disease: a possible link, Eur. J. Int. Med., 2004, vol. 15, pp. 381–386.
Manabe, T., Mizukami, K., Akatsu, H., et al., Influence of pneumonia complications on the prognosis of patients with autopsy-confirmed Alzheimer’s disease, dementia with Lewy bodies, and vascular dementia, Psychogeriatrics, 2016, vol. 16, no. 5, pp. 305–314. doi 10.1111/psyg.12163
Mancuso, R., Baglio, F., Cabinio, M., et al., Titers of HSV-1 antibodies correlate with grey matter volumes in AD, J. Alzheimers Dis., 2014, vol. 38, no. 4, pp. 741–745. doi 10.3233/JAD-130977
Manuelidis, L., Infectious particles, stress, and induced prion amyloids: a unifying perspective, Virulence, 2013, vol. 4, pp. 373–383. doi 10.4161/viru.24838
Marques, F., Sousa, J.C., Sousa, N., and Palha, J.A., Blood–brain-barriers in aging and in Alzheimer’s disease, Mol. Neurodegener., 2013, vol. 8, p. 38.
Maruszak, A., Pilarski, A., Murphy, T., et al., Hippocampal neurogenesis in Alzheimer’s disease: is there a role for dietary modulation?, J. Alzheimers Dis., 2014, vol. 38, pp. 11–38.
Matsumoto, M., Kibe, R. Ooga, T., et al., Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study, Front. Syst. Neurosci., 2013, vol. 7, p. 9. doi 10.3389/fnsys.2013.00009
Mattson, M.P., Cheng, B., Davis, D., et al., Beta-amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity, J. Neurosci., 1992, vol. 12, no. 2, pp. 376–389.
Mattson, M.P., Infectious agents and age-related neurodegenerative disorders, Ageing Res. Rev., 2004, vol. 3, pp. 105–120.
Mayer, E.A., Knight, R., Mazmanian, S.K., et al., Gut microbes and the brain: paradigm shift in neuroscience, J. Neurosci., 2014, vol. 34, no. 46, pp. 15490–15496. doi 10.1523/JNEUROSCI.3299-14.2014
Mayer, E.A., Tillisch, K., and Gupta, A., Gut/brain axis and the microbiota, J. Clin. Invest., 2015, vol. 125, no. 3, pp. 926–938. doi 10.1172/JCI76304
McKimmie, C.S., Johnson, N., Fooks, A.R., and Fazakerley, J.K., Viruses selectively upregulate Toll-like receptors in the central nervous system, Biochem. Biophys. Res. Commun., 2005, vol. 336, pp. 925–933.
McNaught, K.S., Perl, D.P., Brownell, A.L., and Olanow, C.W., Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease, Ann. Neurol., 2004, vol. 56, no. 1, pp. 149–162.
McVey, NeufeldK.A., Mao, Y.K., Bienenstock, J., et al., The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse, Neurogastroenterol. Motil., 2013, vol. 25, pp. 183–188. doi 10.1111/nmo.12049
Miklossy, J., Emerging role of pathogens in Alzheimer’s disease, Expert. Rev. Mol. Med., 2011, vol. 13. e30. doi 10.1017/S1462399411002006
Minter, M.R., Taylor, J.M., and Crack, P.J., The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease, J. Neurochem., 2016, vol. 136, no. 3, pp. 457–474. doi 10.1111/jnc.13411
Mitew, S., Kirkcaldie, M.T., Dickson, T.C., and Vickers, J.C., Altered synapses and gliotransmission in Alzheimer’s disease and ad model mice, Neurobiol. Aging, 2013, vol. 34, pp. 2341–2351. doi 10.1016/j.neurobiolaging.2013.04.010
Montagne, A., Barnes, S.R., Sweeney, M.D., et al., Blood–brain barrier breakdown in the aging human hippocampus, Neuron, 2015, vol. 85, no. 2, pp. 296–302. doi 10.1016/j.neuron.2014.12.032
Moulignier, A., Gueguen, A., Lescure, F.X., et al., Does HIV infection alter Parkinson disease?, J. Acquir. Immune Defic. Syndr., 2015, vol. 70, no. 2, pp. 129–136. http://dx.doi.org/10.1097/QAI.0000000000000677.
Mulligan, V.K. and Chakrabartty, A., Protein misfolding in the late-onset neurodegenerative diseases: common themes and the unique case of amyotrophic lateral sclerosis, Proteins, 2013, vol. 81, pp. 1285–1303. doi 10.1002/prot.24285
Obata, Y. and Pachnis, V., The effect of microbiota and the immune system on the development and organization of the enteric nervous system, Gastroenterology, 2016, vol. 151, no. 5, pp. 836–844. doi 10.10153/j.gastro.2016.07.044
Oh, S.H., Kim, H.N., Park, H.J., et al., Mesenchymal stem cells increase hippocampal neurogenesis and neuronal differentiation by enhancing the wnt signaling pathway in an Alzheimer’s disease model, Cell Transplant., 2015, vol. 24, pp. 1097–1109.
Oleskin, A.V. and Shenderov, B.A., Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota, Microb. Ecol. Health Dis., 2016, vol. 27, p. 30971.
Oleskin, A.V., El’-Registan, G.I., and Shenderov, B.A., Role of neuromediators in the functioning of the human microbiota: “business talks” among microorganisms and the microbiota–host dialogue, Microbiology (Moscow), 2016, vol. 85, no. 1, pp. 1–22.
Pablo, J., Banack, S.A., Cox, P.A., et al., Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease, Acta Neurologica Scandinavica, 2009, vol. 120, no. 4, pp. 216–225. doi 10.1111/j.1600-0404.2008.01150.x
Parashar, A. and Udayabanu, M., Gut microbiota: implications in Parkinson’s disease, Parkinsonism Relat. Disord., 2017, vol. 38, pp. 1–7. doi 10.1016/j.parkreldis.2017.02.002
Prandota, J., Possible link between Toxoplasma gondii and the anosmia associated with neurodegenerative diseases, Am. J. Alzheimers Dis. Other Demen., 2014, vol. 29, pp. 205–214. doi 10.1177/1533317513517049
Prusiner, S.B., Biology and genetics of prions causing neurodegeneration, Ann. Rev. Genet., 2013, vol. 47, pp. 601–623. doi 10.1146/annurev-genet-110711-155524
Putney, J.W., Jr., Presenilins, Alzheimer’s disease, and capacitative calcium entry, Neuron, 2000, vol. 27, no. 3, pp. 411–412.
Rao, S.D., Banack, S.A., Cox, P.A., and Weiss, J.H., BMAA selectively injures motor neurons via AMPA/kainate receptor activations. Exp. Neurol., 2006, vol. 201, no. 1, pp. 244–252. doi 10.1016/j.expneurol.2006.04.017
Rhee, S.H., Pothoulakis, C., and Mayer, E.A., Principles and clinical implications of the brain–gut–enteric microbiota axis, Nat. Rev. Gastroenterol. Hepatol., 2009, vol. 6, no. 5, pp. 306–314. doi 10.1038/nrgastro.2009.35
Ritz, B.R., Paul, K.C., and Bronstein, J.M., Of pesticides and men: a California story of genes and environment in Parkinson’s disease, Curr. Environ. Health Rep., 2016, vol. 3, pp. 40–52.
Rivest, S., Regulation of innate immune responses in the brain, Nat. Rev. Immunol., 2009, vol. 9, pp. 429–439.
Sabatier, J.M., Vives, E., Mabrouk, K., et al., Evidence for neurotoxic activity of tat from human immunodeficiency virus type 1, J. Virol., 1991, vol. 65, pp. 961–967.
Sampson, T.R., Debelius, J.W., Thron, T., et al., Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease, Cell, 2016, vol. 167, no. 6, pp. 1469–1480. doi 10.1016/ j.cell.2016.11.018
Sanchez-Guajardo, V., Barnum, C.J., Tansey, M.G., and Romero-Ramos, M., Neuroimmunological processes in Parkinson’s disease and their relation to a-synuclein: microglia as the referee between neuronal processes and peripheral immunity, ASN Neuro, 2013, vol. 5, pp. 113–139.
Santana, S., Recuero, M., Bullido, M.J., et al., Herpes simplex virus type I induces the accumulation of intracellular beta-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells, Neurobiol. Aging, 2012, vol. 33, no. 2, p. 430. e19–33
Saulnier, D.M., Ringel, Y., Heyman, M.B., et al., The intestinal microbiome, probiotics and prebiotics in neurogastroenterology, Gut Microbes, 2013, vol. 4, no. 1, pp. 17–27. doi 10.4161/gmic.22973
Di Scala, C., Yahi, N., Boutemeur, S., et al., Common molecular mechanism of amyloid pore formation by Alzheimer’s β-amyloid peptide and α -synuclein, Sci. Rep., 2016, vol. 6, p. 28781. doi 10.1038/srep28781
Schwartz, K. and Boles, B.R., Microbial amyloids-functions and interactions within the host, Curr. Opin. Microbiol., 2013, vol. 16, pp. 93–99. doi 10.1016/j.mib.2012.12.001
Selkrig, J., Wong, P., Zhang, X., and Pettersson, S., Metabolic tinkering by the gut microbiome: implications for brain development and function, Gut Microbes, 2014, vol. 5, no. 3, pp. 369–380. doi 10.4161/gmic.28681
Sharon, G., Sampson, T.R., Geschwind, D.H., and Mazmanian, S.K., The central nervous system and the gut microbiome, Cell, 2016, vol. 167, no. 4, pp. 915–932. doi 10.1016/j.cell.2016.10.027
Shenderov, B.A., Probiotic (symbiotic) bacterial languages, Anaerobe, 2011, vol. 17, pp. 490–495.
Silei, V., Fabrizi, C., Venturini, G., et al., Activation of microglial cells by PrP and beta-amyloid fragments raises intracellular calcium through L-type voltage sensitive calcium channels, Brain Res., 1999, vol. 818, no. 1, pp. 168–170.
Smith, M.Z., Wightman, F., and Lewin, S.R., HIV reservoirs and strategies for eradication, Curr. HIV/AIDS. Rep., 2012, vol. 9, no. 1, pp. 5–15. http://dx.doi.org/ 10.1007/s11904-011-0108-2.
Sobol, C.V., Mikroflora i serdechno-sosudistaya sistema v norme i pri patologii (Microflora and the Cardiovascular System in Health and Disease), Lambert Academic Publishing, 2014.
Sobol., C.V., Antineoplastic and antimutagenic effects of a new probiotic product at the cellular level and in rats with transplanted fast-growing Pliss’ lymphosarcoma, Int. J. Probiot. Prebiot., 2015, vol. 10, pp. 133–144.
Sobol., C.V., A novel complementary approach using new probiotic product for the improvement of HIV therapy, in Frontiers in Clinical Drug Research-Anti Invectives, Atta-ur-Rahman, Ed., Sharjah, United Arab Emirates: Bentham Science Publishers, 2017a, vol. 3, pp. 49–121. https:// www.dropbox.com/s/4cykdoth8iuuyiu/9781681083698R. pdf?dl=0.
Sobol, C.V., A new class of pharmabiotics with unique properties, in Soft Chemistry and Food Fermentation, Grumezescu, A.M. and Holban, A.M., Eds., Elsevier, 2017b, vol. 3, pp. 79–112. https://www.sciencedirect. com/science/article/pii/B9780128114124000047.
Sobol, C.V. and Belostotskaya, G.B., Product fermented by Lactobacilli induces changes in intracellular calcium dynamics in rat brain neurons, Biochemistry (Moscow) Suppl. Series A: Membr. Cell Biol., 2016, vol. 10, no. 1, pp. 37–45.
Sobol, C.V. and Sobol, Yu.Ts., Composition and method for producing and use of a fermented hydrolyzed medium containing microorganisms and products of their metabolism, US Patent no. 6953574, 2005.
Sobol., C.V., Belostotskaya, G.B., and Kenworthy, M.W., Calcium signalling in rat brain neurons and differentiation of PC-12 cells induced by application of a probiotic product, Neurophysiology (Ukraine), 2005, vol. 37, pp. 284–293.
Sobol, C.V., Korotkov, S.M., Belostotskaya, G.B., and Nesterov, V.P., The influence of probiotics and probiotic product on respiration of mitochondria and intracellular calcium signal in cells of cardiovascular system, Biochemistry (Moscow) Suppl. Series A: Membr. Cell Biol., 2013, vol. 7, no. 4, pp. 294–301.
Soret, R., Chevalier, J., De Coppet, P., et al., Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats, Gastroenterology, 2010, vol. 138, no. 5, pp. 1772–1782. doi 10.1053/j.gastro.2010.01.053
Soscia, S.J., Kirby, J.E., Washicosky, K.J., et al., The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide, PLoS One, 2010, vol. 5. e9505
Suh, J., Sinclair, E., Peterson, J., et al., Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection, J. Neuroinflammation, 2014, vol. 11, p. 199. http://dx.doi.org/10.1186/s12974-014-0199-y.
Tkachenko, E.I. and Uspenskii, Yu.P., Pitanie, mikrobiotsenoz i intellekt cheloveka (Nutrition, Microbiocenosis, and Human Intelligence), St. Petersburg: SpetsLit, 2006.
Torbick, N., Hession, S., Stommel, E., and Caller, T., Mapping amyotrophic lateral sclerosis lake risk factors across northern New England, Int. J. Health Geogr., 2014, vol. 13, p. 1. doi 10.1186/1476-072X-13-1
Tran, L. and Greenwood-Van Meerveld, B., Age-associated remodeling of the intestinal epithelial barrier, J. Gerontol. A Biol. Sci. Med. Sci., 2013, vol. 68, pp. 1045–1056. doi 10.1093/gerona/glt106
Del Tredici, K. and Braak, H., A not entirely benign procedure: progression of Parkinson’s disease, Acta Neuropathol., 2008, vol. 115, pp. 379–384.
Udit, S. and Gautron, L., Molecular anatomy of the gut-brain axis revealed with transgenic technologies: implications in metabolic research, Front. Neurosci., 2013, vol. 7, p. 134. doi 10.3389/fnins.2013.00134
Valera, E. and Masliah, E., Combination therapies: the next logical step for the treatment of synucleinopathies?, Mov. Disord., 2016, vol. 31, pp. 225–234.
Varatharaj, A. and Galea, I., The blood-brain barrier in systemic inflammation, Brain Behav. Immun, 2017, vol. 60, pp. 1–12. doi 10.1016/j.bbi.2016.03.010
Verma, R., Verma, A.K., Ahuja, V., and Paul, J., Real-time analysis of mucosal flora in patients with inflammatory bowel disease in India, J. Clin. Microbiol., 2010, vol. 48, pp. 4279–4282.
Voloshko, L.N. and Pinevich, A.V., Diversity of cyanobacterial toxins, Astrakhan. Vestn. Ekol. Obraz., 2014, vol. 1, no. 27, pp. 68–80.
Wall, R., Cryan, J.F., Ross, R.P., et al., Bacterial neuroactive compounds produced by psychobiotics, Adv. Exp. Med. Biol., 2014, vol. 817, pp. 221–239.
Wang, X.L., Zeng, J., Feng, J., et al., Helicobacter pylori filtrate impairs spatial learning and memory in rats and increases β-amyloid by enhancing expression of presenilin-2, Front. Aging Neurosci., 2014, vol. 6, p. 66. doi 10.3389/fnagi.2014.00066
Welling, M.M., Nabuurs, R.J., and van der Weerd, L., Potential role of antimicrobial peptides in the early onset of Alzheimer’s disease, Alzheimers Dement., 2015, vol. 11, no. 1, pp. 51–57. doi 10.1016/j.jalz.2013.12.020
Widera, M., Klein, A.N., Cinar, Y., et al., The D-amino acid peptide D3 reduces amyloid fibril boosted HIV-1 infectivity, AIDS Res. Ther., 2014, vol. 11, no. 1, p. 1. doi 10.1186/1742-6405-11-1
Wozniak, M.A., Frost, A.L., Preston, C.M., and Itzhaki, R.F., Antivirals reduce the formation of key Alzheimer’s disease molecules in cell cultures acutely infected with herpes simplex virus type 1, PLoS One, vol. 6. e25152.
Xie, X., Basile, M., and Mash, D.C., Cerebral uptake and protein incorporation of cyanobacterial toxin β-N-methylamino-L-alanine, Neuroreport, 2013, vol. 24, no. 14, pp. 779–784. doi 10.1097/WNR.0b013e328363fd89
Xu, J. and Ikezu, T., The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era, J. Neuroimmune Pharmacol., 2009, vol. 4, no. 2, pp. 200–212.
Yin, H.Z., Yu, S., Hsu, C.-I., et al., Intrathecal infusion of BMAA induces selective motor neuron damage and astrogliosis in the ventral horn of the spinal cord, Exp. Neurol., 2014, vol. 261, pp. 1–9. doi 10.1016/ j.expneurol.2014.06.003
Yu, Y. and Ye, R.D., Microglial Aβ receptors in Alzheimer’s disease, Cell. Mol. Neurobiol., 2015, vol. 35, no. 1, pp. 71–83. doi 10.1007/s10571-014-0101-6
Yu, J.T., Chang, R.C., and Tan, L., Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities, Prog. Neurobiol., 2009, vol. 89, no. 3, pp. 240–255.
Zambrano, A., Solis, L., Salvadores, N., et al., Neuronal cytoskeletal dynamic modification and neurodegeneration induced by infection with herpes simplex virus type 1, J. Alzheimers Dis., 2008, vol. 14, no. 3, pp. 259–269.
Zhang, H., Sun, S., Wu, L., et al., Store-operated calcium channel complex in postsynaptic spines: a new therapeutic target for Alzheimer’s disease treatment, J. Neurosci., 2016, vol. 36, pp. 11837–11850.
Zhao, Y., Dua, P., and Lukiw, W.J., Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer’s disease (AD), J. Alzheimers Dis. Parkinsonism, 2015, vol. 5, no. 1, p. 177.
Zhou, Y., Blanco, L.P., Smith, D.R., and Chapman, M.R., Bacterial amyloids, Methods Mol. Biol., 2012, vol. 849, pp. 303–320.
Zhou, L., Miranda-Saksena, M., and Saksena, N.K., Viruses and neurodegeneration, Virol. J., 2013, vol. 10, p. 172. doi 10.1186/1743-422X-10-172
ACKNOWLEDGMENTS
I am grateful to Doctor of Biology I.V. Shemarova for a number of valuable comments and additions.
The work was supported by the Federal Agency for Scientific Organizations of Russia (topic no. АААА-А18-118012290142-9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Barkhash
Rights and permissions
About this article
Cite this article
Sobol, C.V. Role of Microbiota in Neurodegenerative Diseases. Russ J Dev Biol 49, 297–313 (2018). https://doi.org/10.1134/S1062360418060061
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360418060061