Abstract—
The large Japanese field mouse (Apodemus speciosus) is endemic to the Japanese islands and Kunashir Island in Russia. Variability in the morphology of 722 molars was studied for the first time for the population of this species from Kunashir Island, which allowed us to identify seven new traits for the species. According to data obtained when studying modern and paleontological material (recent, Holocene, Late Pleistocene, Middle Pleistocene), mice of Kunashir Island lack some traits of molars previously described for the large Japanese field mouse from Honshu Island; at the same time, stabilization of the morphology was noted for the remaining traits. Varying frequency was demonstrated for the new traits. This can be caused either by the small number of founders or the passage of the Kunashir population through sharp declines in numbers. It is possible that a change in the frequency of some molar phenes in different historical epochs could be associated with the transition of mice to another type of feed following changes in the climate and the prevailing vegetation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The large Japanese (or red) field mouse (Apodemus (Alsomys) speciosus sensu str. (Temminck 1845)) is an endemic species of the Japanese archipelago, which inhabits mixed forests of four large islands (Hokkaido, Honshu, Shikoku, and Kyushu), several groups of small islands (Sado, Izu, Satsunan), Tsushima Island in Japan, and Kunashir Island of the Kuril Ridge in Russia (Gromov and Erbaeva, 1995; Musser et al., 1996; Musser and Carleton, 2005; Kaneko, 2005; Nakata et al., 2009).
The species was described for the first time as Mus speciosus Temminck 1844 from Kyushu Island, and subsequently it was divided into two species: the East Asian (or Korean) field mouse A. peninsulae (=A. giliacus) (Thomas, 1906) and A. speciosus sensu str. proper (Vorontsov et al., 1977). The latter species was introduced into the composition of Russian fauna under the Russian names “red mouse” (Kostenko, 1976) and “Japanese mouse” (Gromov and Baranova, 1981). “Three indices: coloration of the back, where reddish tones predominate; a white tail tip in 75% of individuals; and an additional tubercle, which forms a loop, in the back of the first molar of the upper jaw (M1), on its inner side” were distinguished as diagnostic traits for A. speciosus (Kostenko, 2000, p. 46; Kostenko et al., 2004, p. 62), or “tubercle t12” according to other nomenclature (Gromov and Erbaeva, 1995, p. 287). The study of the cranial and dental peculiarities of the modern and paleontological material on A. speciosus from several local samples from Honshu Island allowed the determination of additional specific morphological characteristics of the large Japanese field mouse (Kawamura, 1989). It was demonstrated that unlike the species A. argenteus Temminck 1894 and A. peninsulae, A. speciosus has a larger body and cranium size (longer rostrum and less wide posterior part of the nasal bone). The differentiation of A. speciosus sensu str. and A. peninsulae species was later confirmed by genetic and biochemical analysis (Pavlenko et al., 1984) and then by molecular analysis (according to the mtDNA study) (Chelomina et al., 1998; Suzuki et al., 2015).
Based on the morphometric characteristics of the body for A. speciosus sensu str., eight forms and three subspecies were described: A. s. ainu Thomas 1906 from the islands of Hokkaido and Kunashir, A. s. speciosus Temminck 1844 from the large islands Honshu, Shikoku, and Kyushu and the small islands Yakushima (=dorsalis Kuroda 1924), Oshima (=insperatus Kuroda 1938), Dogo (=navigator Thomas 1906), Sado (=sadoensis Thokuda 1941), and Tsushima (=tusimaensis Thokuda 1924). The third subspecies (A. s. miyakensis Imaizumi 1969) was described from two groups of small islands (Imaizumi and Izu) (Corbet and Hill, 1980). Two karyotypes were detected in A. speciosus: “western” (with 2n = 46) and “eastern” (with 2n = 48) (Tsuchiya, 1974). The border between them runs across Honshu Island in its central part. The karyotype of large Japanese field mice from Kunashir Island was described in 1974 and in 1977 (Bekasova and Vorontsov, 1974; Vorontsov et al., 1977), then (capture in 1989) we studied it (Boeskorov et al., 1995; Kartavtseva, 2002; Rubtsov et al., 2015). It had no differences from that of the “eastern” karyotype either in the number of chromosomes or in the nature of their differential staining (Kartavtseva, 2002). A molecular genetic study of intraspecific differentiation of A. speciosus using the mtDNA cytochrome b gene (Chelomina et al., 1998) suggested that the population of Honshu Island was the first to be isolated from others, “then the population of Kunashir Island, and only after that the mice of Hokkaido and two adjacent islands differentiated” (that is, Rishiri and Okushiri) (Chelomina, 2005, p. 150). According to the results of phylogenetic analysis, the A. speciosus populations were divided into two clades: (1) Honshu, Shikoku, Kyushu, and some peripheral islands; (2) Hokkaido and most of the peripheral islands (Suzuki et al., 2004, 2015). It is noteworthy that the molecular genetic differentiation of A. speciosus does not coincide with the karyotype.
The large Japanese field mouse is an excellent model object of study in the monitoring of environmental pollution, medicine, and fundamental biology (Azuma et al., 2009), and for the analysis of the variability of morphological traits in numerous island populations in different landscape, climatic, and ecological conditions. The sizes of the islands, time of isolation, temperature mode, living together or separate with other rodent species, the presence of predators, and other factors (Sakai and Miyao, 1980, 1980a, 1988; Sakai, 1998; Takada et al., 2006; Kageyama et al., 2009; Shintaku et al., 2012; Asahara, 2017; Biswas and Motokawa, 2019; etc.) can determine the occurrence of physiological and morphological differences in the members of certain island populations.
The analysis of the upper and lower molars in modern and fossil individuals (in the interval from the Middle Pleistocene to the Holocene) of A. speciosus samples from the Honshu Island (Kawamura, 1989) revealed a low variability in the frequency of a number of traits, while almost all of them were noted in different historical periods. For other island populations of A. speciosus, variability of the molar phenes is unknown.
As demonstrated by the results of the analysis of molars in the members of species from the genus Apodemus (Kawamura, 1989; Zykov and Izvarin, 2020), it is difficult to carry out species diagnostics of paleontological material without knowledge of modern variability. In this regard, the study of polymorphism in the structure of teeth in mice from modern populations is relevant and is of interest for evolutionary studies.
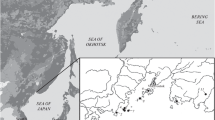
Kunashir Island (inhabited by A. speciosus) has an area of 1495.24 km2 and, together with two other islands (Urup and Iturup), is a part of the southern group of the Great Ridge of the Kuril Islands of Russia. This species was noted for the first time on Kunashir Island by Tokuda (1941). Morphometric data for A. speciosus individuals from the population of this island are given in the work of Vorontsov et al. (1977). The use of metric data of mice of the A. s. ainu subspecies from Hokkaido Island (Kobayashi and Hayata, 1971) allowed individuals from Kunashir Island to be assigned to the same subspecies. Kostenko (2000) had the same point of view, despite the fact that A. speciosus specimens from the Kunashir population were larger than those from Hokkaido (according to unpublished data).
Kunashir Island separated from Hokkaido Island relatively recently, in the Holocene (about 6000 years ago) (Sakaguchi, 1983; Maeda et al., 1994; Sato et al., 1998; Korotky et al., 2005) or, according to other data, about 7500 years ago (Velizhanin, 1976; Bezverkhnii et al., 2002); therefore, their flora and fauna differ little. The time of separation of Kunashir and Hokkaido corresponded to the beginning of the warmest period of the Holocene, although warming in the Japanese archipelago was less pronounced than in other regions of the Far East (Korotky et al., 2000, 2005). At that time, the average annual temperature on Kunashir Island was 2–3°С higher than modern one and the average summer temperature could reach 20°С (Razzhigaeva et al., 2014); mixed coniferous–broad-leaved forests and cool–broad-leaved forests were widespread here. In the Late Holocene, the climate on Kunashir became cooler, and although the island vegetation did not undergo significant changes (Razzhigaeva et al., 2011; Razjigaeva et al., 2002, 2013; Nazarova et al., 2020), these events led to a fragmentation of the populations of many plant and animal species (Razjigaeva et al., 2013).
The isolated position of the population of the large Japanese field mouse on Kunashir (at least since the second half of the Holocene) and the alternation of cold and warm periods could lead to the appearance of morphological peculiarities in the members of this population, as well as to uneven distribution of different phenes inside it. In the present work, we studied the morphological traits of molars in individuals from three A. speciosus samples from Kunashir Island using the classification of main and additional tubercles previously proposed for the large Japanese field mouse from Honshu Island (Kawamura, 1989).
MATERIALS AND METHODS
The collection of crania stored in the Laboratory of Evolutionary Zoology and Genetics and the Laboratory of Theriology of the Federal Science Center of East Asia Terrestrial Biodiversity, Far East Branch, Russian Academy of Sciences, was the material for this study. We studied 722 teeth from 61 individuals from three samples that were collected in different years in the southern part of Kunashir Island (Fig. 1). Sample no. 1 (43°54′55′′ N and 145°39′06′′ E), 25 individuals, collected in 1972, kindly provided by V.A. Kostenko: nos. 4514–4539. Sample no. 2 (44°06′11′′ N and 145°01′34′′ E), 32 individuals, collected by M.V. Pavlenko, 1980: nos. 1-80k–3-80k, 5-80k, 8-80k, 9-80k, 10-80k–14-80k, 16-80k–19-80k, 20-80k–24-80k, 27-80k, 31-80k–33-80k, 35-80k, 36-80k, 43-80k, 45-80k, 47-80k, 50-80k, 51-80k, 1-80knn. Sample no. 3 (coordinates are the same as for sample no. 2), four individuals, collected by M.V. Pavlenko, 1989: nos. 49–89, 51–89, 77–89, 78–79. No individuals with worn teeth were included in the analysis.
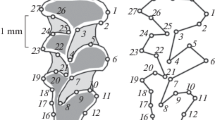
The classification of structures (tubercles and additional formations) of the chewing surface of molars (previously accepted for A. speciosus individuals from Honshu Island (Kawamura, 1988)) or a numerical classification of tubercles (t1, t2, t3, etc.) was used in this work (Fig. 2). The traits of molars and phenes were taken from the work of Kawamura (1989). Schematic images of all molars and their variability in A. speciosus are given in Fig. 3. For each tooth, the traits are indicated by numbers; phenes, by letters. Thus, M1 has eight traits; each trait, a phene. To encode the traits of upper teeth and their phenes, we give a formula, in which first the tooth number is indicated, then (through a hyphen) the trait number and the letter indicating the state of the trait. For example, the formula M1-1A indicates that the first upper molar (M1) has a trait under the serial number 1, and the letter A immediately following it indicates the phene of the absence of the t12 tubercle (posterium cingulum). The M1-1B formula reflects a weak development of the t12 tubercle, while the formula M1-1C reflects its good development.
Schemes of variable morphological structures of molars of Apodemus speciosus individuals previously analyzed in the populations of Honshu Island (according to Kawamura, 1989) and by us on Kunashir Island: (a) eight polymorphic traits M1 (marked with arrows). Arrows and letter designations enclosed in a circle indicate a structure that we found on Kunashir Island; designations enclosed in a square indicate a structure discovered for the first time; teeth with two traits simultaneously are marked with an asterisk. (а),1, three degrees of the t12 tubercle development: A, tubercle is not developed; B, tubercle is poorly developed; C, tubercle is well developed. (а), 2B, spur on t1. (а), 3B, spur on t3. (а), 4B, additional tubercle (prestyle). (а), 5B, platform (precingulum). (а), 6В, separately located t1. (а), 7B, reduced t3. (а), 8B, t4 is separated from t5. (b) Two polymorphic traits M2. (b), 1, three degrees of tubercle t12 development: A, not developed; B, poorly developed; C, well developed. (b), 2, degree of tubercle t3 development: A, no; B, platform; C, poorly developed; D, well developed. (с) Variable signs of M3. (с), 1: A, normal location of plates; B, separately located t8. (с), 2B, additional tubercle (C). (с), 3, rupture of plates: B, lower plate; C, upper plate. (d) Variable traits m1. (d), 1, phenes (A–F) and numbers of additional tubercles are given according to Kawamura (1989). We found the phene marked with the letter C in a circle in the Kunashir population. (d), 2B, circular closure of the enamel of the labial anteroconid tubercle with the formation of two closed spaces of the upper plate. (d), 3B, additional tubercle C5. (d), 4B, connection of two plates (upper and middle). (е) Рhenes (A–H) and numbers of additional tubercles are given according to Kawamura (1989). We found the phene marked with the letter E in a circle on Kunashir Island. (f) Additional tubercles: B, tubercle C1; C, tubercles C1 and C2.
For the first lower molar (m1), the variability in the number of additional tubercles (C) was considered as a single trait m1-1 (Fig. 3d, 1) (retaining the letters that designate phenes) in accordance with the work of Kawamura (1989) to simplify the comparison of our data and the literature.
Minimal (min) and maximal (max) values of the measurements (L, length; W, width) are given in millimeters; the average values, in brackets; n, the number of studied teeth. Photographing of the teeth of individuals from two samples (collected in 1972 and 1989) was carried out under a SteREODiscovery V12 stereomicroscope (Carl Zeiss) using an Axio CamMRc digital camera; images were combined using a Combine ZM program; measurements, in the Axio Vision 4.8.2 program. When processing the material, the equipment of the Center for Collective Use of the Federal Science Center of East Asia Terrestrial Biodiversity, Far East Branch, Russian Academy of Sciences, was used.
RESULTS
The study of the upper and lower molars of A. speciosus individuals from Kunashir Island allowed detection of the traits and phenes both previously described for the large Japanese field mouse of Honshu Island (Kawamura, 1989) and new ones. The average length of the row of upper molars (M1-3) in the specimens of three samples that we studied (Table 1) corresponds to that previously published for the Kunashir population. The sizes of the row of lower molars for combined samples and each tooth are given for the first time.
Upper Molars
М1, first upper molar
This tooth consists of three plates (chevrons or laminas), and each of them consists of three tubercles. The labial tubercle (t3 or labial anterocone) and posterostyle (t7) are always well developed. An additional tubercle (prestyle) in front of the anterior plate and the platform (precingulum) are absent (Fig. 2).
Two out of five previously described phenes (Fig. 3a, 1–5) for M1 individuals from Khonshu Island were found in the Kunashir population (Fig. 3a, 1, 3).
(1) M1-1C, is characterized by a good development of the t12 tubercle (posterium cingulum), which forms a pronounced postero-buccal protrusion from t8 (Figs. 3a, 1C; 4c), which has no contact with t9 (metacone). This tubercle was poorly developed in only one individual (no. 4522) from the sample 1972, M1-1B phene (Figs. 3a, 1B; 4b). The phene M1-1C was detected in all three samples from Kunashir.
(2) М1-3В, a posterior spur of the tubercle t3, is weakly pronounced in one individual (no. 4522) from the sample 1972 (Figs. 3a, 3B; 4b), the spurs on t3 were absent in other teeth studied.
The remaining three phenes noted on Honshu were absent in the population of Kunashir Island: M1-2B, a posterior spur on t1 (Fig. 3a, 2B); M1-4B (Fig. 3a, 4B), the appearance of an additional tubercle (prestyle); and M1-5B (Fig. 3a, 5B), the appearance of the platform (precingulum). Accordingly, no simultaneous presence of two phenes (M1-4B and M1-5B) was detected.
New traits for A. speciosus individuals from Kunashir Island.
(1) М1-6В, separation of the t1 tubercle from t2 (Figs. 3a, 6B; 4c). The frequency of this phene in the samples 1972, 1980, and 1989 varied (0.36, 0.063, and 0.0, respectively).
(2) М1-7В, a decrease in the size of the t3 tubercle and the appearance of a platform, without additional tubercles (Figs. 3a, 7B; 4a). The frequency of the platform appearance in all Kunashir samples is high, the t3 tubercle was not reduced, and the platform was absent only in four mice from the sample 1972 (0.167). In two individuals from the same sample, two phenes (M1-6B and M1-7B) were present simultaneously; that is, the tooth had both a separate t1 tubercle and a platform under t3 (0.083) (Fig. 4c). In other samples, the t3 tubercle was reduced and was always present (1.0).
Photographs of molars of Apodemus speciosus individuals from Kunashir Island: (a) row of upper molars M1–M3 (no. 12-80k), arrow near the M1 tooth indicates a weakly developed tubercle t3 and the platform under it; arrow near the M2 tooth, a developed tubercle t3; (b) M1 (no. 4522), with a well-developed tubercle t3 and a small spine on it, the tubercle t12 is poorly developed; (c) M1 (no. 12-80k) with a well-developed tubercle t12 and a separate tubercle t1; (d) M1 (no. 4536) with a separate tubercle t5 of the middle plate; (e) M3 with a separate tubercle t1; (f) M3 (no. 12-80k), arrow indicates an additional tubercle C; (g) row of lower molars m1–m3 (no. 12–80k) indicating additional tubercles for each tooth; (h) m1 with an additional tubercle C5 above the upper plate and with the connection of two plates, indicated by a large arrow (no. 3-80k); (i) arrow indicates a closure of the enamel of the upper plate m1 with the formation of two closed spaces; (j) m3, two additional tubercles (developed C1 and poorly developed C2) are indicated (no. 19-80k); (k) m3 with a well-developed tubercle C1 (no. 22-80k).
(3) M1-8B, separation of t4 from t5 (Figs. 3a, 8B; 4d). A phene was found in a single individual (no. 4526) from the sample 1972, and we determined it as anomalous (0.04).
M2, second upper molar
This tooth consists of one well-developed t1 tubercle, weakly developed t3, and two plates with three tubercles each (Fig. 3b). The tubercle t7 (posterostyle), as well as the corresponding tubercle on M1, is well developed.
For the second upper molar, we confirmed the variability of two traits: M2-1 (Fig. 3b, 1) and M2-2 (Fig. 3b, 2). No new phenes were found.
(1) M2-1, the phenes of this trait differ in the degree of the tubercle t12 development. М2-1С, good development of the tubercle t12 (Fig. 3b, 1C), was detected in almost all teeth studied; this tubercle was poorly developed in only one individual (no. 4522) from the sample 1972 (M2-1B). The phene M2-1A (no tubercle t12) was not found.
(2) М2-2, the variability is associated with different development of the tubercle t3 (anterocone) (Fig. 3b, 2) adjacent to the tubercle t12 of the first upper molar M1 (Fig. 4a). Previously, Kawamura (1989) visually identified well-developed and poorly developed variants of the tubercle t3. We failed to do this, since, according to our data, the sizes of this tubercle constitute a continuous series of variability within the following limits in the width and length: max 0.26 (0.18), min 0.09; max 0.19 (1.136), min 0.09 (n = 61), respectively. A well-developed tubercle (phene M2-2D) was taken as one having a width of more than 0.2 mm (Fig. 4a); a poorly developed one (phene M2-2C) had a tubercle less than 0.2 mm wide (Fig. 3b, 2C). The frequency of the M2-2D variant was less than that of the M2-2C variant. In the sample 1972, the tubercles t3 were well developed only in four out of 42 teeth (0.095); in the sample 1980, in 19 out of 56 teeth (0.339); and in the sample 1989, all M2 had a poorly developed tubercle t3. Thus, the t3 is always present on the second upper molar in large Japanese field mice in the samples from Kunashir Island that we studied; the degree of its development is mainly weak (0.765). For the phenes M2-2A, the absence of a platform near the tubercle t3 (Fig. 3b, 2A), and M2-2B (Fig. 3b, 2B), we did not detect its presence.
M3, third upper molar
This tooth has a rounded shape and consists of a single tubercle (t1) and two transverse or oblique plates (upper and lower) formed by a fusion of the tubercles t4-t5 and t8-t9, respectively (Figs. 2; 4e).
Previously described traits (according to Kawamura, 1989)
We detected the simultaneous presence of continuous upper and lower plates on M3 (phene M3-1A) in most of the teeth of the large Japanese field mouse on Kunashir (0.964). However, the connection of the tubercles t8 and t9 can be incomplete and look like a weak constriction between them. In the samples studied, the portion of individuals with an incomplete connection of the tubercles t8 and t9 is different; however, we do not consider this variant as a separate phene.
Kawamura attributed a rupture of the upper (phene M3-C) and lower plates (phene M3-3B) (Figs. 3c, 3B; 3c, 3C, respectively) during their connection by the tubercles t4-t8 to anomalous variants. We did not find such phenes. No connection of the plates by the tubercles t4-t8 without their rupture was found either on Honshu or in the present study.
New trait M3-2 is caused by the appearance of an additional tubercle (C) above the tubercle t5 and was found only on one tooth of the individual no. 12-80k (Figs. 3c, 2B; 4f), which allows us to consider it anomalous.
Lower Molars
m1, first lower molar
The structure of the first lower molar includes three plates and one posterior tubercle (postero-central, or posterior cingulum). The front plate has the form of two triangles divided or connected by vertices. The additional tubercles on the buccal side (C) are well developed, C1 is always separated from C2 and the posterior plate, two tubercles (C2 and C3) are connected (phene m1-C) and form a thin independent plate (Figs. 3d; 4g). We found no other five phenes (m1-A, m1-B, m1-D, m1-E, and m1-F) (Fig. 3d), previously described for this tooth and differing in the number and location of the additional tubercles.
New traits are characterized by peculiarities of the fusion of the upper plate tubercles (labial anteroconid, lingual anteroconid, medial anteroconid). Thus, the phene m1-2B, a circular closure of the enamel of the tubercle labial anteroconid with the formation of two closed spaces within the boundaries of the upper plate (Figs. 3d, 2B; 4i), was detected in ten individuals from three samples: 1972 (nos. 4516, 4521), 1980 (nos. 11-80k, 12-10k, 14-80k, 15-80k, 16-80k, 22-80k), and 1989 (nos. 51-89k, 77-89k).
The phene m1-3B is characterized by the presence of the additional tubercle C5 (Figs. 2; 3d, 3B; 4h); it was found only on the left tooth of the individual no. 3-80k.
The phene m1-4B (connection of two plates, upper and middle (Figs. 3d, 4B; 4h)) was found in three individuals: nos. 4519 (1972), 3-80k, and 23-80k (1980).
m2, second lower molar
This tooth includes two plates and one posterior tubercle. A labial anteroconid is present. Two additional tubercles (C2 and C3) (Fig. 3e, E) are always present (phene m2-1E) in individuals of all three Kunashir samples studied (1.0). The degree of their development is different, but is less than on m1 (Fig. 4g). The tubercle C2 is well developed and is not connected to other tubercles. The tubercle C3 is close to the labial tubercle (labial anteroconid) and merges with it when the tooth is abraded. The C3 frequently merges with a neighboring plate by enamel (42%). Seven other phenes of this tooth (Fig. 3e), previously described by Kawamura, were not detected in our material.
m3, third lower molar
This tooth includes one plate and one posterior tubercle (enteroconid), the morphology of which is stable (Fig. 2).
New traits. The appearance of additional tubercles C1 and C2 was detected. The phene m3-B characterizes a tooth with a single additional tubercle C1 (Figs. 3f, B; 4k); m3-C, with two additional tubercles (Figs. 3f, C; 4j). The tubercle C1 is present in all teeth studied, but varies in size: L, min 0.06 (0.124), max 0.23; W, min 0.06 (0.119), max 0.17. The tubercle C2 is smaller than C1; both tubercles were detected together only on one tooth (no. 22-80k) in the sample 1980.
DISCUSSION
A well-developed tubercle t12 on M1 was proposed as one of the species diagnostic traits for A. speciosus (Kostenko, 1984). We found a single individual with a poorly developed t12 in the sample 1972, on the basis of which we assume that the trait can be variable. In the samples 1980 and 1989, all teeth had a well-pronounced tubercle t12.
According to paleontological data (Kawamura, 1989), in the Middle Pleistocene the frequency of M1 teeth with a well-developed tubercle t12 in A. speciosus individuals on Honshu Island was low (0.19); an intermediate variant (that is, poorly developed tubercle) prevailed (0.57) (Figs. 5a, 5c). In the Holocene and at present, the frequency of teeth with well-developed t12 increased to 0.497 and 0.719, respectively. In the Kunashir population, the portion of teeth with a well-developed tubercle was the highest (0.98). It is interesting that three phenes can be distinguished for the tubercle t12 of the first upper molar (well developed, poorly developed, or completely absent) for all time periods on Honshu. Therefore, a well-developed tubercle t12 on M1 cannot be considered as a reliable species diagnostic trait.
Diagrams demonstrating the frequency of tubercles t12 (a–d) on the upper molars M1, M2, and tubercle t3 on the molar M2 (e–f) in Apodemus speciosus individuals from two islands (Kunashir (our data) and Honshu (according to Kawamura, 1989)) in different epochs (modern, Holocene, Late Pleistocene, Middle Pleistocene). The degree of tubercle development is indicated by colors. The variants of the tubercle severity are marked with an arrow (c, d). Tubercle t12 on M1 (c) and M2 (d): A, tubercle is not developed (blue); B, poorly developed (red); C, well developed (green). The variants of t3 tubercle development on M2 (f): A, no tubercle (blue); B, platform (red); C, poorly developed tubercle (green); D, well developed tubercle (purple). Colors in the circles correspond to those in the diagrams; numbers in the sectors of the diagrams, frequency of the trait (%).
The frequency of a well-developed tubercle t12 on M2 (phene M2-1C) in the Kunashir population is similar to that for M1 (phene M1-1C). An increase in the frequency of these phenes is traced in mice from Honshu Island from the Middle Pleistocene to the present (Figs. 5b, 5d). It is possible that most of the mice that were the founders of the Kunashir Island population had well-developed tubercles t12 on the second upper molar or a selection of individuals with such phenes passed for a long time (Figs. 5b, 5d): it is possible that a change in the frequency of some phenes of molars in different historical epochs could have been caused by the transition of mice to a different type of feed, following a change in the climate and prevailing vegetation.
For the upper plate M1 of individuals that we studied, we noted for the first time the separation of the tubercle t1 and a decrease in the size of the tubercle t3. Since no such traits were found in mice of the population from Honshu Island, it can be assumed that they characterize the population of Kunashir Island. Since the separation of the Kunashir from Hokkaido populations occurred relatively recently, the presence of these traits in the Hokkaido population is not excluded.
The separation of tubercles t4 from t5-t6 and t1 from t2-t3 (M1-4B) (which we detected) is also found in other species of the genus Apodemus (Larina and Eremina, 1988). We noted a single t1 with a low frequency (0.127) in A. peninsulae in the Ussuriiskii Reserve, in the southern part of Primorskii krai (Gornikov et al., 2020). The frequency of this trait in the Kunashir population of A. speciosus varied in the samples studied and was maximal in 1972 (0.36).
The variability of the tubercle t3, which is constantly present on the second upper molar, but expressed to varying degrees, is interesting. Thus, the portion of teeth having a well-developed tubercle t3 (phene M2-2D) in the samples 1972, 1980, and 1989 was 0.095, 0.339, and 0, respectively; on average, this index is 0.235. In mice from Honshu Island, four phenotypes were distinguished for this trait, including the absence of the tubercle and platform (Figs. 5e, 5f). Such phenes were absent in the Kunashir population.
It should be noted that the absence of the tubercle t3 on M2 (phene M2-2A) is considered as a species trait for the field mouse (Apodemus agrarius Pallas 1771) (Ruprecht, 1978; Musser et al., 1996; Ge et al., 2019); however, in modern samples of this species from different European populations, this tubercle was detected with a different frequency: from 0.6 to 0.4. For this reason, the diagnostic significance of this trait (especially for the field mouse) was questioned (Ruprecht, 1978). In island and mainland field mouse populations in the southern part of the Russian Far East, the tubercle t3 on the second upper molar was also found with a frequency from 0.167 to 0.333; a large tubercle was detected only in a single mainland population (0.122) (Sheremet’eva et al., 2017). In A. agrarius individuals from four islands in the Sea of Japan, the tubercle t3 on the second upper molar was more common (from 0.6 to 1.0); at the same time, the specimens with a weakly developed tubercle predominated. We assume that the degree of the development of the tubercle t3 on the second upper molar can be taken into account when studying intra- and interpopulation differentiation not only of A. agrarius, but also of A. speciosus.
Despite the fact that we found a weak variability of M3 by the trait of the separation of the tubercle t8 from t9 (phene M3-1B) in the Kunashir population, in general it corresponded to that previously described for the Honshu population. We registered only for the first time the appearance of additional tubercle C in place of reduced t3 (M3-2), regarding this variant as anomalous.
The picture of variability of the upper plate of the first lower molar (phenes m1-2B and m1-4B) and the appearance of the tubercle C1 (phene m1-3B) in it, as well as additional tubercles C1 and C2 (phenes m3-B and m3-C) on the third lower molars (Fig. 3f) in the large Japanese field mice of the Kunashir population, are not typical for this species. However, the high frequency of these unusual variants suggests that they can be controlled by recessive alleles and appear in multiple inbreedings. The same can be assumed for some other morphological peculiarities that we consider as abnormal, for example, the appearance of additional tubercles on m1 (C5) and m3 (C2). It is possible that the A. speciosus population of Kunashir Island was founded by a small number of individuals and/or passed through a “bottleneck.”
Kunashir Island separated from Hokkaido Island in the Early Holocene, while the latter separated from Honshu Island much earlier (12 000 years ago) (Ohshima, 1990). This can predetermine the greater similarity of the teeth of the large Japanese field mice from the islands of Kunashir and Hokkaido when compared with the specimens from Honshu. The peculiarities of A. speciosus molars (detected in this work) supplement the list of morphological and morphometric characteristics of this species, in general, and of the subspecies A. s. ainu. These data allow us to continue the study of individuals from Hokkaido and adjacent small islands.
REFERENCES
Asahara, M., Geographic variation of absolute and relative lower molar sizes in two closely related species of Japanese field mice (Apodemus speciosus and Apodemus argenteus: Muridae, Rodentia), Zool. Sci., 2017, vol. 34, pp. 26–34. https://doi.org/10.2108/zs160103
Azuma, R., Hatanaka, Y., Shin, S.-W., Mura, H., Miyashita, M., et al., Geographic variation in morphological traits of the large Japanese field mouse, Apodemus speciosus (Rodentia, Muridae), from Izu Island group, Japan, Zool. Sci., vol. 26, no. 2, pp. 266–276. https://doi.org/10.2108/zsj.26.266
Bekasova, T.S. and Vorontsov, N.N., Systematic position of Asian wood mice of the genus Apodemus living in the Far East and Siberia, in Materialy I Mezhdunar. teriol. kongr., Tezisy Dokladov (Proc. I Int. Theriol. Congr., Abstracts of Papers), Moscow, 1974, vol. 1, pp. 52–53.
Bezverkhnii, V.L., Pletnev, S.P., and Nabiulin, A.A., Essay on the geological structure and development of the Kuril island-arc system and adjacent territories, in Rastitel’nyi i zhivotnyi mir Kuril’skikh ostrovov (Materialy Mezhdunarodnogo kuril’skogo proekta) (Flora and Fauna of the Kuril Islands (Materials of the International Kuril Project)), Vladivostok: Dal’nauka, 2002, pp. 9–22.
Biswas, J.K. and Motokawa, M., Morphological analysis of static skull variation in the large Japanese field mouse, Apodemus speciosus (Rodentia: Muridae), Mamm. Study, 2019, vol. 44, no. 1, pp. 51–63.
Boeskorov, G.G., Kartavtseva, I.V., Zagorodnyuk, I.V., Belyanin, A.N., and Lyapunova, E.A., Nucleolus-forming regions and B chromosomes of wood mice (Mammalia, Rodentia, Apodemus), Genetika, 1995, vol. 31, no. 2, pp. 185–192.
Chelomina, G.N., Lesnye myshi. Molekulyarno-geneticheskie aspekty evolyutsii i sistematiki (Wood Mice. Molecular Genetic Aspects of Evolution and Systematics), Vladivostok: Dal’nauka, 2005.
Chelomina, G.N., Suzuki, H., Tsuchiya, K., Moriwaki, K., Lyapunova, E.A., and Vorontsov, N.N., Sequencing of the mtDNA cytochrome b gene and reconstruction of the maternal relationships of wood and field mice of the genus Apodemus (Muridae, Rodentia), Russ. J. Genet., 1998, vol. 34, no. 5, pp. 529–539.
Corbet, G.B. and Hill, J.E., A World List of Mammalian Species, London and Ithaca (New York): British Museum (Natural History) and Cornell University Press, 1980.
Ge, D., Feijó, A., Cheng, J., Lu, L., Liu, R., et al., Evolutionary history of field mice (Murinae: Apodemus), with emphasis on morphological variation among species in China and description of a new species, Zool. J. Linn. Soc., 2019, vol. 187, no. 2, pp. 518–534. https://doi.org/10.1093/zoolinnean/zlz032
Gornikov, D.V., Kartavtseva, I.V., Roslik, G.V., and Sheremet’eva, I.N., Variability of the molars of the East Asian mouse Apodemus peninsulae (Rodentia, Muridae), Reg. Probl., 2020, vol. 23, no. 2, pp. 23–31.
Gromov, I.M. and Baranova, G.I., Katalog mlekopitayushchikh SSSR (pliotsen-sovremennost’) (Catalog of Mammals of the USSR (Pliocene–Modern Times)), Leningrad: Nauka, 1981.
Gromov, I.M. and Erbaeva, M.A., Mlekopitayushchie fauny Rossii i sopredel’nykh stran. Zaitseobraznye i gryzuny (Mammals of the Fauna of Russia and Neighboring Countries. Lagomorphs and Rodents), St. Petersburg: Zool. Inst. Ross. Akad. Nauk, 1995.
Kageyama, M., Motokawa, M., and Hikida, T., Geographic variation in morphological traits of the large Japanese field mouse, Apodemus speciosus (Rodentia, Muridae), from the Izu Island group, Japan, Zool. Sci., 2009, vol. 26, no. 4, pp. 266–276. https://doi.org/10.2108/zsj.26.266
Kaneko, Y., Muridae, A Guide to the Mammals of Japan, Abe, H., Ed., Hatano: Tokai University Press, 2005, pp. 125–144.
Kartavtseva, I.V., Kariosistematika lesnykh i polevykh myshei (Rodentia, Muridae) (Karyosystematics of Wood and Field Mice (Rodentia, Muridae)), Vladivostok: Dal’nauka, Dal’nevost. Otd. Ross. Akad. Nauk, 2002.
Kawamura, Y., Quaternary rodent faunas in the Japanese Islands (part 1), Geol. Mineral J. Memoirs Fac. Sci., Kyoto Univ., 1988¸ vol. 53, no. 1, pp. 31–348.
Kawamura, Y., Quaternary rodent faunas in the Japanese Islands (part 2), Geol. Mineral J. Memoirs Fac. Sci., Kyoto Univ, 1989, vol. 54, nos. 1–2, pp. 1–235.
Kobayachi, T. and Hayata, I., Revision of the genus Apodemus in Hokkaido, Ann. Zool. Jpn., 1971, vol. 44, no. 4, pp. 236–240.
Korotky, A.M., Razjigaeva, N.G., Grebennikova, T.A., Ganzey, L.A., Mokhova, L.M., et al., Middle and Late-Holocene environments and vegetation history of Kunashir Island, Kurile Islands, northwestern Pacific, Holocene, 2000, vol. 10, no. 3, pp. 311–331.
Korotky, A.M., Volkov, V.G., Grebennikova, T.A., Razzhigaeva, N.G., Pushkar, V.S., et al., Far East, in Cenozoic Climate and Environmental Changes in Russia, Velichko, A.A., Ed., Book Ser.: Geological Society of America, 2005, vol. 38, Ch. 7, pp. 121–137. https://doi.org/10.1130/0-8137-2382-5.121
Kostenko, V.A., Patterns of biotopic distribution and distribution of rodents in the Far East of the USSR, in Nazemnye mlekopitayushchie Dal’nego Vostoka SSSR (Terrestrial Mammals of the Far East of the USSR), Vladivostok: Dal’nevost. Nauchn. Tsentr Akad. Nauk SSSR, 1976, pp. 3–62.
Kostenko, V.A., Order Rodentia, in Nazemnye mlekopitayushchie Dal’nego Vostoka SSSR (Terrestrial Mammals of the Far East of the USSR), Moscow: Nauka, 1984, pp. 118–215.
Kostenko, V.A., Gryzuny (Rodentia) Dal’nego Vostoka Rossii (Rodents (Rodentia) of the Russian Far East), Vladivostok: Dal’nauka, 2000.
Kostenko, V.A., Nesterenko, V.A., and Trukhin, A.M., Mlekopitayuschie Kuril’skogo arkhipelaga (Mammals of the Kuril Archipelago), GUP Dal’nauka, Dal’nevost. Otd. Ross. Akad. Nauk, 2004.
Larina, E.I. and Eremina, I.V., Catalog of the main variations of craniological characters in rodents, in Fenetika prirodnykh populyatsii (Phenetics of Natural Populations), Moscow: Nauka, 1988, pp. 8–53.
Maeda, Y., Matsuda, I., Nakada, M., Matsushima, Y., Matsumoto, E., and Sato, H., Holocene sea-level change along the Okhotsk Sea in Hokkaido, Jpn. Bull. Yamagata Univ., Nat. Sci., 1994, vol. 13, no. 3, pp. 205–229.
Musser, G.G. and Carleton, M.D., Superfamily Muroidea, in Mammal Species of the World: A Taxonomic and Geographic Reference, Wilson, D.E. and Reeder, D.M., Eds., Baltimore: Johns Hopkins Univ. Press, 2005, 3rd ed., pp. 894–1531.
Musser, G.G., Brothers, E.M., Carleton, M.D., and Hutterer, R., Taxonomy and distributional records of oriental and European Apodemus, with a review of the Apodemus-sylvaemus problem, Bonner Zool. Beitr., 1996, vol. 46, pp. 143–190.
Nakata, K., Saitoh, T., and Iwasa, M.A., Apodemus speciosus (Temminck, 1844), in The Wild Mammals of Japan, Ohdachi, S.D., Ishibashi, Y., Iwasa, M.A., and Saitoh, V.T., Eds., Kyoto: Shoukadoh Book Sellers, 2009, pp. 169–171.
Nazarova, L.B., Razigaeva, N.G., Diekmann, B., Ganzey, L.A., Grebennikova, T.A., et al., Holocene environmental changes in North-western Pacific (Kamchatka–Kuril Region), Transact., Jpn. Geomorphol. Union, 2020, vol. 41, no. 3, pp. 277–293. https://doi.org/10.13140/RG.2.2.31486.10562
Ohshima, K., The history of straits around the Japanese Islands in the Late-Quaternary, Quat. Res., 1990, vol. 29, no. 3, pp. 193–208.
Pavlenko, M.V., Vorontsov, N.N., Bekasova, T.S., and Frisman, L.V., On the species specificity of the electrophoretic spectra of blood proteins in wood mice Apodemus peninsulae and A. speciosus, in Voprosy izmenchivosti i zoogeografii mlekopitayushchikh (Problems of Variability and Zoogeography of Mammals), Vladivostok: Dal’nevost. Nauchn. Tsentr Akad. Nauk SSSR, 1984, pp. 30–42.
Razjigaeva, N.G., Korotky, A.M., Grebennikova, T.A., Ganzey, L.A., Mokhova, L.M., et al., Holocene climatic changes and environmental history of Iturup Island, Kurile Islands, northwestern Pacific, Holocene, 2002, vol. 12, no. 4, pp. 469–480.
Razjigaeva, N.G., Ganzey, L.A., Mokhova, L.M., and Ps-henichnikova, N.F., Meadow landscapes of the Southern Kuriles: origin, age and development, Geogr. Prir. Resur., 2011, no. 3, pp. 96–104.
Razjigaeva, N.G., Ganzey, L.A., Grebennikova, T.A., Belyanina, N.I., Mokhova, L.M., et al., Holocene climatic changes and vegetation development in the Kuril Islands, Quat. Int., 2013, vols. 290–291, no. 21, pp. 126–138. https://doi.org/10.1016/j.quaint.2012.06.034
Razjigaeva, N.G., Ganzey, L.A., Grebennikova, T.A., Belyanina, N.I., Mokhova, L.M., et al., Evolution of landscapes of the Kuril Islands in the Holocene, Izv. Ross. Akad. Nauk, Ser. Geogr., 2014, no. 3, pp. 43–50.
Rubtsov, N.B., Karamisheva, T.V., Bogdanov, A.S., Kartavtseva, I.V., Bochkarev, M.N., et al., Comparative analysis of dna homology in pericentric regions of chromosomes of wood mice from genera Apodemus and Sylvaemus, Russ. J. Genet., 2015, vol. 51, no. 12, pp. 1423–1432. https://doi.org/10.1134/S1022795415120091
Ruprecht, A.N., Taxonomic value of t3 meso-labial cone in M2 of Apodemus Kaup, 1829, Acta Theriol., 1978, vol. 23, no. 37, pp. 546–550.
Sakaguchi, Y., Warm and cold stages in the past 7600 years in Japan and their global correlation, Bull. Dept. Geogr. Univ. Tokyo, 1983, vol. 15, pp. 1–31.
Sakai, E., Geographical variation of molar sizes in two species of Muridae, Apodemus speciosus and Apodemus argenteus in Honshu, Japan, Aichi-Gakuin Dent Sci., 1998, vol. 11, pp. 1–13.
Sakai, E. and Miyao T., Studies on the regional variation of the molars of the small mammals in the Japanese Islands. II. On the molar sizes of the large Japanese field mouse, Apodemus speciosus in Tsushima and Iki, J. Growth, 1980, vol. 19, pp. 1–14.
Sakai, E. and Miyao T., Studies on the regional variation of the molars of the small mammals in the Japanese Islands. III. On the molar sizes of the large Japanese field mouse, Apodemus speciosus in Sado Island and Island of Izu-Oshima, J. Growth, 1980a, vol. 19, pp. 54–67.
Sakai, E. and Miyao, T., The geographical variation of the molar sizes of the large Japanese field mouse, Apodemus speciosus in Chita Peninsula, Aichi prefecture, J. Growth, 1988, vol. 27, pp. 27–45.
Sato, H., Kumano, S., Maeda, Y., Nakamura, T., and Matsud, I., The Holocene development of Kushu Lake on Rebun Island in Hokkaido, Jpn. J. Paleolimnol., 1998, vol. 20, pp. 57–69. https://doi.org/10.1023/A:1007962808715
Sheremetyeva, I.N., Kartavtseva, I.V., Pavlenko, M.V., Kostenko, V.A., Sheremetyev, I.S., et al., Morphological and genetic variability in small island populations of the striped field mouse Apodemus agrarius Pallas, 1771, Biol. Bull. (Moscow), 2017, vol. 44, no. 2, pp. 159–171. https://doi.org/10.1134/S1062359016050113
Shintaku, Y., Kageyama, M., and Motokawa, M., Morphological variation in external traits of the large Japanese field mouse, Apodemus speciosus, Mamm. Study, 2012, vol. 37, no. 2, pp. 113–126. https://doi.org/10.3106/041.037.0202
Suzuki, H., Yasuda, S., Sakaizumi, M., Wakana, S., Motokawa, M., and Tsuchiya, K., Differential geographic patterns of mitochondrial DNA variation in two sympatric species of Japanese wood mice, Apodemus speciosus and A. argenteus, Genes Genet. Syst., 2004, vol. 79, pp. 165–176. https://doi.org/10.1266/ggs.79.165
Suzuki, Y., Tomozawa, M., Koizumi, Y., Tsuchiya, K., and Suzuki, H., Estimating the molecular evolutionary rates of mitochondrial genes referring to quaternary ice age events within inferred population expansions and dispersals in Japanese Apodemus, BMC Evol. Biol., 2015, vol. 15, p. 187. https://doi.org/10.1186/s12862-015-0463-5
Takada, Y., Sakai, E., Uematsu, Y., and Tateishi, T., Morphological variation of large Japanese field mice, Apodemus speciosus on the Izu and Oki Islands, Mamm. Study, 2006, vol. 31, pp. 29–40.
Tokuda, M., A revised monograph of the Japanese Manchou–Korean Muridae, Biogeographica (Trans. Biogeogr. Soc. Jpn.) (Tokyo), 1941, vol. 4, no. 1, pp. 1–152.
Tsuchiya, K., Cytological and biochemical studies of Apodemus speciosus group in Japan, J. Mamm. Soc. Jpn., no. 6, pp. 67–87.
Velizhanin, A.G., Time of isolation of the mainland islands of the northern part of the Pacific Ocean, Dokl. Akad. Nauk SSSR, 1976, vol. 231, no. 1, pp. 205–207.
Vorontsov, N.N., Bekasova, T.S., Kral, B., Korobitsyna, K.V., and Ivanitskaya, E.Yu., On the species identity of Asian mice of the genus Apodemus (Rodentia, Muridae) of Siberia and the Far East, Zool. Zh., 1977, vol. 56, no. 3, pp. 437–450.
Zykov, S. and Izvarin, E., Variations in dental morphologies of yellow-necked mouse (Apodemus flavicollis Melchior, 1834) from Nizhneirginsky Grotto sediments (Middle Urals) in a phylogeographical context, Quat. Int., 2020, vol. 546, no. 30, pp. 152–159. https://doi.org/10.1016/j.quaint.2019.11.007
ACKNOWLEDGMENTS
The authors are grateful to V.A. Kostenko (1938–2021), who provided a collection of mouse crania for the study, as well as a reviewer, whose editorial revision significantly improved the presentation style of the text.
Funding
This work was carried out within a State Assignment of the Ministry of Science and Higher Education of the Russian Federation, topic no. 121031500274-4.
ETHICS APPROVAL AND CONSENT TO PARTICIPATEThis work does not contain any studies involving human and animal subjects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Barkhash
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kartavtseva, I.V., Gornikov, D.V. & Pavlenko, M.V. Morphological Peculiarities of Molars in the Large Japanese Field Mouse (Apodemus speciosus, Rodentia, Muridae) from Kunashir Island. Biol Bull Russ Acad Sci 50, 2119–2130 (2023). https://doi.org/10.1134/S1062359023080101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359023080101