Abstract
The structure of the first upper molar (M1) of the brown bear (Ursus arctos) and he polar bear (U. maritimus) was studied. Seven features of polymorphism were identified, and descriptions were given. The M1 in the mainland populations of U. arctos displays no high morphological variability, whereas the populations of U. arctos from Hokkaido are distinguished by a large number of rare morphotypes and a greater complexity. In general, the M1 in brown bears and polar bears is not a highly variable tooth character compared to the incisors and premolars. In evolutionary terms, variations in the M1 structure are rather weak in the genus Ursus. The brown bear is shown to be reliably distinguished from the polar bear based on the M1 structure. The polar bear teeth vary in a peculiar way that does not coincide with the traditional scenario of tooth change in hypercarnivorous predators. Ursus arctos has a large number of progressive features in the structure of M1, while M1 in U. maritimus combines both progressive and primitive traits, as well as some features characteristic of only this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
This publication is a continuation of the work on the study of the variability of teeth in brown bears (Ursus arctos (L. 1758)) and polar bears (U. maritimus (Phipps 1774)). Earlier, the results of a study on the variability of incisors and fourth premolars in these species were published (Gimranov and Kosintsev, 2017; Gimranov, 2018). In this work, attention will be paid to the upper first molar (M1). To date, the variability of qualitative dental traits in the order of carnivorous mammals Carnivora has been described in some representatives of the families Canidae (Szuma, 2007, 2011; Tedford et al., 2009), Mustelidae (Wolsan, 1988, 1989), and Felidae (Sotnikova and Nikolskiy, 2006). There are few works that describe the variability of dental characteristics in representatives of the family Ursidae (Rabeder, 1999; Baryshnikov, 2007). There are no statistical data on the structure of teeth of modern brown bears and polar bears. Of all the elements of the skeleton, teeth are most often preserved intact in the deposits from the Quaternary. Without studying the structural features of the teeth of modern taxa, it is impossible to interpret the changes in dental structures that occurred on a geological time scale. More details on the relevance of research and the choice of brown bears and polar bears in the study of the variability of the teeth of carnivorous mammals can be found in our previous work (Gimranov and Kosintsev, 2017). There is also a review of the sources on the problem of tooth variability in representatives of the genus Ursus. The objectives of this study include a description of the dental features of the upper first molar (M1), compilation of morphotypical schemes, calculation of the index of complexity of the tooth crown, and description of specific diagnostic features.
MATERIALS AND METHODS
The craniological collections of the Zoological Museum, Moscow State University (Moscow); the Zoological Institute, Russian Academy of Sciences (St. Petersburg); the Museum of the Institute of Economics and Life, Ural Branch, Russian Academy of Sciences; the Zoological Museum of Tomsk State University; the collection funds of the Institute of Economics and Life, Siberian Branch, Russian Academy of Sciences; the Hokkaido University Museum (Sapporo, Japan); the Hokkaido Museum (Sapporo, Japan); the Department of Archeology, University of Tokyo (Tokoro, Japan); and the Shiretoko Museum (Shari, Japan) were studied. The geographical distribution of the studied samples of bears is given in the work on the variability of incisors (Gimranov and Kosintsev, 2017).
The localities of the studied individuals (skulls) of the brown bear were combined into large samples. Mountainous Caucasus: Republic of Azerbaijan (n = 3), Georgia (n = 2), Republic of Abkhazia (n = 2), Republic of Dagestan (n = 3), Karachai-Cherkess Republic (n = 3), Republic of North Ossetia–Alania (n = 16), Chechen Republic (n = 2); the plain Caucasus: the Republic of Adygea (n = 55), Krasnodar krai (n = 8); center of the European part of Russia: Kostroma oblast (n = 4), Moscow oblast (n = 1), Smolensk oblast (n = 4), Tver oblast (n = 6), Yaroslavl oblast (n = 1); Northern European part of Russia: Arkhangelsk oblast (n = 5), Vologda oblast (n = 11), Leningrad oblast (n = 38), Murmansk oblast (n = 5), Novgorod oblast (n = 14), Pskov oblast (n = 8), Republic of Karelia (n = 3); Southern Urals: Republic of Bashkortostan (n = 10), Orenburg oblast (n = 1); Northern and middle Urals: Komi (n = 31); Sverdlovsk oblast (n = 18); Perm oblast (n = 3), Tyumen oblast (n = 3), Khanty-Mansi Autonomous Okrug (n = 3), Yamalo-Nenets Autonomous Okrug (n = 1), Northern Urals without exact locality (n = 11); Western Siberia: Novosibirsk oblast (n = 3), Tomsk oblast (n = 22), Tyumen oblast (n = 29); Altai: Republic of Altai (n = 19); Baikal region: Irkutsk oblast (n = 16); Central Siberia: Krasnoyarsk krai (n = 22); Eastern Siberia: Republic of Sakha (n = 9), Magadan oblast (n = 3), Chukotka Autonomous Okrug (n = 2); Primorye: Primorskii krai (n = 16); Kamchatka: Kamchatka krai (n = 115); islands of the Far East: Sakhalin oblast (n = 10); Japan: western part of Hokkaido (n = 40), central part of Hokkaido (n = 55), eastern part of Hokkaido (n = 56), no exact locality of Hokkaido (n = 27). The total sample by species is 719 individuals. The total number of the teeth studied is 1398 specimens.
The localities and number of the studied individuals (skulls) of the polar bear: the Yamal group includes specimens from the Arkhangelsk oblast (n = 27) and the Yamalo-Nenets Autonomous Okrug (n = 18); the Taimyr group consists of specimens collected from the northern part of Krasnoyarsk krai (n = 69); the “Siberia (northeast)” group includes specimens from the northern part of the Republic of Sakha (n = 26) and from the Chukotka Autonomous Okrug (n = 58); and the “Arctic Ocean” group consists of specimens that do not have exact localities (n = 32). The total sample by species is 230 individuals. The total number of the teeth studied is 458 specimens.
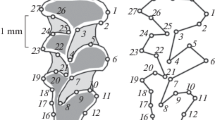
The characteristics of the selected morphotypes are given in Table 1. When describing M1 morphotypes, we studied the shape of the base of the buccal part of the tooth crown, the presence of para- and metastyles (Fig. 1a), the presence of proto- and post-metaconules (Fig. 1b), the presence of the entmetaconule (Fig. 1c), and the development of the lingual cingulum (Fig. 1d). When describing the structure of the M1 crown, most of the feature names were used according to Rabeder (Rabeder et al., 2009), with some modifications regarding entmetaconulus (entMec) according to Jiangzuo (Jiangzuo et al., 2019) and metaconulus (Mec) (personal communication of Qigao Jiangzuo), as well as the author’s additions concerning the protoconul (Prc) and postmetaconul (postMec). The complexity index was calculated based on the frequency of occurrence of simple and complex morphotypes (Rabeder, 1999). The value of the factor for each morphotype is given in Table 1. The samples were not separated by sex. In our previous works, we analyzed premolars in brown bear individuals with a known sex in order to assess the degree of manifestation of sexual dimorphism in the frequencies of morphotypes (Gimranov, 2018). It was shown that there are no statistically significant differences between males and females. The samples from the Hokkaido Island were grouped according to the groups identified on the basis of molecular genetic data (Hirata et al., 2013). Statistical assessment of differences in the proportions of morphotypes between the mainland and island populations of the brown bear, as well as between two species of bears, was carried out using the χ2 test. The χ2 values were calculated using the PAST package (Hammer et al., 2001). Skulls of brown bears and polar bears with different morphotypes of the right and left teeth were not found during this study.
M1 morphotypes of brown bears and polar bears (see Table 1 for the names of tooth elements): (a) buccal section (paracone–metacone), (b) lingual section (protoconule), (c) distal section (entmetaconule), and (d) lingual cingulum (in points).
RESULTS
The morphotypes of the buccal (paracone–metacone) section of M1. In all populations of U. arctos, the B3 morphotype is significantly dominant (Table 2). In the brown bears of the Caucasus, the European part of Russia, and the Urals, morphotypes B1 and B2 (2.9–7.9%) are found, which are absent in the individuals from the Asian part of Russia, except for one case in Altai. In the European part of Russia, morphotype C is rare in brown bears (1.6–6.0%), while in the Asian part its values are rather high (9.3–25.0%). However, in the population of U. arctos inhabiting Kamchatka, the C morphotype was encountered once (0.9%). In Hokkaido, all the brown bear teeth studied show the B3 morphotype, with the exception of one case in western Hokkaido. Therefore, no significant statistical differences in the frequencies of M1 buccal morphotypes were found between the samples of brown bears from the mainland and Hokkaido Island (Table 3). In U. maritimus, the dominant morphotypes are A3 (77.3%). Significant differences were found between brown bears and polar bears (Table 3). Note that, in the samples of U. maritimus from Yamal and northeastern Siberia, the A2 morphotype (13.3–15.4%) prevails over the A1 morphotype (2.2–7.7%). Polar bears from the Taimyr Peninsula have an inverse ratio of rare morphotypes: A1 (11.6%) prevails over A2 (4.3%). The differences between the polar bear samples are not significant.
The morphotypes of the lingual (protoconal) section of M1. In all representative samples of the brown bear, A1 is the dominant morphotype (76.3–95.7%). Rare morphotypes A2 and A3 (4.1 and 8.4%) do not have a pronounced geographical pattern in the frequency distribution (Table 4). Only in U. arctos, which lives in the north of the European part of Russia, was the A4 morphotype found (0.3%). The brown bear populations living on the island of Hokkaido do not show clear differences in the frequencies of the identified morphotypes. At the same time, island bears noticeably differ from mainland bears in the frequent presence of the A3 morphotype (15.6–29.7%) and the rare occurrence of the A2 and A4 morphotypes (0.7% each). Despite this, no significant statistical differences in the frequencies of the M1 lingual morphotypes between the Hokkaido brown bear and the mainland brown bear were found (Table 3). The morphotypic diversity in U. maritimus is lower than in U. arctos. Significant differences were found between the brown bears and the polar bears (Table 3). In the polar bear, only the A1 and A2 morphotypes were found, of which the A2 morphotype dominates significantly (73.8–86.7%). The frequency of occurrence of the A2 morphotype ranges from 13.3 to 26.2% (Table 4). The samples of U. maritimus are quite similar to each other, and no significant statistical differences were found between them.
The morphotypes of the distal (entmetaconule) section of M1. In the brown bear, a fairly close ratio of the frequencies of morphotypes of the distal section is observed; the A2 morphotype is dominant in almost all samples (Table 4). An exception is the sample of bears from the Urals, where the A1 morphotype dominates (74.6%). The brown bears from the island of Hokkaido differ from mainland brown bears in the almost complete dominance of the A2 morphotype (98.9%). The A1 morphotype was found in only two cases. Therefore, according to the structure of the distal part of M1, the populations of U. arctos inhabiting the island of Hokkaido differ significantly from other populations (Table 3). In U. maritimus, the A2 morphotype was not found (Table 4). Significant statistical differences were obtained between the brown bear and the polar bear in terms of the frequency of occurrence of the discussed morphotypes (Table 3).
The degree of development of the lingual cingulum of M1. In the mainland populations of the brown bear, no geographical pattern was found in the structure of the lingual cingulum (Table 4). The cingulums with a development of two and three points dominate: in some regions, teeth with a poorly developed cingulum are more common (two points), in other regions there are more individuals with a moderately developed cingulum (three points). Cingulums with a development of one and four points are rare. A well-developed cingulum of five points was noted in three cases of U. arctos only in the Urals. In general, the island populations of the brown bear have the most commonly encountered, most poorly developed lingual cingulum (one point). A similar structure of the cingulum is dominant in the populations of the central part of Hokkaido (39.2%). The bears from the eastern part of the island have specific features: only they have a developed form of the lingual cingulum, corresponding to four points. Significant statistical differences were obtained between the samples of brown bears from the mainland and from the island of Hokkaido at the level of p = 0.0005 (Table 3). Significant statistical differences were found between the samples of brown bears and polar bears (Table 3). In the polar bear, the most poorly developed lingual cingulum (one point) is the most common. It should be noted that the polar bears of Yamal have a high frequency of occurrence (42.2%) of a poorly developed lingual cingulum (two points), while in other samples this figure is lower (23.8–28.1%). The lingual cingulum of an average degree of development (three points) was noted only in the populations of U. maritimus living in northeastern Siberia and in populations without a precisely established locality. The differences between the polar bear samples are not significant.
Complexity of teeth. As the description of morphotypes, the characteristics of their complexity are given separately for each part of M1 (Table 5, Fig. 2). The buccal and lingual sections of M1 in mainland brown bears appear approximately equally complicated in all the samples studied. We can note very low rates of complexity in the Altai group, which may be due to the small sample size. The most complicated is the buccal section of M1 in the populations of brown bears in Primorye and Western Siberia, the lingual section in the populations of bears in Western Siberia and the Northern European part of Russia, and the distal section in bears inhabiting Altai and Western Siberia. The lingual cingulum is the most complicated in brown bears in central Russia and Kamchatka. The structure of the M1 crown in mainland brown bears is rather similar. However, the populations of the brown bear from the island of Hokkaido differ significantly from the mainland animals by an increase in the complexity of all parts of the tooth. Inside the island, bears living in the western and eastern parts of the island have the most complicated M1 (Table 5, Fig. 2). In the polar bear, there are no significant differences in the structure of M1 between the samples either. Note that this species has the highest degree of complexity of the lingual section. The buccal section of M1 is generally less complicated than in brown bears. The complexity of the distal section in polar bears is comparable to that of brown bears from the island of Hokkaido. U. maritimus has the least degree of development (complexity) of the M1 lingual cingulum among all samples studied (Table 5, Fig. 2).
The level of complexity of M1 in the samples of the brown bear and the polar bear. Brown bear: (1) Mountainous Caucasus, (2) Plain Caucasus, (3) Northern European part of Russia, (4) Central European part of Russia, (5) Northern and middle Urals, (6) Western Siberia, (7) Altai, (8) Kamchatka, (9) Primorye , (10) Hokkaido Island (west), (11) Hokkaido Island (center), (12) Hokkaido Island (east), (13) Hokkaido Island (no exact locality). Polar bear: (14) Yamal, (15) Taimyr, (16) Siberia (northeast), (17) Arctic Ocean (no exact locality).
DISCUSSION
The brown bear. Previously, we managed to establish some geographical pattern in the variability of the structure of the crowns of incisors and fourth premolars (Р4 and р4) in U. arctos (Gimranov and Kosintsev, 2017; Gimranov, 2018). The structure of the M1 crown in mainland brown bears is quite similar. However, the populations of the brown bear from the island of Hokkaido differ significantly from the mainland animals by an increase in the complexity of all parts of the tooth. It can be concluded that M1 in the brown bear is a slightly variable tooth compared to incisors and premolars (Gimranov and Kosintsev, 2017; Gimranov, 2018). There are more variable features on M1 than on incisors and premolars, so there is an impression of its (M1) sufficient variability. However, each individual trait exhibits weak variability in the samples studied. The brown bear can be reliably differentiated from the polar bear based on the structure of M1. Differentiating features are the absence of a protoconulus, the presence of an entmetaconulus, and a developed lingual cingulum. The brown bear can be reliably distinguished from the polar bear by the structure of the buccal part of M1, namely, by the absence of a deep notch between the paracone and the metacone.
The polar bear. In the polar bear, there are no significant differences in the structure of M1 between the samples. It can be concluded that M1 in the polar bear, as well as in the brown bear, is a slightly variable tooth compared to incisors and premolars (Gimranov and Kosintsev, 2017; Gimranov, 2018). The polar bear can be reliably differentiated from the brown bear based on the structure of M1. The differentiating features are the presence of a protoconulus, the absence of an entmetaconulus, and a very weak (in this case, reduction) lingual cingulum. Furthermore, the polar bear can be reliably distinguished from the brown bear by the structure of the buccal part of M1, namely, by the deep notch between the paracone and the metacone.
The polar bear separated from the common branch with brown bears relatively recently (Hailer et al., 2012; Bidon et al., 2014), but in a short time acquired a large number of unique adaptations, including the reduction of the dental system (Baryshnikov, 2007; Slater et al., 2010). The rapid adaptation of the teeth of U. maritimus during the transition to a completely predatory lifestyle is also evidenced by previously obtained data (Gimranov, 2018): P4 acquires a piercing predatory appearance with one or two apexes (loss of the protocone as the main pressing element, increase in the cutting function of the tooth). As in P4 (Gimranov, 2018), in M1 the styles are located in one line with the main tubercles (paracone and metacone). It can be assumed that styles lengthen the main blade of the tooth and are involved in the process of cutting food. However, this conclusion is inconsistent with the results obtained. In U. maritimus, a high proportion of rare morphotypes of the buccal part of M1 with a reduction of one of the stylids (18%) was noted. The development of conules on P4 and M1 has a functionally opposite meaning to styles. These bumps in bears are involved in the process of crushing food. The protoconulus is often present in polar bears (80%).
In the transition to the obligate carnivore, it can be expected that the polar bear, like other hypercarnivorous predators (Felidae), will consolidate and develop such elements as stylids and will get rid of such elements as horses on P4, M1, or M2. But we are seeing a different situation. The described scenario is to some extent true for P4, with the proviso that the tooth of U. maritimus is unique in its piercing rather than cutting function. For M1, the formulated scenario is not suitable, because this tooth in the polar bear does not develop cutting functions. There is tooth reduction due to the loss of styles, entmetaconule, and reduction of the lingual blade. In this case, the tooth increases the pressing function due to the protoconulus. The reduction of teeth in the polar bear occurs as a result of the loss of their main functions. Researchers believe that this is due to the consumption of soft food, which is based on pinnipeds (Slater et al., 2010). Therefore, an interesting feature of these animals is that, despite the purely predatory feeding strategy, their teeth are modified according to a peculiar scenario. The incisors are more adapted to predation (Gimranov and Kosintsev, 2017), as are the canines, which are greatly enlarged (Baryshnikov, 2007), the premolars acquire a piercing appearance (Gimranov, 2018), and M1 loses additional elements associated with cutting food. Why M1 in the polar bear has a protoconulus, which is not characteristic of the brown bear, remains unclear.
An interesting feature of the structure of M1 in the studied species of bears is that, unlike the fourth premolars, in which the proportion of asymmetric morphotypes is quite large (Gimranov, 2018), the first molar does not have asymmetric structural variants. Note that five specimens of U. maritimus obtained from the Wrangel Island and one individual from Komsomolskaya Pravda Island lacked the studied teeth on both sides of the jaw. We have not recorded obvious traces of intravital loss of M1. In U. arctos, such pathologies were not found.
Evolutionary changes of M1 in the genus Ursus. One of the most ancient representatives of the genus Ursus is U. minimus (Devèze de Chabriol et Bouillet, 1827), which appeared at the turn of the Miocene and Pliocene (Baryshnikov, 2007; Wagner, 2010). Some finds of small Pliocene bears from Eurasia and North America are sometimes assigned to a separate genus Protarctos (Kretzoi 1945), which includes four species (Wang et al., 2017). During the Pliocene, the diversity of the genus Ursus increases: U. thibetanus (G. Cuvier 1823) and U. americanus (Pallas 1870) appear; U. etruscus (G. Cuvier 1823) appears in the Late Pliocene (Baryshnikov, 2007; Krause et al., 2008; Bon and Elalouf, 2010; Wagner, 2010), all four of the above species belong to the subgenus Euarctos. U. deningeri (von Reichenau 1904) and U. dolinenesis (Garcia et Arsuaga 2001) appeared in the Early Pleistocene (Kurtén, 1968; García and Arsuaga, 2001; Wagner and Čermák, 2012), which may be the earliest in the cave bear lineage (subgenus Spelearktos). At the same time, U. etruscus or U. dolinensis may be the ancestors of the line of brown bears (subgenus Ursus). At the moment, the subgenus Spelearctos includes six species (Baryshnikov and Puzachenko, 2019; Barlow et al., 2020), which can be conditionally divided into four large groups: deningeroid bears (U. deningeri, U. deningeroides (Mottl 1964)), the Caucasian cave bear (U. kudarensis (Baryshnikov 1985)), the Ural cave bear (U. kanivetz (Vereshchagin 1973)), the European cave bear (U. spelaeus (Rosenmüller 1794)), and two species of small cave bears (U. rossicus (Borissiak 1930) and U. savini Andrew 1922)).
M1 in U. minimus demonstrates the absence or weak expression of the parastyle and metastyle, the absence of the protoconule and postmetaconule, a developed lingual cingulum, an almost undeveloped entmetaconule, and a convex shape of the buccal margin of the crown base (Baryshnikov, 2007; Wagner et al., 2011). Similar features in the structure of M1 are also characteristic of representatives of the genus Protarctos (Wang et al., 2017). The above described states of characters can be considered basal or primitive for the genus Ursus. The tooth structure of U. thibetanus and U. americanus is very similar to that of U. minimus (Baryshnikov, 2007; Wagner et al., 2011). The Himalayan bear often has a parastyle and a metastyle, a convex shape of the base of the buccal margin of the crown, and the absence of a protoconulus and postmetaconulus (unpublished data of the author). Furthermore, according to our observations, the modern Himalayan bear often lacks a mesocone and the lingual cingulum is often greatly reduced. The baribalus is characterized by the presence of a parastyle and metastyle, a slightly convex or even shape of the base of the buccal margin of the M1 crown, the absence of a protoconulus and a postmetaconule, and a very weak development of the entmetaconule and lingual cingulum (unpublished data of the author).
The structure of teeth in U. etruscus and U. dolinensis is similar to each other and to U. arctos. U. etruscus has underdeveloped styles on M1 (usually one of two) and has an entmetaconulus in about half of the cases, a well-developed lingual cingulum, a curved buccal margin of the crown base, and the presence of a protocone, mesocone, and metaconulus without additional elements (Mazza and Rustioni, 1992; Baryshnikov, 2007; Petrucci and Sardella, 2009; Wagner et al., 2011; Koufos et al., 2018; Medin et al., 2017; Jiangzuo et al., 2017; Medin et al., 2019). M1 of U. dolinensis is similar to M1 of U. etruscus in many of the above characters, and, unlike the latter, both styles are developed in U. dolinensis (Garcia and Arsuaga, 2001).
On the whole, the structure of teeth in cave bears is rather peculiar and differs from other members of the genus Ursus (Baryshnikov, 2007). Early deningeroid cave bears already had a number of features that distinguished them from the Etruscan bear. We are talking about characters such as developed para- and metastyles, a developed platform of the entmetaconule, the presence of a postmetaconule, and the average degree of development of the lingual cingulum (Baryshnikov, 2007; Rabeder et al., 2009; Wagner and Cermak, 2012). Late Pleistocene cave bears (U. spelaeus sensu lato) have well-developed para- and metastyles, a well-developed metaconule platform, a post-metaconule, a well-developed lingual cingulum, and sometimes a bifurcated mesocone (Grandal d’Anglade, 1993; Baryshnikov, 1998, 2007; Rabeder, 1999). The lesser or Russian cave bear U. rossicus differs from other cave bears in having more elaborate tooth crowns. Despite this, the structure of M1 in U. rossicus is very similar to that of other Late Pleistocene cave bears (Borisyak, 1932; Baryshnikov, 2007).
The structure of M1 of the Pleistocene U. arctos, which is sometimes distinguished as a separate subspecies of U. a. priscus is very similar to the modern brown bear (Capasso Barbato et al., 1990; Rabeder et al., 2009; Baryshnikov, 2010; Marciszak et al., 2015, 2019).
In evolutionary terms, M1 in representatives of the genus Ursus changes little compared to premolars (Gimranov, 2018). The main elements of the tooth that vary on an evolutionary time scale in members of the genus Ursus are the styles, postmetaconule, entmetaconule, and lingual cingulum. The absence or weak development of styles as well as the entmetaconule is a primitive state. Also archaic is the well-developed lingual cingulum. The presence of well-developed styles and an entmetaconnula is a progressive state of the characters, as is a reduced cingulum. Apparently, the presence of a postmetaconulus (characteristic of cave bears) and a protoconulus (characteristic of a polar bear) can apparently be considered a progressive state. U. arctos has a lot of progressive characteristics in the M1 structure, while in U. maritimus M1 combines both progressive and primitive features, along with some features unique to this species. This can be explained by the transition from an omnivorous or even herbivorous type of food (brown bear) to obligate predation.
CONCLUSIONS
For the first upper molar M1 of brown bears and polar bears, seven features with variability were identified and described: the shape of the base of the buccal edge of the crown, the presence of para- and metastyles, the presence of the entmetaconulus, postmetaconulus, and protoconulus, and the development of the lingual cingulum. The structure of M1 in the populations of U. arctos inhabiting the mainland is very similar. The island populations of U. arctos have a greater number of rare morphotypes and, as a result, an increase in the value of the index of complexity. A combination of primitive (developed entmetaconule) and progressive (presence of postmetaconule and reduced lingual cingulum) characteristics distinguishes U. arctos from Hokkaido Island from the mainland U. arctos. In general, M1 in brown bears and polar bears is a slightly variable tooth compared to incisors and premolars.
The brown bear can be reliably differentiated from the polar bear based on the structure of M1. Characteristic features of U. arctos are the absence of a protoconnule, the presence of an entmetaconnula platform, and a developed lingual cingulum. Characteristic features of U. maritimus are a deep incision of the buccal margin of the crown base between the paracone and the metacone, the presence of a protoconule, the absence of an entmetaconule, and a strongly reduced lingual cingulum. Although U. maritimus is an almost entirely obligate carnivore, the scenario of tooth change in this species does not match similar scenarios for other hypercarnivorous predators. The teeth of a polar bear are modified in a peculiar way: the incisors become more adapted to capture prey, like the fangs, which are greatly enlarged, the premolars acquire a piercing appearance, and M1 loses additional elements associated with cutting food, while developing pressing elements.
In evolutionary terms, M1 in representatives of the genus Ursus changes little. The main elements of the tooth that vary on an evolutionary time scale in members of the genus Ursus are the styles, postmetaconule, entmetaconule, and lingual cingulum. U. arctos has a lot of progressive characteristics in the M1 structure, while M1 of U. maritimus combines both progressive and primitive features, along with some features unique to this species. This can be explained by the “return” from the omnivorous or herbivorous type of food (brown bear) to active predation, which bears “abandoned” in the process of evolution.
REFERENCES
Barlow, A., Paijmans Johanna, L.A., Alberti, F., Gasparyan, B., Bar-Oz, G., et al., Middle Pleistocene cave bear genome calibrates the evolutionary history of Palearctic bears, Curr. Biol., 2020 (in press). https://ssrn.com/abstract= 3523359.https://doi.org/10.2139/ssrn.3523359
Baryshnikov, G., Cave bears from the Paleolithic of the Greater Caucasus, in Quaternary Paleozoology in the Northern Hemisphere, Saunders, J.J., Styles, B.W., and Baryshnikov, G.F., Eds., Springfield: Illinois State Museum Scientific Papers, 1998, pp. 69–118.
Baryshnikov, G.F., Fauna Rossii i sopredel’nykh stran (Fauna of Russia and Adjacent Countries), vol. 1: Mlekopitayushchie (Mammals), no. 5: Medvezh’i (Carnivora, Ursidae) (Bears (Carnivora, Ursidae)), St. Petersburg: Nauka, 2007.
Baryshnikov, G.F., Late Pleistocene brown bear (Ursus arctos) from the Caucasus, Russ. J. Theriol., 2010, vol. 9, no. 1, pp. 9–17.
Baryshnikov, G.F. and Puzachenko, A.Y., Morphometry of upper cheek teeth of cave bears (Carnivora, Ursidae), Boreas, 2019, vol. 48, pp. 581–604.
Bidon, T., Janke, A., Fain, S.R., Eiken, H.G., Hagen, S.B., et al., Brown and polar bear Y chromosomes reveal extensive male-biased gene flow within brother lineages, Mol. Biol. Evol., 2014, vol. 31, pp. 1353–1363.
Bon, C. and Elalouf, J.M., Cave bear genomics in the Paleolithic painted cave of Chauvet-Pont d’Arc, in Evolutionary Biology—Concepts, Molecular and Morphological Evolution, Pontarotti, P., Ed., Berlin: Springer, 2010, pp. 343–356.
Borissiak, A.A., A new race of the cave bear from the Quaternary deposits of the North Caucasus, Tr. Paleozool. Inst., 1932, vol. 1, pp. 137–201.
Capasso, BarbatoL., Minieri, M.R., Petronio, C., and Vigna Taglianti, A., Strutture dentarie di Ursus arctos e di Ursus spelaeus della grotta di Monte Cucco (Sigillo, Perugia, Italia), Boll. Soc. Paleontol. Ital., 1990, vol. 29, no. 3, pp. 321–333.
Garcia, N. and Arsuaga, J.L., Ursus dolinensis: a new species of Early Pleistocene ursid from Trinchera Dolina, Atapuerca (Spain), Earth Planet. Sci., 2001, vol. 332, pp. 717–725.
Gimranov, D.O., Morphotypic characteristics of fourth premolars of brown (Ursus arctos) and polar (Ursus maritimus) bears (Carnivora, Ursidae), Zool. Zh., 2018, vol. 97, no. 2, pp. 205–223.
Gimranov, D.O. and Kosintsev, P.A., Morphotypic variability of incisors of brown (Ursus arctos) and polar (Ursus maritimus) bears (Carnivora, Ursidae), Zool. Zh., 2017, vol. 96, no. 5, pp. 547–562.
Grandal d’Anglade, A., El Oso de las cavernas en Galicia: El yacimiento de Cova Eiros, Nova Terra, 1993, vol. 8, pp. 1–285.
Hailer, F., Kutschera, V.E., Hallström, B.M., Klassert, D., Fain, S.R., et al., Nuclear genomic sequences reveal that polar bears are an old and distinct bear lineage, Science, 2012, vol. 336, pp. 344–347.
Hammer, O., Harper, D.A.T., and Ryan, P.D., Paleontological statistics software package for education and data analysis, Palaeontol. Electron., 2001, vol. 4, no. 1, pp. 1–9.
Hirata, D., Mano, T., Abramov, A.V., Baryshnikov, G.F., Kosintsev, P.A., et al., Molecular phylogeography of the brown bear (Ursus arctos) in Northeastern Asia based on analyses of complete mitochondrial DNA sequences, Mol. Biol. Evol., 2013, vol. 30, pp. 1644–1652.
Jiangzuo, Q., Liu, J., Wang, Y., Jin, C., Liu, S., et al., New materials of Ursus etruscus from Jinyuan cave of Luotuo Hill, Dalian and a brief review of Ursus cf. etruscus in China, Quat. Sci., 2017, vol. 37, no. 4, pp. 828–837.
Jiangzuo, Q., Liu, J., and Chen, J., Morphological homology, evolution, and proposed nomenclature for bear dentition, Acta Palaeontol. Pol., 2019, vol. 64, no. 4, pp. 693–710.
Koufos, G.D., Konidaris, G.E., and Harvati, K., Revisiting Ursus etruscus (Carnivora, Mammalia) from the Early Pleistocene of Greece with description of new material, Quat. Int., 2018, vol. 497, pp. 222–239.
Krause, J., Unger, T., Nocon, A., Malaspinas, A.-S., and Kolokotronis, S.-O., Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene–Pliocene boundary, BMC Evol. Biol., 2008, vol. 8, pp. 1–12.
Kurtén, B., Pleistocene Mammals of Europe, London: Weidenfeld and Nicolson, 1968.
Marciszak, A., Stefaniak, K., Mackiewicz, P., and Ridush, B., Ursus arctos L., 1758 from Bukovynka Cave (W Ukraine) in an overview on the fossil brown bears size variability based on cranial material, Quat. Int., 2015, vol. 357, pp. 136–148.
Marciszak, A., Schouwenburg, C., Lipecki, G., Talamo, S., Shpansky, A., et al., Steppe brown bear Ursus arctos “priscus” from the Late Pleistocene of Europe, Quat. Int., 2019, vol. 534, pp. 158–170.
Mazza, P. and Rustioni, M., Morphometric revision of the Eurasian species Ursus etruscus Cuvier, Palaeontogr. Ital., 1992, vol. 79, pp. 101–146.
Medin, T., Martínez-Navarro, B., Rivals, F., Madurell-Malapeira, J., Ros-Montoya, S., et al., Late Villafranchian Ursus etruscus and other large carnivorans from the Orce sites (Guadix-Baza basin, Andalusia, southern Spain): taxonomy, biochronology, paleobiology, and ecogeographical context, Quat. Int. B, 2017, vol. 431, pp. 20–41.
Medin, T., Martínez-Navarro, B., Madurell-Malapeira, J., Figueirido, B., Kopaliani, G., et al., The bears from Dmanisi and the first dispersal of early Homo out of Africa, Sci. Rep., 2019, vol. 9, no. 17752. https://doi.org/10.1038/s41598-019-54138-6
Petrucci, M. and Sardella, R., Ursus etruscus Cuvier, 1823 from the Early Pleistocene of Monte Argentario (Southern Tuscany, Central Italy), Boll. Soc. Paleontol. Ital., 2009, vol. 48, no. 2, pp. 89–94.
Rabeder, G., Die evolution des Höhlenbärengebisses, Mitteilungen der kommission für quartärforschung der österreichischen akademie der Wissenschaften, 1999, vol. 11, pp. 1–102.
Rabeder, G., Pacher, M., and Withalm, G., Early Pleistocene bear remains from Deutsch-Altenburg (Lower Austria), Mitteilungen der kommission für quartärforschung der österreichischen akademie der Wissenschaften, 2009, vol. 17, pp. 1–135.
Slater, G.J., Figueirido, B., Louis, L., Yang, P., and Van Valkenburgh, B., Biomechanical consequences of rapid evolution in the polar bear lineage, PLoS One, 2010, vol. 5, no. 11, art. ID e13870.
Sotnikova, M.V. and Nikolskiy, P., Systematic position of the cave lion Panthera spelaea (Goldfuss) based on cranial and dental characters, Quat. Int., 2006, no. 3, pp. 218–228.
Szuma, E., Geography of dental polymorphism in red fox Vulpes vulpes and its evolutionary implications, Biol. J. Linn. Soc., 2007, vol. 90, pp. 61–84.
Szuma, E., Ecological and evolutionary determinants of dental polymorphism in the arctic fox Vulpes (Alopex) lagopus, Ann. Zool. Fenn., 2011, vol. 48, pp. 191–213.
Tedford, R.H., Wang, X., and Taylor, B.E., Phylogenetic systematics of the North American fossil Caninae (Carnivora: Canidae), Bull. Am. Mus. Nat. Hist., 2009, vol. 325, pp. 1–218.
Wagner, J., Pliocene to early middle Pleistocene ursine bears in Europe: a taxonomic overview, J. Natl. Mus. Nat. Hist. Ser., 2010, vol. 179, pp. 197–215.
Wagner, J. and Čermák, S., Revision of the early Middle Pleistocene bears (Ursidae, Mammalia) of Central Europe, with special respect to possible co-occurrence of spelaeoid and arctoid lineages, Bull. Geosci., 2012, vol. 87, pp. 461–496.
Wagner, J., Čermák, S., and Horáček, I., The presence of Ursus ex gr. minimus-thibetanus in the Late Villányian and its position among the Pliocene and Pleistocene black bears in Europe, Quaternaire. Hors-série, 2011, vol. 4, pp. 39–58.
Wang, X., Rybczynski, N., Harington, C., White, S., and Tedford, R., A basal ursine bear (Protarctos abstrusus) from the Pliocene High Arctic reveals Eurasian affinities and a diet rich in fermentable sugars, Sci. Rep., 2017, vol. 7, no. 1. https://doi.org/10.1038/s41598-017-17657-8
Wolsan, M., Dental polymorphism in the genus Martes (Carnivora: Mustelidae) and its evolutionary significance, Acta Theriol., 1989, vol. 34, pp. 545–593.
Wolsan, M., Morphological variations of the ferst upper molar in the genus Martes (Carnivora, Mustelidae), Mem. Mus. Natl. Hist. Nat. (France), Ser. C: Sci. Terre, 1988, vol. 53, pp. 241–254.
ACKNOWLEDGMENTS
The author thanks the staff of the laboratory of theriology of the Zoological Museum of the Moscow State University; the staff of the laboratory of theriology of the Zoological Institute of the Russian Academy of Sciences; the head of the museum of the Institute of Economics and Life, Ural Branch, Russian Academy of Sciences, N.G. Erokhin; an employee of the Laboratory of Phylogeny and Faunogenesis, Institute of Economics and Life, Siberian Branch, Russian Academy of Sciences, D.E. Taranenko; and Director of the Zoological Museum, Tomsk State University, S.S. Moskvitin for the opportunity to work with the collection material. Thanks are due to T. Amano, R. Masuda, Y. Amaike, and T. Akiyama (Hokkaido University) for their help in organizing and conducting research in Japan (Hokkaido Island). The authors are also grateful to the curators of the Japanese museum collections: F. Takaya and M. Kato (Botanic Garden, Field Science Center for Northern Biosphere, Hokkaido University), K. Omote (Hokkaido Museum), D. Natsuki (Graduate School of Humanities and Sociology, University of Tokyo), T. Murakami (Shiretoko Museum), and Director of the Shiretoko Museum Mr. M. Yamanaka.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 19-04-00111-a) and by a grant from the President of the Russian Federation for young Russian scientists (MK-1130.2019.4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest. This article does not contain any studies involving animals or human participants performed by the author.
Additional information
Translated by N. Smolina
Rights and permissions
About this article
Cite this article
Gimranov, D.O. Morphotypic Characteristics of the First Molar (M1) of the Brown Bear (Ursus arctos) and the Polar Bear (Ursus maritimus) (Carnivora, Ursidae). Biol Bull Russ Acad Sci 49, 961–974 (2022). https://doi.org/10.1134/S1062359022070056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022070056