Abstract
A survey of the expedition on the R/V Akademik Mstislav Keldysh in the waters south of the southernmost tip of Argentina, in the Drake Passage, as well as in the vicinities of the Antarctic Peninsula, the Scotia Sea, and the northern part of the Weddell Sea, was undertaken during two trips: January 16–February 6 (hereafter, January) and February 8–March 3 (hereafter, February), 2020. We propose a new method for analyzing the results of ecological studies and, based on this method, present new information on the ecology and spatial distribution of marine mammals and seabirds of the Antarctic. A number of marine mammal and seabird species showed similar ecological fingerprints, indicating their similar spatial distributions and the same relations to the environmental conditions regardless of their systematic position. The fin whale, the humpback whale, the snow petrel, the Adélie penguin, the Antarctic petrel, and the southern fulmar showed similar specific patterns of ecological fingerprints in January, showing the association of these species to the sea areas with icebergs and/or cut ice. In February, the allocation of the whale species to icy areas weakened what was mirrored in their ecological fingerprints, while all the above-mentioned bird species still preserved this association. The ecological fingerprints clearly circumscribe the broadness of the abiotic ecological niche occupied by individual species or species clusters. Ecological fingerprints are capable of showing changes in species distribution areas in certain periods (the southern royal albatross), in the strategy of the area usage patterns (the Antarctic fur seal), as well as in other ecological features, including those not yet considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In December 2019, on behalf of the Government of the Russian Federation, the Program for Comprehensive Field Research of the Ecosystems of the Atlantic Sector of the Southern Ocean was launched. More than ten institutes participate in the Program, the head of which is the Shirshov Institute of Oceanology, Russian Academy of Sciences (Morozov et al., 2019, 2020). Within the framework of this Program, there is a Project of the Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, “Resource Research of the Ecosystem of the Southern Ocean (Atlantic Sector of the Antarctic”). Thus, the task of the project is to study the biota of the Antarctic in the current conditions of climate change. The scientific work of the R/V Akademik Mstislav Keldysh in the waters south of the southern tip of Argentina, the Drake Passage, the vicinity of the Antarctic Peninsula, the Scotia Sea, and the northern part of the Weddell Sea had two stages: from January 16 to February 6 and from February 8 to March 3, 2020. The period of February 6–8 was devoted to a short visit at the port of Ushuaia, Argentina. The total length of the route was approximately 6000 km at each stage; in total, the vessel covered approximately 12 000 km in Antarctic waters.
There are many studies on the ecology of Antarctic marine mammals, mainly cetaceans (Joiris and Dochy, 2013; Friedlaender at al., 2006; Kasamatsu et al., 2000; Mondreti et al., 2020; etc.). Also, many works have been devoted to the ecology of birds in Antarctica (for example, Lyver et al., 2011; Ainley et al., 2010; Santora et al., 2009; Mondreti et al., 2020; Serratosa et al., 2020; etc.). However, individual ecological parameters of the environment are usually considered separately. In this study, we would like to rise to a higher level (meta-level) of consideration of the environmental factors and make an attempt to generalize the assessment of their impact in order to concentrate the influence of environmental factors on a small number of meta-parameters (in our case, two meta-parameters), to try to understand better the adaptations, ecological opportunities, and in some cases, ecological preferences of certain species of marine mammals and birds.
Previously, we used factor analysis to present the preliminary results of studies of the influence of various environmental factors on the occurrence of marine mammals and seabirds (Kharitonov et al., 2021, 2021a, 2021b). In this report, we have reached the next level of generalization of the results of this analysis. The “ecological fingerprint method” we propose here appears to be a novel approach. It can be useful to minimize a large amount of data into 1–2–3 numerical indicators, for example, the main components (Korosov, 1996). However, in addition to the numerical characteristic indicators, we also would like to have a visual information characteristic, something like a visual index, i.e., a type of graph (diagram) that would allow one quickly to get an idea of the ecological features of biological objects at a given time and site, including the spatial distribution of these objects. The method of ecological fingerprints allows to see and evaluate the totality of environmental factors associated with certain groups of animals and time periods. The purpose of this work is to propose a new method for analyzing ecological results and, using this method as an example, to present new information about the ecology of marine mammals and birds in Antarctica.
MATERIALS AND METHODS
Field Observations
Observations and records on the occurrence of marine mammals and birds were carried out from the vessel along the routes: (1) January 16–February 6, 2020, entrance to the Beagle Channel–Drake Passage–vicinity of the tip of the Antarctic Peninsula–South Shetland Islands–South Orkney Islands–back to the Antarctic Peninsula–Drake Passage–Beagle Passage, further in the article this period will be called “January”; (2) February 8–March 3, 2020, Beagle Strait–Drake Passage–tip of the Antarctic Peninsula–Powell Basin–tip of the Antarctic Peninsula– Drake Passage–Beagle Channel–Ushuaia, hereinafter this period will be called “February” (Fig. 1).
The observations were carried out during daylight hours, mainly from the direction-finding deck of the ship, by two observers who stood on the starboard and port sides of the ship. The duration of observations was associated with the change of watches on the ship and for each pair of observers was four hours; however, in the morning and evening, depending on the time of sunrise and sunset, the observation periods could be shorter. The observers, in addition to binoculars, were equipped with modern cameras with long-focus lenses. Almost all marine mammals encountered were photographed, and the vast majority of birds encountered were also photographed. During the observations, the species, the number of individuals in the group, their behavior, the presence of ice (icebergs), and general weather indicators were recorded. Throughout the entire route of the vessel, three field GPS navigators recorded the coordinates, so the location of each encounter with biological objects in the ocean is known.
In total, data on 1142 encounters with marine mammals and 5425 encounters with seabirds were collected for this area of the Atlantic sector of the Southern Ocean. Representatives of 23 species or systematic groups of marine mammals and representatives of 42 species or systematic groups of seabirds were noted. However, for ecological analysis we use only those species for which the collected material was sufficiently extensive. There are only three such species among marine mammals: the Antarctic fur seal (Arctocephalus gazella), humpback whale (Megaptera novaeangliae), and fin whale (Balaenoptera physalus). Among birds, 18 species or groups of species met these criteria: the Adélie penguin (Pygoscelis adeliae), chinstrap penguin (Pygoscelis antarcticus), Wilson’s storm petrel (Oceanites oceanicus), black-bellied storm petrel (Fregetta tropica), wandering albatross (Diomedea exulans), southern king albatross (Diomedea epomophora), light-mantled albatross (Phoebetria palpebrata), black-browed albatross (Thalassarche melanophris), gray-headed albatross (Thalasarche chrysostoma), southern giant petrel (Macronectes giganteus), northern giant petrel (Macronectes halli), Antarctic fulmar (Fulmarus glacialoides), Cape petrel (Daption capense), Antarctic petrel (Thalassoica antarctica), snow petrel (Pagodroma nivea), two species of prions (Pachyptila sp.),Footnote 1 the white-chinned petrel (Procellaria aequinoctialis), and the Antarctic skua (Stercorarius antarcticus).
Additional Instrumental Methods
The depth of the ocean during the route was recorded according to the readings of the Kongsberg EA600 scientific echo sounder; the radiation frequency was 12 kHz. However, it was not always possible to take depth readings for every point where birds and mammals were encountered. The echo sounder did not work during heavy seas, or while staying at the “stations,” at the points of collecting the hydrological samples. In these cases, we calculated the position of the nearest point where the depth was determined, and these data were used for analysis. Usually the nearest point was at a distance of less than 3 km from the encounter points of biological objects.
The work of the Davis Vantage Pro 2 weather station, managed by a team of employees of the Ilyichev Pacific Oceanographic Institute, Far East Branch, Russian Academy of Sciences, provided us with general weather parameters, recorded every 5 min. The water temperature at different depths was recorded only during the taking of hydrological samples using the Rosetta system (SBE9 and SBE19).
Statistical processing was carried out using the Statistica-12 software package, StatSoft Inc. In addition, to compare means, instead of the generally accepted Student’s and Mann–Whitney tests, we used the Bailey test (Plokhinskii, 1978). The great advantage of this test is that it is suitable for all types of distributions, not just for the normal one, and it has more statistical power than the Mann–Whitney test, since the latter is non-parametric (rank), while the Bailey test is parametric. The Bailey test has the same statistical power as Student’s t test, but does not contain the requirement of a mandatory normal distribution of data. The MapInfo-12.5 program was used for cartographic works.
Method of Ecological Fingerprints
For visual assessment and comparison of a set of ecological and environmental parameters of individual species of mammals and birds, we built special spider plots, which in this case can be called the ecological fingerprints of objects. As objects we used either individual species found over the entire period of work (January–February), or the same species, but for a shorter period of time: January or February. Ten environmental parameters were chosen: latitude, longitude, depth, sea waves, air temperature, air humidity, wind speed, gust wind speed, wind chill factor, and atmospheric pressure. All these parameters were equally used by us earlier in the factor analysis (Kharitonov et al., 2021). In this report, we place the values of these parameters on one diagram in order to visualize more conveniently the complex of environmental factors associated with different biological objects. For each parameter, the general minimum and maximum values were selected for the entire (two-month) period of the expedition, the maximum value of each parameter corresponded to the outermost circle of the spider plot, and the minimum values of each axis (each parameter) were in the center of the diagram circle. These minimum and maximum levels for each parameter were determined according to the following principle: we use the minimum and maximum values that were noted for the period under consideration, regardless of whether the species under consideration was observed at such parameters or not. Since observations were made only during daylight hours, the general minimum and maximum were determined only for daylight hours. For example, we determined the minimum and maximum levels of air temperatures during the period under consideration, but only during daylight hours. Each particular species was not necessarily observed at the minimum and maximum temperatures of the period under consideration; it could be observed in a narrower temperature range. In these ranges, each species had its own real minimum and maximum temperatures at which it was observed. So, from a set of observations, it was possible to calculate the average value of the parameter at which each species was observed. Similar calculations were made for each parameter. Latitude and longitude in this study are considered in numerical form, i.e., not degrees and minutes with an indication of the hemisphere, but degrees with decimals and with a minus sign. The minus sign for latitude means south latitude, and for longitude, west longitude. Therefore, more western values of longitude and more southern values of latitude are located in the region of minima on the diagrams.
Considering that different parameters are similarly used in factor analysis, we decided to increase the level of generalization of the parameter values, i.e., to establish some identical conditional units, into which we then translate the parameter values. For this, the values in the range from the general minimum to the general maximum were taken equal to 100 conditional units. Thus, it turned out that, within each parameter, each such conditional unit contained different real units (degrees of geographical coordinates, Beaufort scale points, degrees Celsius, millimeters of mercury, etc.), and a different number of units depending on the parameter. However, now all parameters had the same measurement scale. After that, it was possible to find the minimum, maximum, and average for all parameters in conditional units. Thus, instead of 30 values for ten parameters, in our case we get only three values, which we then compare between species and between months. However, it turned out that in most cases the consideration of minima is not necessary; the average and maximum values of all parameters expressed in conditional units are especially promising for the species. These values make it possible to understand better how a species in the period considered fit into the limits of environmental parameters that were noted in a given place and at a given time. In addition, for each case, there is also the diagram described above (ecological fingerprint), the form of which also makes it possible to speak about the specific patterns of the species distribution and behavior under the environmental conditions observed during the period under consideration.
A special computer program was created in Visual Basic-3 (Kharitonov, 2021). Based on the data from the input text file containing information on the environmental parameters, general minimums and maximums for each parameter, and a set of specific data, the program output a drawing of the ecological fingerprint presented in the input file of the object, and all necessary calculations are carried out in real and conditional units of measurement for each parameter.
RESULTS
In February, the survey area was slightly less spread in the longitudinal direction to the east than in January: in February, the vessel did not visit the vicinity of the South Orkney Islands. However, in February the vessel sailed more than 100 km to the west than in January; the latitudinal coverage for both months was almost the same (Fig. 1, Table 1). The ranges of values for most environmental parameters, on the contrary, were somewhat narrower in January than in February (Table 1). For the depths, sea waves, wind speeds, and gust wind speeds, as well as atmospheric pressure, the differences were small; however, the maximum level of air temperature (and, accordingly, the wind chill factor) in February was significantly higher than in January (Table 1). The maximum humidity values in both months were similar; however, the minimum values in January were significantly higher, which means, in general, higher air humidity in this month (Table 1). Thus, we can expect that according to the measured parameters, January in Antarctica is colder and wetter than February. In addition, the northern boundary of floating ice in January passed further north than in February, when it was closer to Antarctica (Figs. 2, 3). So, January in the Antarctic corresponds to July in the high Arctic, and February corresponds to August.
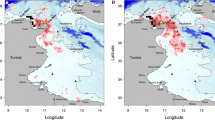
Observations of some of bird species according to the ice conditions: (a) snow petrel Pagodroma nivea; (b) Adélie penguin Pygoscelis adeliae; (c) Antarctic petrel Thalassoica Antarctica; (d) Antarctic fulmar Fulmarus glacialoides. The purple line indicates the route in January; the red line, in February. Turquoise triangles indicate areas of icebergs and cut ice in January; white triangles, in February. The symbols “bird” and “penguin,” colored in green, indicate encounters in January, colored in yellow, encounters in February.
Only general minimum and maximum levels for two months were used to construct ecological fingerprints, for the compatibility of the results of January and February (Table 1). The main results obtained, i.e., the ecological fingerprints, the average values of the factors in conditional units, and the average maximum values of the factors in the same conditional units, are presented in Table 2.
Table 2. (Contd.)

Table 2. (Contd.)

Table 2. (Contd.)

Table 2. (Contd.)

DISCUSSION
Limitations for the Use of Ecological Fingerprints
It should be kept in mind that the ecological fingerprint does not represent all the adaptations of a certain species or population. A fingerprint shows how a species or population fits within the range of environmental parameters observed at a given location and over a given period of time. Representatives of the same species in different places, which are currently characterized by different values of the ecological and environmental parameters, can fit into the range of these parameters and can be distributed over the water area in a completely different way. For example, the occurrence of the black-browed albatross in January is positively related to atmospheric pressure, while in February this relation is negative (Kharitonov et al., 2021a), which affected the decrease in the maximum of this parameter for this species in February (Table 2). Satellite tracking of this species has shown that the species indeed prefers warm air; however, it often uses the cold regions of Antarctica for feeding (Wakefield et al., 2011).
Moreover, the environmental parameters in some cases can be interchangeable and substitutable. For this area of Antarctica, it is important to determine the spatial distribution and occurrence of marine mammals and birds depending on the water temperature. However, the water temperature was recorded only during hydrographic measurements, but not constantly (Morozov et al., 2020, 2020a). These data were provided by E.G. Morozov. These measurements were unevenly distributed in time and space, and thus, it is practically impossible to link these results with all our observations of birds and mammals. However, the analysis showed an almost functional relationship between water temperature and air temperature in the Antarctic during our work: the correlation coefficient between these temperatures is 0.95 (N = 113 for the simultaneous measurements of the air and surface water temperatures). In addition, the average differences between air temperature and water temperatures were 0.4403 ± 0.14778°C and 0.4431 ± 0.1714°C, for January (N = 62) and February (N = 51), respectively. This indicator does not differ significantly between the indicated months (t = 0.0124, P = 0.99); so it was absolutely the same. In addition, over the entire observation period, no sharp intrusions of cold or warm air into the study area were detected. Thus, air temperature can be used instead of water temperature, and the identified relation of seabirds and mammals to air temperature automatically extends to the surface water temperature.
Interpretation of Ecological Fingerprints
To interpret ecological fingerprints, it is useful to divide them into groups according to the following traits: (1) visual similarity of ecological fingerprints; (2) similarity of mean values; (3) similarity of maximum values; (4) similarity of the difference between the maximum and average values. Moreover, for the abovementioned points 2, 3, and 4, we consider not only numerical values, but, as well as for the point 1, the shape of the graphs themselves.
The first environmental feature that ecological fingerprints can indicate is a preference for sea areas with special conditions. A number of similar diagrams (whale diagrams are similar to those of some bird species) could be revealed. The ecological fingerprints are a vivid reflection of an environmental feature of species such as the ratio of Antarctic species (birds and mammals) to ice conditions and their spatial distribution depending on the distribution of floating ice. The fingerprints of the fin whale and the humpback whale for January, as well as the Adélie penguin, Antarctic petrel, snow petrel, and Antarctic fulmar for both months turned out to be biased in the upper left sector of the graph, and also have a plot deflection on the air temperature axis. The latter indicates the confinement of these biological objects to low air temperatures and hence water temperatures. Mapping of the bird and whale encounter points shows that this type of ecological fingerprint is observed in all marine organisms (whether birds or mammals) if their presence during the period under consideration is associated with ice: icebergs or cut ice fields.
The ecological fingerprints of the humpback whales and fin whales in January are very similar. Mapping the meeting points of both species showed that representatives of both species encountered mainly in the ice area this month (Fig. 2). In the area of floating ice, in both January and February, almost all weather indicators (except for air humidity), as well as sea waves, were less important than in areas free from floating ice (Table 3). For this table, the average values of the parameters in icy and ice-free areas in January and February are obtained on the basis of data on records of birds only, without taking into account encounters with marine mammals. This was done to avoid repetitions in the calculations, since the moments of encounters with marine mammals often coincided in time with the sightings of birds. The air humidity in areas of floating ice was higher than in ice-free areas. Although fin whales were clearly biased to the eastern sector of the study area and humpback whales, to the western sector, the ice “equalized” these species and leveled them in the ecological fingerprints. By February, the humpback whales had moved away from the northeast, while the fin whales moved further west. At the same time, humpback whales mostly remained in the area of icebergs, while fin whales were found everywhere, in the icy and ice-free area. In addition, most humpback whales by February had not only reduced their range in the east of their former range, but had also increased their presence in the western part of the area studied, which turned out to be ice-free in February. As a result, both species of whales were characterized by less ice-biased ecological fingerprints in February. In addition, the fin whales, which seemed to be not confined to the ice area this month, have completely different ecological fingerprints. In February humpback whales have a greater influence of ice in their ecological fingerprints: a smaller maximum in the area of atmospheric pressure and a larger deflection of the line of averages in the area of air temperatures (although the air temperature maximum levels in both whale species turned out to be close) (Table 2). However, in the ecological fingerprints of humpback whales, the average air humidity was significantly lower in January. This phenomenon is probably related to the more western distribution of humpback whales, and the air humidity in the west of the study area was less than in the east (Pearson’s correlation coefficient r = 0.49, N = 3165, P = 0.0002). Indeed, visually ice conditions in the west were not as hard as in the east, although a detailed quantitative assessment of the thickness of the ice fields was not carried out.
Some birds also had the same ice-biased nature of ecological fingerprints: the Antarctic petrel, Adélie penguin, snow petrel, and, surprisingly, the Antarctic fulmar, a widespread species the spatial distribution of which absolutely does not seem to be associated with ice (Fig. 3). Adélie penguins in February mainly were represented by juveniles who, possibly, need to rest on ice. The Antarctic petrel is known to be confined to areas with icebergs, since these birds prefer to rest on icebergs but not on the water (Delord et al., 2020). However, as can be seen from the ecological fingerprints, these birds are not always biased toward the icebergs, but can be quite far from them: in February, the ecological fingerprints of this species are already less icy than in January. Snow petrels are generally biased to the ice that was in the eastern part of the surveyed area in both January and February (Fig. 3). The Southern fulmar was characterized by the ice-biased nature of the ecological fingerprints in both January and February. However, according to the species distribution in the sea (Fig. 3), these birds do not prefer ice itself; apparently, they simply prefer (or, if necessary, form food aggregations) cold ocean areas with high air humidity.
On the contrary, prions in February, when there was less ice, turned out to be more “icy.” Usually, prions have a wide range of feeding grounds (Quillfeldt et al., 2014). The light-backed albatross also turned out to be more “icy” in February, when the ice distribution decreased. A similar trend was also observed in the white-chinned petrel. Perhaps this could be explained by the food preferences of the species. We have previously suggested that prions, the light-mantled albatross, and the white-chinned petrel may be biased to the Powell Basin and Hesperides Basin in February, as krill (Euphausia superba) swarms with only limited numbers of Salpa tompsoni were recorded there (Kharitonov et al., 2021a). Furthermore, this area was more “icy” in February (Fig. 3).
The second environmental feature indicated by ecological fingerprints is the range of the distribution area. For solving this issue, it is not necessary to plot meeting points on the map, which is not always easy. The southern royal albatrosses were not numerous in January and were recorded in a narrow area in the west of the study area, which is indicated well by the January ecological fingerprints of this species: the longitude of the meeting sites is close to the general minimum values.
A third feature indicated by ecological fingerprints is the variety of ecological and environment conditions, suitable for a species. The maxima show the possible places of the presence of a given species, while the averages show the conditions it is most likely to be in. For example, the Antarctic skua is characterized by high maximum values of all the parameters, which means its adaptation to a wide range of conditions. However, its average values are much lower than the maxima (Table 2); i.e., representatives of this species preferred much milder conditions, far from extreme. Antarctic skuas are quite rare birds in Antarctic waters far from the coast. However, some widespread oceanic species also have a similar feature: Wilson’s storm-petrel, the southern giant petrel, and the black-browed albatross. The species with the widest environmental preferences, according to the maxima, is the black-browed albatross (Table 2). No wonder representatives of this species are sometimes found in the northern hemisphere in the vicinity of Novaya Zemlya and Franz Josef Land (Pokrovskaya et al., 2018). According to the ecological fingerprints, the southern giant petrel, also a very numerous and widespread species in the Antarctic, although it is a competitor of the black-browed albatross (Kharitonov et al., 2021a), is still not as “ecologically broad” a species as the latter.
The ecological fingerprint method revealed an interesting feature of the competition patterns between the black-browed albatross and the southern giant petrel. According to our data, in January the competition between these species was not too strong and their linear density was similar, 10.6 and 9.5 encounters per 100 km of the route, respectively. In February, the competition was obviously stronger; the linear density of the black-browed albatross decreased to 7.4 records per 100 km of the route; in the southern giant albatross, it almost tripled compared to January, to 3.85 records per 100 km (Kharitonov et al., 2021a). The ecological fingerprints of these species by months show practically no similarity in January, while in February the fingerprints of these species became more similar (although we expected the further divergence with increased competition) (Fig. 4). The patterns of the maxima practically coincided, and the averages became much closer (Table 2, Fig. 4). It is not clear what is the cause and what is the effect here, but it is a really challenging task for further research.
Superimposed ecological fingerprints of the black-browed albatross (dark maxima and averages) and the southern giant petrel (light maxima and averages) in January (left) and February (right). Axes designations as in Table 2.
Among the marine mammals, the Antarctic fur seal was found to be the most ecologically broad species, even in comparison to the two species of whales. However, fur seals narrowed their ecological indicators by February compared to January (Table 2). Apparently, this is due to the different nature of the use of the water area by this species. In January, these animals were recorded in groups and aggregations and large groups of fur seals were noted near the tip of the Antarctic Peninsula and neighboring islands, near the South Shetland and South Orkney islands; i.e., in January seals were both far in the sea and close to land. By February fur seals became more common far from land and their distribution in the ocean became random (Kharitonov et al., 2021). The changes in the nature of the distribution and, accordingly, ecological fingerprints in these months are very likely associated with the breeding behavior of these animals: in January they were still close to rookeries, while in February Antarctic fur seals begin to migrate to the north (Dikii and Peklo, 2012).
The northern and southern giant petrels are closely related species that have quite similar two-month ecological fingerprints. However, this mainly concerns the average values; the ecological maximum for the southern giant is clearly wider (Table 2). However, for separate months, the ecological fingerprints of these species vary significantly. In January, the northern giant petrel fully corresponds to its name “northern”: in terms of latitudes, it was found further to the north and also to the west than the southern giant petrel (Table 2). Moreover, the species prefers higher air temperatures and stronger winds and gusts (or being tolerant to such conditions). In warmer February, northern giant petrels spread further south, on average even somewhat further south than the southern giant petrel. At the same time, in contrast to the southern one, the northern giant petrels avoided extremely high temperatures, which resulted in the slightly lower average air temperatures of the encounter points of this species. The southern giant petrel turned out to be more tolerant not only to the range of temperatures, but also to the range of atmospheric pressure and air humidity (Table 2). These species are known to differ in dispersal areas and feeding spectra. In addition to plankton, both species also feed on carrion. Male southern giant petrels even prey on live birds, which is not typical for northern giant petrels (Cooper et al., 2001; Shirihai, 2008).
Although the general ecological limits widened from January to February (Table 1), the values of the ecological and environmental parameters narrowed in many birds. This mainly affected the decrease of maximum levels. This phenomenon has been observed in the following species: the chinstrap penguin, Wilson’s storm petrel, the grey-headed albatross, the Cape petrel, prions, and the white-chinned petrel. The last three species have greatly decreased their maxima with almost unchanged averages. The reason for such phenomena is still not entirely clear and requires further investigation.
The wandering albatross has fairly wide ecological limits and is widely distributed in Antarctica. However, it is clearly seen that this species is strongly biased to low temperatures and, accordingly, a stronger effect of the wind chill factor. In its distribution range, it is somewhat similar to the southern royal albatross; it tends to the western part of the studied area of Antarctica. The species is tolerant to waves or even prefers hard seas, which is probably why the species have been frequently recorded in the Drake Passage. At the same time, it was characterized by somewhat lower humidity than the ice-biased species.
The black-bellied storm petrel was characterized by interesting ecological fingerprints: in almost all parameters, it was found in a narrower range of conditions than the closely related species, Wilson’s storm petrel. The black-bellied storm petrel has lower maxima and, surprisingly, higher minima than Wilson’s storm petrel. Perhaps that is the reason for the higher average values of the most parameters for the black-bellied storm petrel (in comparison to Wilson’s storm petrel). While Wilson’s storm-petrel is numerous and widespread, both in areas of icebergs and iceberg-free areas, the black-bellied storm petrel was recorded much less frequently; this species clearly avoids areas with ice.
CONCLUSIONS
The proposed method of ecological fingerprints provides visualization of the environmental parameters that are difficult to come up with. This makes it possible to assess more accurately the ecological and environmental characteristics of species or groups of animals in different places and in different seasons, and to identify the features that may remain poorly detected by other methods, including the method of factor analysis.
Very similar ecological fingerprints of some species of marine mammals and seabirds indicate the same relations to environmental conditions of different species, regardless of their systematic affiliation. Fin whales, humpback whales, snow petrels, Adélie penguins, Antarctic petrels, and Antarctic fulmars in January were characterized by specific patterns of ecological fingerprints, indicating that these species are confined to water areas with icebergs and cut ice. In February, the association of whales with the ice weakened, which became evident from their ecological fingerprints, while it remained practically the same for the bird species listed above. The ecological fingerprints demonstrate the wide range of the abiotic ecological niche occupied by specific species and groups. They can show a change in distribution areas in certain periods (as for the southern royal albatross) and a change in the strategy of using the water area (as for the Antarctic fur seal), as well as others, including yet undiscovered ecological and environmental features.
It should be emphasized that this report is the first one that considers and uses the method of ecological fingerprints. Therefore, it is unlikely that we have been able to extract all the information that ecological fingerprints can provide. Further development of the method should allow this.
Notes
In fact, we recorded two species, the Antarctic prion (Pachyptila desolata) and the slender-billed prion (Pachyptila belcheri). The small size of these birds and their chaotic flight make it very difficult to identify them during the field observations. Sometimes even the analysis of photographs does not allow us precise attribution of the bird to a certain species, because the size of the white eyebrow and the shape of the beak vary among prions. In this regard, in order to avoid errors, we consider both species together.
REFERENCES
Ainley, D., Russell, J., Jenouvrier, S., Woehler, E., Lyver, P.O., Fraser, W.R., and Kooyman, G.L., Antarctic penguin response to habitat change as Earth’s troposphere reaches 28°C above preindustrial levels, Ecol. Monogr., 2010, vol. 80, no. 1, pp. 49–66.
Cooper, J., Brooke, M.L., Burger, A.E., Crawford, R.J.M., Hunter, S., and Williams, T., Aspects of the breeding biology of the Northern Giant Petrel (Macronectes halli) and the Southern Giant Petrel (M. giganteus) at sub-Antarctic Marion Island, Int. J. Ornithol., 2001, vol. 4, pp. 53–68.
Delord, K., Kato, A., Tarroux, A., Orgeret, F., Cotté, C., Ropert-Coudert, Y., Cherell, Y., and Descamps, S., Antarctic petrels ‘on the ice rocks’: wintering strategy of an Antarctic seabird, R. Soc. Open Sci., 2020, vol. 7, no. 4, pp. 1–8.
Dikii, I.V. and Peklo, A.M., Seals of the Argentine Islands (Antarctic), Sb. Tr. Zool. Muz., 2012, no. 43, pp. 104–116.
Friedlaender, A.S., Halpin, P.N., Qian, S.S., Lawson, G.L., Wiebe, P.H., Thiele, D., and Read, A.J., Whale distribution in relation to prey abundance and oceanographic processes in shelf waters of the Western Antarctic Peninsula, Mar. Ecol. Progr. Ser., 2006, no. 317, pp. 297–310.
Joiris, C.R. and Dochy, O., A major autumn feeding ground for fin whales, southern fulmars and grey-headed albatrosses around the South Shetland Islands, Antarctica, Polar Biol., 2013, vol. 36, pp. 1649–1658.
Kasamatsu, F., Matsuoka, K., and Hakamada, T., Interspecific relationships in density among the whale community in the Antarctic, Polar Biol., vol. 23, no. 7, pp. 466–473.
Kharitonov, S.P., Spider graph software, executable file spider_g.exe, 2021.
Kharitonov, S.P., Tretyakov, A.V., Mischenko, A.L., Konyukhov, N.B., Artemyeva, S.M., Pilipenko, G.Yu., Mamayev, M.S., and Dmitriyev, A.E., Spatial distribution, species composition, and number of marine mammals at Argentine Sea, Drake Passage, east of Antarctic Peninsula and the Powell Basin in January–March 2020, in Advances in Polar Ecology. Antarctic Peninsula Region of the Southern Ocean. Oceanography and Ecology, Morozov, E.G., Flint, M.V., and Spiridonov, V.A., Eds., 2021, pp. 316–330.
Kharitonov, S.P., Mischenko, A.L., Konyukhov, N.B., Dmitriyev, A.E., Tretyakov, A.V., Pilipenko, G.Yu., Artemyeva, S.M., and Mamayev, M.S., Spatial distribution, species composition, and number of seabirds at Argentine Sea, Drake Passage, east of Antarctic Peninsula and the Powell Basin in January–March 2020, in Advances in Polar Ecology. Antarctic Peninsula Region of the Southern Ocean. Oceanography and Ecology, Morozov, E.G., Flint, M.V., and Spiridonov, V.A., Eds., 2021a, pp. 331–344.
Kharitonov, S.P., Tretyakov, A.V., and Mischenko, A.L., Meat in the ocean: how much and who is to blame?, in Advances in Polar Ecology. Antarctic Peninsula Region of the Southern Ocean. Oceanography and Ecology, Morozov, E.G., Flint, M.V., and Spiridonov, V.A., Eds., 2021b, pp. 345–355.
Korosov, A.V., Ekologicheskie prilozheniya komponentnogo analiza (Ecological Applications of Component Analysis), Petrozavodsk: Petrozav. Univ., 1996.
Lyver, P.O’B., MacLeod, C.J., Ballard, G., Karl, B.J., Barton, K.J., Adams, J., and Ainley, D.G., Intra-seasonal variation in foraging behavior among Adelie penguins (Pygocelis adeliae) breeding at Cape Hallett, Ross Sea, Antarctica, P.R. Wilson, Polar Biol., 2011, vol. 34, pp. 49–67.
Mondreti, R., Davidar, P., Ryan, P.G., Thiebot, J.B., and Gremillet, D., Seabird and cetacean occurrence in the Bay of Bengal associated with marine productivity and commercial fishing effort, Mar. Ornithol., 2020, vol. 48, pp. 91–101.
Morozov, E.G., Flint, M.V., Spiridonov, V.A., and Tarakanov, R.Yu., Multidisciplinary program of field studies of the ecosystem in the Atlantic sector of the Southern Ocean (December 2019–March 2020), Oceanology, 2019, vol. 59, no. 6, pp. 989–991.
Morozov, E.G., Spiridonov, V.A., Molodtsova, T.N., Frei, D.I., Demidova, T.A., and Flint, M.V., Investigations of the ecosystem in the Atlantic sector of Antarctica (cruise 79 of the R/V Akademik Mstislav Keldysh), Oceanology, 2020, vol. 60, no. 5, pp. 721–724.
Morozov, E.G., Frei, D.I., Polukhin, A.A., Krechik, V.A., Artem’ev, V.A., Gavrikov, A.V., Kas’yan, V.V., Sapozhnikov, F.V., Gordeeva, N.V., and Kobylyanskii, S.G., Mesoscale variability of the ocean in the northern part of the Weddell Sea, Oceanology, 2020a, vol. 60, no. 5, pp. 573–588.
Plokhinskii, N.A., Matematicheskie metody v biologii (Mathematical Methods in Biology), Moscow: Mosk. Univ., 1978.
Pokrovskaya, I.V., Pokhelon, A., Gommershtadt, O.M., and Vaiss, I., Repeated records of the black-browed albatross Thalassarche melanophris in Russian Arctic waters, Russ. Ornitol. Zh., 2018, vol. 25, no. 1663, pp. 4375–4378.
Quillfeldt, P., Phillips, R.A., Marx, M., and Masello, J., Colony attendance and at-sea distribution of thin-billed prions during the early breeding season, J. Avian Biol., 2014, vol. 45, pp. 315–324.
Santora, J.A., Reiaa, C.S., Cossio, A.M., and Veiti, R.R., Interannual spatial variability of krill (Euphausia superba) influences seabird foraging behavior near Elephant Island, Antarctica, Fish. Oceanogr., 2009, vol. 18, no. 1, pp. 20–35.
Serratosa, J., Hyrenbach, K.D., Miranda-Urbina, D., Portflitt-Toro, M., Luna, N., and Luna-Jorquera, G., environmental drivers of seabird at-sea distribution in the eastern South Pacific Ocean: assemblage composition across a longitudinal productivity gradient, Front. Mar. Sci., 2020, vol. 6, no. 838, pp. 1–13.
Shirihai, H., The Complete Guide to Antarctic Wildlife. Birds and Marine Mammals of the Antarctic Continent and the Southern Ocean, Princeton: Princeton Univ. Press, 2008.
Wakefield, E.D., Phillips, R.A., Trathan, P.N., Arata, J., Gales, R., Huin, N., Robertson, G., Waugh, S.M., Weimerskirch, H., and Matthiopoulos, J., Habitat preference, accessibility, and competition limit the global distribution of breeding black-browed albatrosses, Ecol. Monogr., 2011, vol. 81, pp. 141–167.
ACKNOWLEDGMENTS
The authors are grateful to the head of the expedition E.G. Morozov (Shirshov Institute of Oceanology, Russian Academy of Sciences, Moscow) for his assistance in organizational aspects during the expedition, as well as for the prompt provision of the necessary data (water temperature, current directions, etc.), the collection of which was not part of the task of the authors. We also thank the managers of the scientific echo sounder device of the research vessel, D.I. Frei and A.V. Gavrikov (Shirshov Institute of Oceanology, Russian Academy of Sciences, Moscow) for providing us data on the depths along the route of the ship. In fact, this work is not so simple. Access to meteorological data was kindly provided by the employees of the Ilyichev Pacific Oceanographic Institute (Far East Branch, Russian Academy of Sciences), E.A. Mar’ina and Ya.I. Rudykh. M.S. Mamaev participated in the collection of factual material, mainly on marine mammals.
Funding
This work was carried out according to a State Assignment, project no. 0089-2019-0021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by T. Kuznetsova
Rights and permissions
About this article
Cite this article
Kharitonov, S.P., Tretyakov, A.V., Mishchenko, A.L. et al. Observations on Marine Mammals and Seabirds in the Antarctic: Ecological Fingerprints of Seaside Distributions during the 79th Voyage of the R/V Akademik Mstislav Keldysh. Biol Bull Russ Acad Sci 49, 1244–1259 (2022). https://doi.org/10.1134/S1062359022080088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022080088