Abstract
Information on the fauna and distribution of the rotifers of the family Trichocercidae in the Urals is summarized based both on the author’s original research and an analysis of literature sources. Data on their localities, biology, morphology, quantitative development, and indicative properties in the Urals’ water bodies are presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
This work is a further summary of the material on rotifers of the order Ploima in the Urals. It summarizes the data accumulated over a century on the fauna and ecology of the Rotifera of the Urals based on materials published in the period from 1910 to the present, as well as the author’s own research conducted in the water bodies of the Southern Urals and the Trans-Urals from 1986 to 2019. Previous works (Rogozin, 2018, 2019, 2020) considered the family Brachionidae. This article is dedicated to another large family, Trichocercidae Harring 1913. For the species with enough quantitative data, the individual indicator significance and indicator weight have been calculated (the latter can also be considered as an indicator of stenobiontness/eurybiontness, Rogozin, 2018b). They characterize the specific features of the species biology (relation to water temperature (Rogozin et al., 2015), saprobity (Rogozin, 2018a), and trophic type of reservoir (Rogozin, 2018b)). Obtained on local material, these data are most objective in the regional geographical conditions. In the absence of such information, we use literature data, the application of which, of course, is more limited.
The family Trichocercidae currently includes three genera: Ascomorphella Wiszniewski 1953, Trichocerca Lamarck 1801, and Elosa Lord 1891 (Kutikova, 1970; Shiel and Koste, 1992). Representatives of the first two genera are found in water bodies of the Urals.
Genus Ascomorphella Wiszniewski 1953. A monotypic genus with a single representative, A. volvocilola (Plate 1886).
Ascomorphella volvocilola (Plate 1886)
The only single specimen of the species was found by Oparina (1923) in Peschanoe Lake in the coast region of the Kama River (near Perm). It was found in the colonial algae Volvox globator L. 1758. The total length of the specimen was 138 µm.
It is one of the few parasitic rotifer species; it inhabits the volvox. The specifics of the habitat may explain the exceptional rarity of A. volvocilola finds in the region. As was noted by Kirk (1998), the successful introduction and reproduction of rotifers in an algae colony (in oospores developing in spring) require a high abundance of volvox in a particular water body for a very long time. This, as a rule, is not realized in the Ural reservoirs, although volvox is widespread here, it is not abundant and even quite rare. The vegetative season is too short to contribute to A. volvocilola success. In more southern regions, this rotifer can significantly affect Volvox populations (Ganf et al., 1983).
Sládeček (1983) assigned the species to beta-mesosaprobes (individual saprobic index 1.5).
A. volvocilola is distributed in all zoogeographic zones, except for the Antarctic and the Pacific. In Russia, it is known from many, mainly western, regions.
Genus Trichocerca Lamarck 1801. This extensive genus includes more than a hundred species, 25 of them are found in the Urals. Two of them, T. gracilis (Tessin 1890) and T. sejunctipes (Gosse 1886), are currently considered as species inquirenda (Segers, 2007).
Trichocerca bicristata (Gosse 1887)
Two finds in the Urals were from Peschanoe Lake and the Kur’ya River in the coastal region of the Kama (Oparina, 1923) and in the floodplain lakes of the Kama (Vershinin, 1953). The abundance of the species was given verbally: “a few” and “single specimens.” The representatives of the species were found in July and August. According to the measurements made by Oparina, the length of the body is 289 µm, the width is 120 µm, and the length of the left toe is 240 µm. So, the size corresponds to the average values for the species.
The species inhabits thickets of aquatic vegetation, detritus, and sand and occasionally was found in plankton (Kutikova, 1970). According to other data, it is a pelagic species (Morales-Baquero et al., 1989). In small, overgrown water bodies, it can reach a high abundance and may dominate (Green, 2001). Apparently eurythermal, it occurs at temperatures from 8 to 24.5°С (Shiel and Koste, 1992). This is also evidenced by the finds of the species at the latitude of the Polar Urals on the Yamal Peninsula (Bogdanov et al., 1997). According to some reports (Xue et al., 2014), it prefers eutrophic waters rich in nutrients. According to Sládeček (1983), it is an oligosaprobe (1.0) with a high indicator weight.
The species is distributed throughout Russia. It occurs in all zoogeographic regions, except for Antarctica.
Trichocerca bidens (Lucks 1912)
This species was repeatedly recorded in the lakes of the Ilmenskaya group in the Southern Urals in the foothills of the Ilmenskii Range (Argayash, Bolshoi Kisegach, Bolshoye Miassovo, Sirikkul, etc.) by the expeditions of A.O. Towson and N.V. Bondarenko; these data refer to the end of the 1930s and have not been published. Kutikova (1970) mentioned this species for Chelyabinsk oblast; however, it is impossible to specify the published source she used. In published materials, this species is indicated for the Middle Cis-Urals (the Kama River and floodplain lakes, (Tauson, 1946, 1947; Vershinin, 1953)). In all cases, only single individuals were found. Our only find of T. bidens was made in Bolshoe Miassovo Lake. The size: body length 180–186 µm, length of toes 52–55 µm. The species occurred in the pelagic zone in July, in the upper water layer (0–10 m) warmed up to 22°С. The population density is 600–800 ind./m3.
The species inhabits peat bogs and shallow overgrown and swampy water bodies (Kutikova, 1970). It can be confined to the sapropel zone in small lakes up to 11 m deep (Jersabek, 1995) or to fluvial silt deposits with loam and macrophyte detritus in shallow water (Ermolaeva, 2015). Apparently, it is a eurythermal species, since it is found in large numbers both in relatively cold waters (8–12°С) and in those heated above 30°С (Chittapun et al., 2007). It is an eurybiont species also in relation to the oxygen concentration and pH of the water (Jersabek and Bolortsetseg, 2010). Studies of high-mountain water bodies of Mexico have shown that the species is indifferent to the nutrient content and turbidity (Muñoz-Colmenares and Sarma, 2017). Sládeček (1983) classified it as an oligo-beta-mesosaprobe species (1.3) with a good indicator weight.
In Russia, it is widespread throughout the territory. It occurs in all zoogeographic regions, except for Antarctica.
Trichocerca brachyura (Gosse 1851)
The first find in the Urals was recorded in the shallow overgrown Chernen’koe Lake in the foothills of the Ilmenskii Range in the Southern Urals (unpublished data by A.O. Tauson, 1937). Later it was encountered in the Cis-Urals in the Kama River (Tauson, 1946, 1947) and in the Middle Urals in Shartash Lake (Balabanova, 1949).
It inhabits small overgrown reservoirs, bogs, and psammon (Kutikova, 1970). It may be a dominant in rotifer communities in eutrophic water bodies with low transparency (Geng et al., 2005). Nevertheless, Sládeček (1983) classified it as an oligosaprobe species (1.1) with a high indicator weight, which may result from its association with bog waters. Flow seems to have a negative effect on the development of T. brachyura (Czerniawski and Domagała, 2010).
Widespread in Russia. It occurs in all zoogeographic regions of the Earth.
Trichocerca capucina (Wierzejski et Zacharias 1893)
(Fig. 1a)
The first and very numerous finds of the species in the South Ural lakes of the Kasli and Ilmen groups (Akakul, Alabuga, Argayash, Bolshie Kasli, Bolshoe Miassovo, Maloye Miassovo, Kirety, Sungul, Uvildy, etc.) date back to the beginning of the last century (Furman and Tiebo, 1910). These findings were subsequently repeatedly confirmed (Maslennikova, 1941; Podlesny and Troitskaya, 1941; Rogozin, 2009). The species is also found in water bodies of the forest–steppe Trans-Urals (Makartseva, 1978; Ogorodnikova, 1977). The southernmost finds in the Urals were recorded in the Ural River, in the steppe regions of Orenburg oblast (Muraveisky, 1923; Akatova, 1954). Quite a lot of finds are known from the Middle Urals and the Cis-Urals, especially in the Kama River and the lakes of its basin (Balabanova, 1949; Krasnovskaya, 1949; Tauson, 1934, 1946; etc.).
According to our data, the body length is 370–398 µm; left toe, 110–115 µm; right toe, 33–37 µm. Oparina (1923) provided a body length of 260 µm and the left toe length of 80 µm.
It is a planktonic species found in the pelagial and phytal zones (Kutikova, 1970). Our data confirms this: although the rotifer was also found in small overgrown water bodies, the main habitat in the Urals is the pelagial zone of large lakes. Over the entire data array (more than 560 samples), the occurrence of T. capucina is 10%, including more than 80% in the pelagic zone. It is a termophilic species, like most Trichocerca species (Segers, 2003), which is confirmed by our data on Ural water bodies (Rogozin et al., 2015). Trichocerca capucina is a thermobiont (2.4), extremely rare in cold waters (with a temperature not lower than 8°С). Therefore, both in the Urals and in other regions of the Earth with a temperate climate, it is a “summer” species (Herzig, 1987). In very cold and slowly warming water bodies, it can reach its maximum development in autumn (Makarov et al., 2019). In the lakes of the Southern Urals, T. capucina in the vast majority of cases was found in July and August, during the period of maximum water heating, and only occasionally in June and September. In the deep lakes (over 20 m), T. capucina was concentrated mainly in the upper warmest water layers (0–10 m) and never descends below a depth of 15 m. Similar results were obtained in warmer regions of the planet (Baloch et al., 1998). This confirms the rotifer as an epilimnic species (Matveeva, 1986) and corresponds to the properties of a thermobiont inhabiting temperate climate ecosystems. As for other properties of the species, T. capucina is a stenobiont halophobe avoiding mineralization above 200 mg/dm3 (Bielańska-Grajner and Cudak, 2014).
The highest population density, 14 500 ind./m3 with a biomass of 0.103 g/m3, was recorded in Bolshoe Miassovo Lake in the eastern foothills of the Ilmenskii Range in the Southern Urals in August. The average abundance of T. capucina is small, amounts to 1930 ± 872 ind./m3, and often does not exceed 1000 ind./m3. Most Ural authors estimate the abundance of T. capucina according to the visual scale as “not abundant” or “single” (Maslennikova, 1941; Makartseva, 1978; etc.), sometimes mass reproduction is noted at the end of summer (Argayash Lake, Makartseva, 1978). The low abundance can be explained by various reasons. First, it is a predatory rotifer (feeds by sucking out the eggs of other planktonic rotifers, Schmidt-Rhaesa, 2014) and, due to such specifics of feeding, it does not reach a high abundance. Second, stenobionty with a pronounced preference for well-warmed waters greatly shortens the duration of vegetation in cold Ural water bodies and does not allow T. capucina to reach a high abundance. Third, the species is pelagic, preferring relatively large water bodies; while in the Ural climate, the pelagial waters warm up especially slowly. The first reason is obviously the main one, because a consistently low abundance of T. capucina has also been recorded even in year-round warm waters (Ramírez García et al., 2002).
According to our data, T. capucina is a mesotrophic species (individual index 1.3) with a fairly high stenobiont index (3.62). This data differs from the materials of a number of authors who consider the genus Trichocerca, including T. capucina, to be confined to eutrophic waters (Ruttner-Kolisko, 1974; Bērziņš and Pejler, 1989; Geng et al., 2005). Such a contradiction can be associated both with a more accurate method of quantitative determination of indicator properties (Rogozin et al., 2015; Rogozin, 2018b) in relation to the trophic state of the reservoir and with differences in these properties in different natural and geographical zones. Despite the thermophilicity and, consequently, the occurrence in the period of the highest productivity of water bodies, in the conditions of the Urals, the species is consistently confined to oligo- and mesotrophic lakes. The revealed saprobic properties of T. capucina are fundamentally different from Sládeček’s data for Europe. Sládeček (1983) classified the species as an oligosaprobe (1.2) with a high indicator weight; in the Urals, it seems to be a beta–alpha–mesosaprobe species (2.1), tending to moderately muddy waters. There is no particular contradiction here with the mesotrophicity of the species, since saprobity is not a complete analogue of trophicity, although these concepts are sometimes used as synonyms (Shitikov et al., 2003). The contradiction with Sládeček’s data on saprobity is quite typical for data on the water bodies of the Urals and has already been discussed by us in a separate paper (Rogozin, 2018a).
The species is known throughout Russia. It occurs in all zoogeographic regions, except for the Pacific and Antarctic.
Trichocerca cavia (Gosse 1886)
This species was mentioned by Kutikova (1970) as occurring in Chelyabinsk oblast (Southern Urals); however, it is impossible to find the original source of this information; the authorship, site, and time of that find are still unknown. The second and so far the last find of T. cavia in the Urals was made by us in the overgrown shallow Bolshoi Tatkul Lake (eastern foothills of the Ilmenskii Range in the Southern Urals) in the coastal zone among Ceratophyllum, water milfoil, and elodea. According to our data, the body length is 90–117 µm and the length of the toes is 30–35 µm. There are no data on the quantitative development, as only single individuals were encountered.
Published data on the biology of T. cavia are scarce. Like many other species of the genus, it lives mainly in overgrown, swampy water bodies, often among submerged mosses. Sometimes it can be found in plankton and can be very numerous in thickets (Ejsmont-Karabin, 1995). According to Sladeček (1983), it is a good indicator of oligo-beta-mesosaprobity.
The species is known in many regions of Russia from the Center to Eastern Siberia. It occurs in all zoogeographic regions, except for the Pacific and Antarctic.
Trichocerca collaris (Rousselet 1896)
The only mention of a find of this species in the Urals was found in the handwritten report of a student of Moscow State University, N.V. Bondarenko (1938), who worked on Lake Ilmenskoe (foothills of the Ilmenskii Range in the Southern Urals, vicinity of the city of Miass). A reference to this record is given in my monograph (Rogozin, 1995). Trichocerca collaris is known in many reservoirs of Central Russia, in the Volga region, and in the Far North. Like the previous one, this species is an inhabitant of overgrown reservoirs and swamps, where it occurs among microalgal mats and mosses (Riccia and Sphagnum). Apparently, it is eurythermal (range from 8 to 28°С) and occurs, for example, both in the lower reaches of the Yenisei River (Grese, 1957) and in the upper reaches of the Nile (Iskaros et al., 2008). It is usually confined to acidic waters (with pH up to 3.7) (Pejler and Bērziņš, 1993; Jersabek and Bolortsetseg, 2010). Like many other “swamp” species, it is oligo-beta-mesosaprobe species (Sládeček, 1983).
The species is known in many regions of Russia from the Center to Eastern Siberia. It is found in all zoogeographic regions except for the Pacific and Antarctic.
Trichocerca cylindrica (Imhof 1891)
The first finds of this species in the South Ural lakes of the Kasli group (Argayash, Bolshiye Kasli, and Kirety) date back to the beginning of the last century (Furman and Tiebo, 1910). Subsequently, this species was also found in other lakes of the eastern foothills of the Southern Urals: Arakul and Bolshoe and Maloe Miassovo (Drabkova and Sorokin, 1979; Lyubimova, 1981; Makartseva, 1978; Rogozin, 1995), as well as in many other reservoirs of the Ilmenskaya group of lakes (manuscripts by A.O. Tauson, N.V. Bondarenko, and others). It is also found in the lakes of the forest–steppe Trans-Urals—Kundravinskoe Lake (Kozlova, 1966), Smolino Lake (Rechkalov and Marushkina, 2005), Argazinskoe Reservoir on the Miass River (Kozlova and Shilkova, 1966), and in steppe water bodies (Lubimova, 1975). The southernmost records for the Urals were for the Ural River in the steppe regions of Orenburg oblast (Akatova, 1954). Quite a lot of finds were recorded for the Middle Urals and the Cis-Urals, especially in the Kama River and the lakes of its basin (Tauson, 1934, 1935, 1946; Vershinin, 1953). The northernmost finds of T. cylindrica in the Urals were in the tributaries of the Lower Ob River (Bogdanov et al., 2005). According to our data, the body length is 300–320 µm; left toe, 250–262 µm; and right toe, 18–22 µm.
It is a planktonic species that also inhabits the phytal zone (Kutikova, 1970). The occurrence throughout our samples in the lakes of the Southern Urals is less than 1.5% and that of them in the pelagial of lakes, 75%. The biology of the species is similar to that of T. capucina considered above. It is a thermobiont (2.4), confined to the period of maximum water heating (August–early September); it stays in the epilimnion, not diving down below 10 m, or in shallow bays. An inverse dependence of the average daily abundance of T. cylindrica on water temperature was noted (Ermolaeva et al., 2016). Despite the pronounced thermophilicity of T. cylindrica, it is now generally accepted that its twin-species, T. chattoni (de Beauchamp 1907), inhabits subtropical and tropical regions, while T. cylindrica is a “cold-water species” (cit. ex. Segers, 2003), although it is more correct to consider T. cylindrica a warm-water species, but confined to water bodies of a temperate climate.
Mäemets (1983) considered T. cylindrica as an indicator eutrophic species for Estonian lakes, and Andronikova (1996) followed him, considering the literature data. According to our data, T. cylindrica is a typical mesotrophic species with an individual trophic index of 1.0 and the highest indicator significance (5.0); it is a stenobiont. Although the overwhelming majority of works do not provide quantitative data on T. cylindrica, only one of the T. cylindrica records (including unpublished materials) made for the Urals refers to eutrophic water bodies, the other recorded findings were made in meso- and oligomesotrophic water bodies. Consequently, in the Urals, T. cylindrica can be considered not only an eutrophy-indicator species, but in general a species that prefers eutrophic conditions. The controversy of the eutrophic indicator status of T. cylindrica was also discussed earlier (Pejler and Bērziņš, 1993).
According to our data, the individual saprobic index of T. cylindrica is 1.4 (with an indicator significance of 3.62). This is consistent with those given by Sládeček (1983) of 1.2 and 4, respectively.
According to our data, the highest abundance of T. cylindrica is 1600 ind./m3, and the average for the season is 1200 ± 210 ind./m3. The species was found only in August and September when the water warmed up the most. The summer–autumn maximum in the development of the species (with similar quantitative indicators) was also noted in the lakes of North America (Stemberger et al., 1979) and in northern India (Irfan et al., 2013).
It feeds on algae, mainly on chrysophytes (Barrabin, 2000), and on eggs of other planktonic rotifers (Schmidt-Rhaesa, 2014).
In the lakes of the Southern Urals, T. cylindrica sometimes may be one of the main plankton species. Lyubimova (1981) reports a 60% level of occurrence of the species in the oligotrophic lake Arakul (eastern foothills of the Southern Urals) with an average biomass for the season of 0.003 g/m3. According to her data (Lyubimova, 1975), T. cylindrica is one of the mass species in the ponds of the Chesmenskii fish farm in the forest–steppe zone in the southern part of Chelyabinsk oblast. According to the data of Leningrad hydrobiologists (Makartseva, 1978; Drabkova and Sorokin, 1979), Trichocerca cylindrica is one of the leading forms of plankton in Argayash Lake (forest–steppe of the Trans-Urals). In general, the species is not abundant (600–900 ind./m3) and reaches its maximum development in the Ural lakes only at the end of summer (Kozlova, 1966; Kozlova and Shilkova, 1966). However, T. cylindrica is often characterized by a very high occurrence at a low abundance, which was also noted by foreign researchers (Stanachkova et al., 2017).
The species is known in many regions of Russia from Karelia to the Far East. It occurs in all zoogeographic regions, except for the Pacific and Antarctic.
Trichocerca dixonnuttalli (Jennings 1903)
(Fig. 1b)
This species was first recorded in the Ilmenskoe and Maloye Miassovo lakes of the eastern foothills of the Southern Urals in the mid-1930s by members of the expedition of A.O. Tauson (as Diurella inermis; unpublished data), and then in the lower reaches of the Ural River (Akatova, 1954). In fact, it is already outside the Urals, even in its broadest sense. We recorded this species in the plankton of Bolshoe Miassovo (Rogozin, 1995, mentioned as T. inermis) and Malyi Terenkul (Rogozin, 2009a) lakes in the foothills of the Ilmenskii Range. According to our data, the body length is 89–113 μm; left toe, 38–48 μm; and right toe, 23–27 μm.
The scarce data only for two lakes do not allow us to conclude anything about the biology of the species. It was found only in the summer months or in early autumn (from June to early September). The abundance varied from 300 to 24 000 ind./m3 and was maximum in July in the littoral zone. Taking into account the season of occurrence and trophic types of lakes, we can assume the thermophilic properties of T. dixonnuttalli and its confinement to mesoeutrophic waters. Based on the available data, we can classify T. dixonnuttalli as a beta-mesosaprobe, while according to Sládeček (1983) it is a well-pronounced oligosaprobe. According to the literature data, it occurs in reservoirs ranging from ultraoligotrophic to eutrophic, both among vegetation and in the pelagic zone (Jersabek and Bolortsetseg, 2010), as well as in psammon and moss (Kutikova, 1970; Naberezhny, 1984).
The species is widely distributed in the European part of Russia and the North Caucasus. Apparently it is absent or rare to the east of the Urals. It occurs in all zoogeographic regions, except for the Pacific and Antarctic.
Trichocerca elongata (Gosse 1886)
(Fig. 2a)
The first finds were recorded in the Middle Urals near Perm (Oborinskii pond, Oparina, 1923). Later it was found in the Kama River (Tauson, 1946) and lakes of its floodplain (Vershinin, 1953), in Bolshoi Kisegach, Sirikkul, and Chernenkoe lakes (data of A.O. Tauson and N.V. Bondarenko of the late 1930s, not published) and in Bolshoe Miassovo, Maloe Miassovo, and Bolshoi Elanchik lakes (author’s data) of the eastern foothills of the Southern Urals. It was also found in the Ural River (Akatova, 1954) and the Miass River (Shershnevskoe Reservoir, author’s data). The northernmost records are from the tributaries of the Lower Ob River (Bogdanov et al., 2004). According to our data, the body length is 357–395 µm; left toe, 280–300 µm; and right toe, 55–59 µm. Oparina (1923) provided a body length of 340 µm and the left toe length of 272 µm.
The species inhabits coastal water bodies, predominantly phytals and is often found among filamentous algae, aquatic mosses, and semi-submerged macrophytes (Kutikova, 1970; Jersabek and Bolortsetseg, 2010). According to our data, it is a stenobiont thermophilic species (2.1), found in warm waters during the warm season (from July to September). An inverse dependence of the average daily abundance of T. elongata on water temperature was noted (Ermolaeva et al., 2016). As Mexican researchers have shown, the temperature regime affects T. elongata cumulatively with the concentration of dissolved oxygen and chlorophyll a (Contreras et al., 2009). In addition to the water temperature, the pH value (pH) is also essential for T. elongata, however, only for the representatives inhabiting the littoral zone. In the pelagial, the concentrations of nitrates and phosphates are significant for the species (Vázquez-Sánchez et al., 2014). This agrees with the data of Chinese researchers who noted the preference of T. elongata for nutrient-rich lakes (Xue et al., 2014). Apparently, it is an euryhaline species, as it occurs not only in fresh and even ultrafresh waters (the lakes of the Urals), but also in saline ones (Saygi et al., 2011). It feeds on filamentous green algae (Oedogoniales), and possibly it is a monophagous species (Pourriot, 1970).
According to our data, T. elongata belongs to stenobiont mesotrophic species (individual trophic index 1.0). In relation to organic water pollution, it is a beta-mesosaprobe species (1.8). Here is a significant difference from the data for Central Europe (Sládeček, 1983), where the rotifer acts as an oligosaprobe species (1.0).
The highest abundance of the T. elongata population was recorded in September in the mesotrophic Lake Bolshoi Elanchik, 12 000 ind./m3. In general, this indicator varies between 400 and 2000 ind./m3. Like other species of the genus, this one is usually not numerous, apparently due to the rather narrow ecological range and its specific feeding habits. In warmer climates, this rotifer can be one of the dominant plankton species, especially in eutrophic waters (Xue et al., 2014), where it demonstrates good resistance to predation by planktivorous fish (Yoshida et al., 2003).
The species is known throughout Russia. It occurs in all zoogeographic regions, except for the Pacific and Antarctic.
Trichocerca gracilis (Tessin 1890)
Two finds are known in the Urals: in the Kama River (Tauson, 1946) and in the lakes of its floodplain (Vershinin, 1953). Currently, most researchers have recognized T. gracilis as a species inquirenda due to an unsatisfactory description (Segers, 2007). Unfortunately, it is not possible to determine which of the valid species was mentioned by A.O. Towson and N.V. Vershinin. However, we mention T. gracilis because future research will sooner or later answer this question.
Trichocerca iernis (Gosse 1887)
Two finds of this species are known in the Urals, in the Cis-Urals in the Kama River (Tauson, 1947) and in the Middle Urals, in Shartash Lake in Yekaterinburg (Balabanova, 1949). Data on the quantitative development have not been provided.
The species inhabits aquatic vegetation (Kutikova, 1970), in particular, Ceratophyllum (Green et al., 1984) and Utricularia (Jersabek and Bolortsetseg, 2010). In summer, it can reach a significant abundance, more than 300 000 ind./m3 (Gürbüzer et al., 2017). Although the species is recognized as eurythermal (Jersabek and Bolortsetseg, 2010), the distribution and development of T. iernis populations are positively affected by the water temperature, as well as the relatively high mineralization and alkaline conditions (pH up to 8.76, Jersabek and Bolortsetseg, 2010). At the same time it avoids nutrient-rich waters (Adamczuk et al., 2015). According to other data, this rotifer prefers shallow water bodies rich in dissolved organic matter (Arimoro and Oganah, 2010) like Oligosaprobe species (Sládeček, 1983).
The species is cosmopolitan, and is not found only in Antarctica. In Russia, it is known mainly to the west of the Urals. The rarity of its finds in the Urals could most likely be explained by the poor knowledge of the phytal zone of the majority of even well-studied water bodies.
Trichocerca longiseta (Schrank 1802)
(Fig. 2b)
The first records of the species were made as early as 1923 in the Ural region in Istochnoe, Osinovoe, and Peschanoe lakes and in the Yurchim River in Perm krai (Oparina, 1923). Then it was discovered in the Kama River (Tauson, 1947) and floodplain lakes of its middle part (Vershinin, 1953). In the southern Ural region, it can be found the Ural River (Akatova, 1954). In the Southern Urals, it was found in the lakes of the eastern foothills (Bolshoe Miassovo, Rogozin, 1995; Bolshoi Ishkul and Turgoyak, unpublished data of the author), and in the western foothills, in Minyarskii pond on the Sim River (Rogozin, 2007). Its range spreads up to the Polar Urals (lakes of the eastern macroslope and tributaries of the Lower Ob River, Bogdanov et al., 2004, 2005). According to our data, its body length is 221–355 µm; anterior spines are 13–18 µm and 46–49 µm; left toe, 112–186 µm; and right toe, 22–26 µm. Oparina (1923) reports the length of the body as 306 µm, the length of the left toe as 170 µm, and the largest anterior spine as 51 µm.
Like most other species of the genus, it inhabits the phytal zone in the coastal zone of water bodies, psammon, and is less common in plankton (Kutikova, 1970). A number of researchers consider it a periphytic species; it lives among filamentous green algae, sphagnum mosses, silt, and coarse-grained detritus in stagnant and flowing waters (Jersabek and Bolortsetseg, 2010). According to some studies, T. longiseta retains an equally high abundance both in the pelagic area and among macrophytes when the latter invade the open water zone (Sipaúba-Tavares et al., 2017). It prefers well-heated waters with an alkaline reaction and a high content of dissolved oxygen (Duggan et al., 1998).
Single records of the findings of the species do not allow us to make a conclusion about the ecology of the species in the Ural region. There are no quantitative data on it in the works of Ural hydrobiologists. According to the literature data, T. longiseta is a eurythermal, euryhaline species (Jersabek and Bolortsetseg, 2010), which inhabits clear waters (oligosaprobe, according to Sládeček, 1983). In our samples, the number of rotifers ranged from 50 to 1100 ind./m3. The species can reach a high population density and act as a subdominant in zooplankton communities (Kim and Joo, 2000).
It is distributed throughout Russia. The relative rarity of finds in the Urals is associated, as in cases with other Trichocerca, with poor knowledge of the inhabited biotopes and the relative low abundance. It occurs in all zoogeographic regions of the Earth, except for Antarctica.
Trichocerca porcellus (Gosse 1886)
(Fig. 3a)
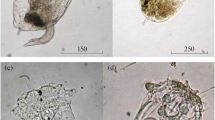
Trichocercidae from the South Ural water bodies: (a) Trichocerca porcellus (Gosse, 1886) from the Argazinskoe Reservoir on the Miass River; (b) T. rattus f. carinata (Ehrenberg 1830) from Sugoyak Lake; (c) T. similis (Wierzejski, 1893) from Bolshoi Elanchik Lake; (d) T. tenuior (Gosse 1886) from the Malyi Kizil River.
The first finds of this species were recorded in the early 1920s in Dikoe, Istochnoe, and Lasvinskoe lakes of the Kama River basin (Oparina, 1923). Later it was discovered in the Kama River and its floodplain lakes (Tauson, 1946, 1947; Vershinin, 1953). In the late 1930s thanks to fishery research carried out by hydrobiologists on the lakes of the Ufaleisk-Kasli and Kisegach-Miassovo groups in the eastern foothills of the Southern Urals (Bolshoi Irtyash, Bolshoi Ishkul, Bolshie Kasli, Kirety, Maloe Miassovo, Silach, Sinara, Sungul, etc.), numerous finds of this species were made (Maslennikova, 1941; unpublished materials by N.V. Bondarenko and A.O. Tauson). The latest detections of the species were made by the author in Arakul, Bolshoi Elanchik, Itkul, Bolshoe Miassovo, and Malyi Terenkul lakes of the eastern foothills of the Southern Urals and in the Argazinskoe Reservoir on the Miass River recently. According to our data, the body length is 123–157 µm; left toe, 48–57 µm; and right toe, 38–42 μm. Oparina (1923) provided a body length of 140–170 µm and length of toes of 50 µm.
According to the literature data, T. porcellus is an inhabitant of near-bottom areas and phytals of lakes, swamps, psammon, and periphyton; it also occurs in the pelagic zone (Kutikova, 1970). As an euplanktonic species, it was noted in eutrophic water bodies (Jersabek and Bolortsetseg, 2010); it is consistent with our own data: in Malui Terenkul Lake the species was found only in the plankton community. At the same time, it was noted in the pelagic zone of mesotrophic and oligotrophic lakes (Arakul, Bolshoi Elanchik, and Itkul). This is consistent with the data of Matveeva (Matveeva, 1986) on the mesotrophic Glubokoe Lake in Moscow oblast, where T. porcellus appeared in the pelagial zone in the 1980s; in South America, it often becomes one of the dominant species in oligotrophic water bodies (Schmid-Araya, 1993). In Bolshoye Miassovo Lake, it was found both in the psammon of the coastal zone and in the epilimnion of the pelagic zone. In general, in the studied lakes of the Urals, it behaves more like an euplankton species. According to our data, its body length is 130–144 μm, the left toe is 45–48 μm, and the right toe, 37–40 μm.
In relation to the temperature factor, the rotifer can be considered an eurythermal species (indicator weight, essentially meaning stenobiontic type, is very low, 1.33) with a slight preference for cold waters (individual thermoindex, 1.3). In the literature it is also characterized as a eurythermal species (Jersabek and Bolortsetseg, 2010). In relation to the trophism of the water body, in the Urals T. porcellus is a mesoeutroph (individual indicator value 1.6 with an average indicator weight of 2.96), which corresponds to the data on Estonian lakes (Mäemets, 1983). In relation to organic pollution, it is a beta-mesosaprobe species (individual indicator significance, 1.7 with a good indicator weight, 3.98), while in Europe it behaves like an oligosaprobe (1.2 according to Sládeček, 1983). According to the data of other researchers, T. porcellus generally demonstrates the features of a eurybiont with an indifferent attitude to environmental factors such as temperature and water transparency, mineralization, dissolved oxygen content, and concentration of nutrients (Adamczuk et al., 2015). Its resistance to acidic waters has also been noted (Jersabek and Bolortsetseg, 2010).
Obviously, due to its ecological plasticity, T. porcellus is one of the most abundant and widespread species of the genus in the Urals. The maximum abundance recorded by us is 10 800 ind./m3 in August, in the upper section of the Argazinskoe reservoir. The average for the entire array of samples is 2820 ± 1614 ind./m3. The first encounters of the species occur in June, and the main development occurs in the middle of summer, as well as in late autumn. For example, in the Argazinskoe Reservoir, the average abundance of the species in November was over 5500 ind./m3. Such seasonal dynamics results from the eurythermy of T. porcellus, which is also reflected in the vertical distribution of the species: it is abundant both in the well-heated epilimnion and in colder near-bottom water layers. This also confirms the data on the eurybiont nature of the species in relation to dissolved oxygen. So, in Bolshoe Miassovo Lake in August, the abundance of T. porcellus in the surface water layer (0–5 m) was 1000 ind./m3 at a temperature of 21.3°C and 105% oxygen saturation, and in the hypolimnion (15–20 m depth), it was 3800 ind./m3 at 8.3°С and 2% oxygen content.
The species is widespread in Russia, from the Arctic to the southern regions. It is distributed in all zoogeographic regions, except for the Pacific and Antarctic.
Trichocerca pusilla (Lauterborn 1898)
The first find of the species was recorded on the southern border of the region in the Ural River in Orenburg (Muraveiskii, 1923). Then it was found in many lakes of the eastern foothill limnological region of the Southern Urals: Bolshoi Irtyash, Bolshoi Ishkul, Bolshie Kasli, Kirety, Kundravinskoe, Maloe Miassovo, Silach, Sinara, Sirikkul, Sungul, etc. (Maslennikova, 1941 and unpublished materials of A.O. Towson). We found this species in Serebryi and Tabankul lakes of the South Urals. In the Middle Urals, it was found in the Kama River and floodplain lakes of its middle course (Kerentseva et al., 1946; Tauson, 1947; Vershinin, 1953). According to our data, the length is 65–105 µm and the left toe is 38–48 µm.
It is an euplankton species; however, it can also be found in potamoplankton among macrophytes (Kutikova, 1970; Jersabek and Bolortsetseg, 2010). Most of the finds of T. pusilla in the Urals were made in pelagic plankton. Unfortunately, the authors did not indicate the number of rotifers; visual estimates of abundance in unpublished works are “single” or “few.” Our own data are insufficient and do not allow the author to give a quantitative assessment of the ecological properties of the species in the Ural water bodies. Taking into account the summer occurrence (in most cases, June–August), the species can be assumed to be thermophilic. We recorded T. pusilla at a water temperature of at least 18°С. According to the literature, it is a summer or summer–autumn species (Herzig, 1987) and lives at temperatures above 10°C (Jersabek and Bolortsetseg, 2010) or even 12°C (May et al., 2001). There are notes about a temperature optimum for the development of the species in the range of 25–29°С (Yin et al., 2018). Fussmann (1993) calls it a “summer” stenothermic species. The importance of the temperature factor for T. pusilla has also been shown by other studies; in addition to warm waters, it also prefers a high oxygen content (Adamczuk et al., 2015). Although it is believed that the species is euryhaline (Jersabek and Bolortsetseg, 2010) and tolerates mineralization up to 4 g/m3 (Bielańska-Grajner and Cudak, 2014), in the Urals it has only been found in fresh waters.
Trichocerca pusilla was assigned to mesoeutrophic (Mäemets, 1983) or typically eutrophic species (Kuczyńska-Kippen and Pronin, 2018; Yin et al., 2018). Some scientists even refer T. pusilla to an indicator species of eutrophic conditions (Cannon and Stemberger, 1978). The abundance of the species positively correlates with the content of nitrates, nitrites, and phosphates in water (Plangklang et al., 2019). However, according to other data, T. pusilla avoids a high content of nitrogen and phosphorus (Adamczuk et al., 2015). The vast majority of lakes in the Urals in which it is found belong to the mesotrophic or oligomesotrophic type. However, as already mentioned, it was found in them singly or in small abundance. Our findings in the mesoeutrophic Serebryannoe Lake and in eutrophic Tabankul Lake show the abundance of the species ranging from 15 000 to 38 000 ind./m3. In the mesotrophic Chinese Xianhu Lake with signs of eutrophy, T. pusilla was recorded in abundance of over 10 000 ind./m3, and it was one of the dominant species (Wen et al., 2017). Obviously, this rotifer prefers eutrophic waters, but also lives in other types of water bodies up to oligomesotrophic ones. Moreover, its indicator properties correspond to oligosaprobity (1.3, according to Sládeček, 1983).
The abundance of representatives of Trichocerca over 10 000 ind./m3 is very significant for the Ural water bodies; however, T. pusilla may be more abundant. According to May et al. (2001), the species feeds on colonial diatoms of the genus Aulacoseira, the development of which mainly determines the number of rotifers, which reaches a peak of 1–3 million ind./m3 during the “blooming” period and falls to 100 000 ind./m3 during the depression of algae. Aulacoseira are widespread and abundant in the water bodies of the region; however, such outbreaks of T. pusilla are not observed here. Perhaps the fact is that the development of these diatoms in the lakes of the Urals occurs at a water temperature below the optimum level for T. pusilla.
The species is widespread in Russia. It is distributed in all zoogeographic regions of the Earth, except for the Antarctic.
Trichocerca rattus (Müller 1776)
(Fig. 3b)
This species is represented in the Urals by two forms: in addition to the typical T. rattus f. rattus (Müller 1776), there is T. rattus f. carinata (Ehrenberg 1830). The first find in the Urals was recorded in Peschanoe Lake near Perm (Oparina, 1923, typical form). In the Middle Urals, it was later found in the Kama River (Kerentseva et al., 1946, typical form; Tauson, 1946, T. rattus f. carinata). In the Southern Urals, both forms are also known: in Karmakkul, Savelkul, and Sirikkul lakes of the foothills of the Ilmenskii Range and in the forest–steppe Kundravinskoe Lake (unpublished data by A.O. Tauson and our own data) as a typical fort; in the forest–steppe Sugoyak Lake in the vicinity of Chelyabinsk, as T. rattus f. carinata (own data). Trichocerca rattus spreads up to the Polar Urals, where it is found in the reservoirs of the Kara River basin (Bogdanov, 2003) and in the lakes of its eastern macroslope (Bogdanov et al., 2004). According to our data, the typical body length is 182–187 µm, and that of the left toe, 170–172 µm; f. carinata body length is 157–168 µm, and its left toe, 142–148 µm. Oparina (1923) gives a body length of 204 µm for a typical form, and 153 µm for its left toe.
It is a periphytic and benthic species, found mainly in swampy water bodies, rarely in plankton and psammon (Jersabek and Bolortsetseg, 2010). In Savelkul Lake (foothills of the Ilmenskii Range in the Southern Urals), it was found by us in sandy peaty soil overgrown with fontinalis (Fontinalis antipyretica) and reeds (Phragmites australis) at a depth of 0.2 m. In the forest–steppe Sugoyak Lake in the vicinity of Chelyabinsk, it is found in the coastal wetland among reeds, Eleocharis mamillata, and sedges (Carex spp.).
The lack of data and the absence of literature data on the quantitative occurrence of the species in the water bodies of the region do not allow us to assess the ecological properties of the species in the Urals. According to published sources, it is a eurythermal species with some preference for cold waters (Jersabek and Bolortsetseg, 2010) or even cryophilic species (Segers, 2003), which is consistent with data on the distribution of the species up to the Polar Urals (a rare case for representatives of the genus Trichocerca). It is one of the few rotifer species found in Antarctica. With respect to the oxygen content and pH of the water, the species exhibits features of an eurybiont (Jersabek and Bolortsetseg, 2010). It feeds on colonial filamentous algae, breaking the filaments and sucking out the cell contents (Ecology and General Biology, 2014). Like many other species confined to marsh habitats, T. rattus is a pronounced oligosaprobe (1.0–1.1, Sládeček, 1983).
According to our observations, the abundance of T. rattus varies from 600 to 9000 ind./m3 and it is more numerous near the surface of the substrate than in open water. This agrees with the data of Harney et al. (2013): in one of the Indian ponds, the abundance of the species in the periphyton reached 8000–9000 ind./m3. According to visual estimates, the abundance of T. rattus in the unpublished works of Ural hydrobiologists is defined as “single,” “very few,” and “not abundant,” while in Sirikkul Lake it is one of the main species of zooplankton in terms of occurrence.
The species is widespread in Russia. It is distributed in all zoogeographic regions of the Earth.
Trichocerca rousseleti (Voigt, 1902)
The first find was recorded for Malyi Kisegach Lake in the eastern foothills of the Southern Urals (1938, unpublished materials by N.V. Bondarenko). In the Cis-Urals, it was found in the Kama River and its floodplain lakes (Tauson, 1946; Vershinin, 1953). The authors do not provide quantitative data on T. rousseleti.
The rarity of finds of this species in the Urals is surprising, since it inhabits the biotope most studied by hydrobiologists, the plankton of lakes and rivers, where it is often one of the dominant species (Yoshida et al., 2000; Haberman and Künapp, 2002). In 35 years of studying the South Ural plankton, we have not encountered it.
Apparently, it is an eurythermal species. On the one hand, it belongs to typical summer lakes in the northern hemisphere (Herzig, 1987) and reaches its maximum abundance in summer when the water is warmest, as, for example, in large Estonian lakes (Haberman and Künapp, 2002), when in August it is up to 20% of all zooplankton abundance (Haberman, 1995). On the other hand, it is noted as cold-water (Segers, 2003). The abundance of T. rousseleti can be regulated by copepods, in particular Eurytemora affinis (Brandl, 2005).
It was noted as an indicator of eutrophy by Estonian researchers (Haberman and Künapp, 2002; Haldna and Haberman, 2014), but it occurs in lakes of all trophic types from eutrophic to ultraoligotrophic (Jersabek and Bolortsetseg, 2010). Malyi Kisegach Lake, where the only find of T. rousseleti was recorded in the Southern Urals, is oligomesotrophic. It is unlikely that the species can be considered an indicator of eutrophy; at least it could not be an indicator species outside of Estonia. The oligosaprobe (1.0) status of the species with a high indicator weight (5) (Sládeček, 1983) is also inconsistent with the characterization of an indicator of eutrophy.
It is distributed throughout Russia, as well as in all zoogeographic regions, except for the Neotropical, Antarctic, and Pacific regions.
Trichocerca ruttneri Donner 1953
The only record of the species in the Urals was made by the author in a swampy lacustrine derivative in the Bolshoe Miassovo Lake basin the eastern foothills of the Ilmenskii Range in the Southern Urals. Dimensions: body length 120 µm; left toe, 51 µm; right toe, 22 µm. It mainly inhabits the plankton of lakes (Kutikova, 1970), where it can be one of the numerous and dominant species (Dagne et al., 2008; Dorak, 2013). In the above-mentioned work by Dagne et al., T. ruttneri reaches its maximum abundance (up to 200 000 ind./m3) in June, and this occurs in the subequatorial climate. As a summer species, the abundance of which significantly depends on the water temperature, T. ruttneri is also mentioned in the last cited work devoted to water bodies of the subtropics. Segers (2003) considers it as a warm water species. In Estonian water bodies, it was found only in June and July (Viro and Haberman, 2005). Obviously, the species is a thermobiont found at temperatures of 13.5°C and above (Jersabek and Bolortsetseg, 2010). Possibly, the warm-loving nature of the species, with its affinity for areas of subtropical, tropical, and subequatorial climate, explains the rarity of its finds both in the Urals and on the territory of Russia. Nevertheless, T. ruttneri is a globally distributed species, unknown only in the Pacific and Antarctic.
Trichocerca sejunctipes (Gosse 1886)
The only find in the Urals was recorded in the eastern foothills of the Ilmenskii Range in the Southern Urals in Maloe Terenkul Lake (Rogozin, 2009a). The species was designated as species inquirenda by Segers (2007). Accurate identification of the species requires further study.
Trichocerca similis (Wierzejski 1893)
(Fig. 3c)
All the findings of this species were made only in the Southern Urals in the lakes of the eastern foothills and the adjacent forest–steppe. The first reports date back to the mid-1970s, when T. similis was discovered by an expedition of Leningrad hydrobiologists in Argayash, Maloe Miassovo, and Kundravinskoe lakes (Makartseva, 1978; Drabkova and Sorokin, 1979). Later it was found in other lakes of this area: in Bolshoe Miassovo (Rogozin, 2000), Tabankul (Rogozin, 2006), Uvildy (Rogozin, 2009), Bolshoi Elanchik, Elovoe, Itkul, Bolshoi Ishkul, Bolshoi Kisegach, Malyi Terenkul, and Turgoyak lakes, and also in the Argazinskoe and Shershnevskii reservoirs on the Miass river (own data).
According to our data, the body length is 128–154 µm; the length of the left toe, 39–49 µm; and the length of the right toe, 24–32 µm. The length of the anterior spines is 24–30 µm.
It is a euplankton species of freshwater lakes and ponds, including waterlogged ones (Jersabek and Bolortsetseg, 2010). According to some reports, it prefers polyhumic water bodies (Mäemets, 1983; Pejler and Bērziņš, 1993) and can even be considered an indicator of such waters (Steinberg, 2003). Often found among aquatic vegetation, in potamoplankton (Kutikova, 1970; Jersabek and Bolortsetseg, 2010). All our finds of the species in the Urals belong to the pelagic and, more rarely, littoral areas of rather large lakes and, as a rule, are not associated with either vegetation or swampy waters. This is one of the most common planktonic rotifers from Trichocercida, the second in frequency of occurrence after T. capucina (7.6% of the entire array of samples).
In relation to the temperature factor, according to our studies, this rotifer is a thermobiont (the species thermal index is 2.8 with a stenobiont index 4.03). It can be found in summer from July to September (extremely rarely in June and October), and mainly in August, when the water in the Ural lakes reaches its maximum heating. As a rule, in August the species has not only the highest occurrence level, but also is the most numerous. In a less severe climate, the seasonal development of T. similis is much longer, but even there, T. similis is characterized by a summer–autumn peak in abundance, when the species can be one of the dominant zooplankton species (Eckert and Walz, 1998; Ramírez-Garćia et al., 2002), including in strongly heated (over 30°С) water bodies (Plangklang et al., 2019). At the same time, there is evidence that the species is eurythermal (Jersabek and Bolortsetseg, 2010), and even that the abundance of T. similis decreases with increasing water temperature (Nandini et al., 2005). Nevertheless, under the conditions of the Urals, T. similis manifests itself as a pronounced thermobiont. This is confirmed not only by the seasonal dynamics, but also by the vertical distribution of T. similis in the water column, which we studied in Bolshoe Miassovo Lake (foothills of the Ilmenskii Range in the vicinity of Miass). The vertical distribution is obviously determined by the thermobiont nature of the species: the highest abundance was recorded in the epilimnion (Fig. 4a); below the thermocline, the abundance of the species sharply decreases and becomes minimal in the bottom, coldest layers. Similar results were obtained in studies of water bodies in Mexico (Nandini et al., 2008). In general, as shown by Devetter (1998), the temperature factor is one of the most significant for T. similis.
The abundance of the species also correlates positively with the concentration of dissolved oxygen (Plangklang et al., 2019). This rotifer prefers fresh waters, although it can also be found in brackish waters (Bielańska-Grajner and Cudak, 2014). We found T. similis only in fresh and even ultra-fresh lakes of the Southern Urals. Among other abiotic environmental factors, T. similis is favorably affected by an increased content of phosphates and ammonium, and this rotifer apparently avoids water bodies with high transparency (Adamczuk et al., 2015, Czerniawski et al., 2013). The latter is not surprising, since T. similis, according to the literature data, is confined to eutrophic conditions (Frutos et al., 2009), and can even occur in hypertrophic water bodies (Mäemets, 1983). However, in the Urals, the rotifer manifests itself as a mesoeutroph (individual indicator value 1.5 with indicator weight 3.55). This also corresponds to the fact that it is an alpha-mesosaprobe under the conditions of the Urals (indicator significance 3.0, indicator weight 4.92). According to Sládeček (1983), T. similis is an oligosaprobe species in water bodies of Europe (indicator significance 1.3, indicator weight 4). Oligosaprobity does not associate well with confinement to eutrophic and hypertrophic waters and, in this case, is most likely associated with the habitation of T. similis in the humified water bodies of Europe. Obviously, the biology of the species differs in different landscape–geographical zones of the Earth.
Like other species of the genus, T. similis is an algal feeder with a wide range of consumed algae; in polyhumous water bodies it feeds on golden algae (Chrysophyta) (Pejler and Bērziņš, 1993), which are abundant in such waters.
According to many researchers, being one of the usual components of zooplankton, T. similis is usually not numerous and its abundance is usually 1000–1200 ind./m3 (Ramírez-Garćia et al., 2002; Güher, 2019; Nandini et al., 2008). Over the entire array of our samples, the average abundance of the species was 10 836 ± 9302 and the maximum abundance was 206 400 ind./m3 (hypereutrophic Tabankul Lake in the eastern foothills of the Ilmenskii Range), which significantly exceeds the value usually indicated for T. similis.
If we consider the seasonal dynamics of the species, we can once again note the summer (July–August) population peak, which is atypical for most planktonic rotifers (Fig. 4b). Usually during this period there is a competitive exclusion of rotifers by cladocera, which reach the greatest abundance in the middle of summer. In this case, as we see (Fig. 4b), there is no competition for food resources, but on the contrary, the synchronous dynamics of T. similis and Cladocera is observed, which is apparently determined by a similar response to the temperature factor. According to the available data, T. similis does not experience competitive displacement typical of rotifers from small cladocera (Eckert and Walz, 1998) and daphnia (Gilbert, 1989), which is confirmed by our observations. It is known that predatory cladocera and larvae of Chaboorus significantly affect the population of T. similis (Devetter, 1998; Wallace and Starkweather, 1983); however, the summer abundance of adult copepods and coretra in the studied lakes is very low. The question of how T. similis avoids competitive exclusion by cladocera requires additional research.
It is a widespread species known throughout the European territory of Russia, and some finds were recorded in the Urals, Siberia, and the Far East. It is found in all zoogeographic regions of the Earth, except Antarctica.
Trichocerca stylata (Gosse 1851)
The first finds of the species in the Urals were made at the beginning of the 20th century (Furman and Tiebo, 1910) in Bolshoy Irtyash, Kazhakul, Bolshie Kasli, Kirety, and other lakes of the eastern foothills of the Southern Urals. Later it was found in almost all the lakes of this region studied belonging to the Kasli and Kisegach-Miassovo lake systems (Maslennikova, 1941; unpublished materials by N.V. Bondarenko, A.O. Tauson, and our own data). It was recorded in the forest–steppe Trans-Urals (Kundravinskoe Lake, Makartseva, 1978). It was also found in more southern regions: in the basins of the Ural and Sakmara rivers in Orenburg oblast (Muraveisky, 1923). In the Cis-Urals, it is registered in the Kama River and the lakes of its basin (Oparina, 1923; Tauson, 1934; Kerentseva et al., 1946; Vershinin, 1953); in the Middle Urals, it was found in Bolshoi Shartash Lake in the vicinity of Yekaterinburg (Balabanova, 1949).
The sizes of the studied individuals from Bolshoi Kisegach Lake are body length 131–152 µm, the length of the left toe is 43–47 µm, and the right toe is rudimentary. Oparina (1923) provided the length of the body as 130 µm, the length of the left toe as 35–43 µm, and the length of the anterior spine as 17 µm.
It is a euplankton species in fresh lakes and rivers, less common in potamoplankton, in marshy and brackish waters, according to some data, halophobe species (Kutikova, 1970; Jersabek and Bolortsetseg, 2010).
In all works on the Urals in which T. stylata is mentioned, its abundance is not indicated; our data are insufficient to determine the autoecological characteristics of the species. According to the literature data, this, like most Trichocerca, is a summer stenothermic–warm water species (Herzig, 1987; Bērziņš and Pejler, 1989). There are also data on the spring maximum development of T. stylata (May and O’Hare, 2005). In the Ural lakes and rivers, it was found mainly in July and August during the period of maximum water heating. It has also been noted that it is confined to neutral and weakly alkaline waters (Bērziņš and Pejler, 1987), which, by the way, are characteristic of the foothill water bodies of the Urals.
Some authors consider T. stylata an indicator of hypertrophic conditions (Rosińska et al., 2019), although the species is characterized by a negative reaction to a high nitrate content in water (Mantovano et al., 2019); and according to Sladeček (Sládeček, 1983), T. stylata is an oligosaprobe species (indicator significance 1.3, indicator weight 4). All finds in the Southern Urals were made in lakes of the mesotrophic or oligotrophic type, and it has never been found in eutrophic or even more hypertrophic lakes.
The only finding of T. stylata with quantitative data was made by us in the oligotrophic Bolshoi Kisegach Lake in the eastern foothills of the Ilmenskii Range in the Southern Urals in August in the littoral zone (abundance 8400 ind./m3, biomass 0.0075 g/m3). In most of the lakes of the Southern Urals, according to the authors’ estimates, the abundance of T. stylata is characterized as “single” or “rare.” One of the main forms of zooplankton in terms of occurrence in the amount of “many,” the species was found only in Kundravinskoe (forest–steppe, with increased mineralization) and Bolshoi Ishkul lakes (ultrafresh foothill) (Rogozin, 1995). As one of the dominant species, it was noted by many authors in water bodies of different natural and geographical zones (May and O’Hare, 2005; Frutos et al., 2009; Mantovano et al., 2019; etc.).
In Russia, it is ubiquitous. It is found in all zoogeographic regions of the Earth, except Antarctica.
Trichocerca sulcata (Jennings 1894)
This species was found in the eastern foothills of the Ilmenskii Range in the Southern Urals in Argayash and Ilmenskoe lakes (unpublished data from A.O. Tauson) and in the Kama River (Towson, 1946).
An inhabitant of the coastal zone of water bodies among aquatic vegetation (Kutikova, 1970), it can be considered as a quiescent plankton (Miracle et al., 1995). It is classified as an oligosaprobe species (1.1) with a high indicator weight (5) (Sládeček, 1983).
It is rarely found in Russia, but is known at least from European territory to Altai. It is distributed in the Holarctic, Neotropical, and Australo-Polynesian zoogeographic regions.
Trichocerca tenuior (Gosse 1886)
(Fig. 3d)
The first findings of this species in the Urals in the reservoirs of the Kama basin and in the Kama River itself date to the early 1920s (Oparina, 1923). It was later repeatedly recorded in these habitats (Tauson, 1946, 1947; Vershinin, 1953). In the eastern foothills of the Southern Urals, we found it in Bolshoi Ishkul and Bolshoe Miassovo lakes (data not published), in the Argazinskoe Reservoir on the Miass River (Rogozin, 2013), and in the Malyi Kizil River (tributary of the Ural River; own data). It is still unknown in other areas, but it was found near the borders of the Polar Urals on the Yamal Peninsula (Bogdanov et al., 1997). According to our data, the body length is 170–187 µm, the length of the left toe is 54–55 µm, and the length of the right toe is 35–37 µm. Oparina (1923) noted a body length of 170 µm, the left toe length of 69 µm, and that the right toe of 40 µm.
Trichocerca tenuior is an inhabitant of psammon and periphyton in the littoral of water bodies, in ponds and swamps, and is rarely found in open water (Kutikova, 1970; Segers, 2003). It was found both in the summer months during the period of strong water heating and in late autumn when the water cools below 8°C, which corresponds to its eurythermy (Jersabek and Bolortsetseg, 2010; Bertani et al., 2011). This is also confirmed by its distribution to the Arctic. It prefers neutral waters with pH 7 (Bērziņš and Pejler, 1987). The distribution and development of the species is affected by the flow of the water body: when it increases in the littoral zone, T. tenuior disappears from plankton (Vizer et al., 2016). Trichocerca tenuior belongs to oligo-beta-mesosaprobes (Sládeček, 1983); the indicator properties of the species are low.
The largest abundance of T. tenuior recorded by us was 1200 ind./m3 (upper section of the Argazinskoe Reservoir). For most phyto- and psammophilous species of Trichocerca, this is a common value; according to other studies, T. tenuior may be one of the dominant species in the phytal zone (Duggan et al., 1998).
In Russia it is widespread. The species is found everywhere except Antarctica.
Trichocerca tigris (Müller 1786)
(Fig. 5a)
For the first time this species was identified in the Urals in the Kama River and floodplain lakes of its basin in the late 1940s–early 1950s (Tauson, 1946, 1947; Vershinin, 1953). In the Southern Urals, the species was found by the author in Bolshoe Miassovo Lake (eastern foothills of the Ilmenskii Range) in a small overgrown bay in July. It is unknown in other regions of the Urals, but was found near the borders of the Polar Urals in the lower Ob and the Gulf of Ob (Ermolaeva, 2017).
According to our data, the body length is 231 µm, and that of the toes is 105–107 µm. There are no data on the quantitative development in the Ural water bodies.
Like many other Trichocerca, it inhabits stagnant and flowing waters, swamps (Kutikova, 1970), and temporary reservoirs (Kulikova, 2015). It occurs among detritus, in sand, in mosses, and on macrophytes in the periphyton (Jersabek and Bolortsetseg, 2010). It may be a part of the commensal complex on bivalves (Bołtruszko, 2010). In many European water bodies, T. tigris is one of the dominant species in the littoral zone (The Production Ecology…, 2009). Like other species of the genus, it is an oligosaprobe with a good indicator weight (Sládeček, 1983).
The species is widespread in Russia and has a cosmopolitan distribution, including the Antarctic.
Trichocerca weberi (Jennings 1903)
(Fig. 5b)
This species was recorded for the first time in the Urals, in a bog on the banks of the Kama River (Oparina, 1923). Further few finds were made in the Southern Urals: in Bolshoe Miassovo Lake in the foothills of the Ilmenskii Range (Rogozin and Shchetinina, 1989) and in the Argazinskoe Reservoir on the Miass River (Rogozin, 2013). According to our data, the body length is 92–97 µm; the toe length, 34–36 µm. Oparina (1923) provided a body length of 125 µm.
It inhabits ponds and bogs (Kutikova, 1970), littoral of lakes among macrophytes, mosses, detritus, less often in open water, in the pelagic zone (De Smet, 1993; Jersabek and Bolortsetseg, 2010). We found the species representatives in the pelagic area of water bodies at a depth of 3 to 22 m at a temperature of 6.9 to 24.9°С. The wide temperature range of occurrence of the species was also noted previously; some authors consider it a eurythermal species (Jersabek and Bolortsetseg, 2010). We consider T. weberi as a cryophilic species (1.0) with a stheno/eurybiont index of 2.0, which prefers waters with a temperature not exceeding 13°С. Indirectly, such biological properties of the species are confirmed by the fact of its distribution beyond the Arctic Circle (Ermolaeva, 2017). This rotifer lives in a wide range of oxygen concentrations (from 2 to 13 mg/L), and the optimum concentration seems to be 8 mg/L (Bērziņš and Pejler, 1989). The pH range (5.96–8.95) at which the species is found is also wide (Jersabek and Bolortsetseg, 2010), which allows it to live in both acid marsh and alkaline waters during the period of mass algae bloom. There is apparently no information on the association of T. weberi with waters of a certain trophicity. We found the species in mesotrophic conditions; there are data on its occurrence in eutrophic and dystrophic (obviously, oligodystrophic) water bodies (Jersabek and Bolortsetseg, 2010). Sládeček (1983) identifies T. weberi as an excellent indicator of oligosaprobic waters (1.1).
Trichocerca weberi is one of the few carnivorous rotifers; it reaches its greatest abundance under conditions of mass development of detritivorous species and algophages (Cervantes-Martinez and Gutiérrez-Aguirre, 2015). The number of T. weberi, like most species of the genus, is usually small. We have registered values from 600 to 3700 ind./m3. Such indicators correspond to the data of other researchers: in the water bodies of Poland, 1000 ind./m3 (Goździejewska and Tucholski, 2011), and in water bodies of Mexico, under favorable conditions, it can reach an abundance of 5000 ind./m3 (Cervantes-Martinez and Gutiérrez-Aguirre, 2015). The tychoplankton character of the species is confirmed by the data on its vertical distribution, which we studied in the summer period in Bolshoe Miassovo Lake in the eastern foothills of the Ilmenskii Range in the Southern Urals (Fig. 6). Despite the occurrence throughout the entire water column, except for the surface layer, T. weberi is concentrated in deep layers, well below the thermocline. The highest abundance of the species (3700 ind./m3) was recorded in the same lake at a depth of 10 m in the littoral zone (the bottom is 10.5 m).
The species is widespread in Russia and has a cosmopolitan distribution (except Antarctica).
The genus Trichocerca in the Urals is represented by species that are widespread both in Russia and throughout the planet. Most of them are inhabitants of small, often swampy water bodies and bogs, the overgrown littoral of lakes and rivers, living in periphyton, psammon, and bottom detritus, and only a few belong to euplankton species or occasionally occur in plankton. The works of the Ural hydrobiologists are mainly devoted to the study of plankton. The combination of these circumstances leads to the fact that a significant number of species of Trichocerca have been found in the Urals; however, the findings of most of them are rare, and information on the quantitative development is fragmentary or completely absent. Only a few euplankton species (T. capucina, T. cylindrica, T. porcellus, and T. similis) have been relatively well studied. It should also be taken into account that rotifers of the Trichocercidae family rarely form numerous populations, and the probability of finding them in routinely processed hydrobiological samples is not very high. Autecological characteristics of the Ural representatives of Trichocerca are given in Table 1.
The distribution of most species of the genus tends to the southern regions of the Urals. Obviously, this is due to the thermophilicity of many of them. Even those Trichocerca representatives that are recognized as eurythermal in the rotatoriological literature, in fact may not be so. The presence of a species in a wide range of temperatures does not necessarily mean that it is eurythermal; what matters is how its abundance and occurrence are distributed along the gradient of the temperature factor (Rogozin et al., 2015). According to our data, this distribution for Trichocerca is shifted towards warm waters.
The lack of research in the Urals of many characteristic habitats of Trichocercidae suggests that the list of species of this family will still be replenished with new finds. Most likely, T. intermedia (Stenroos 1898), which was recorded in the adjacent territory in Northern Kazakhstan in the overgrown eutrophic Sitovo Lake (Ermolaeva, 2013); T. lophoessa (Gosse 1886) and T. mucosa (Stokes 1896), known from the water bodies of the Volga region; and T. uncinata (Voigt 1902), which inhabits pondweeds in the phytal zones of lakes, psammon, and swamp habitats, and is found in water bodies of both west and east of the Urals, will be found.
This study once again showed how poorly the hydrofauna of the Urals has been studied. In fact, this result of a 110-year study of local water bodies provides only the very initial basis for further work to identify the biological diversity of this vast territory.
REFERENCES
Adamczuk, M., Mieczan, T., Tarkowska-Kukuryk, M., and Demetraki-Paleolog, A., Rotatoria–Cladocera–Copepoda relations in the long-term monitoring of water quality in lakes with trophic variation (E. Poland), Environ. Earth Sci., 2015, vol. 73, pp. 8189–8196.
Akatova, N.A., Study of the zooplankton of the Ural River and some floodplain water bodies in the vicinity of Yanvartsevo Village, West-Kazakhstan region, Tr. Zool. Inst. Akad. Nauk SSSR, 1954, vol. 16, pp. 517–531.
Andronikova, I.N., Strukturno-funktsional’naya organizatsiya zooplanktona ozernykh ekosistem raznykh troficheskikh tipov (Structural and Functional Organization of Zooplankton in Lake Ecosystems of Different Trophic Types), St. Petersburg: Nauka, 1996.
Arimoro, F.O. and Oganah, A.O., Zooplankton community responses in a perturbed tropical stream in the Niger delta, Nigeria, Open Environ. Biol. Monit. J., 2010, vol. 3, pp. 1–11.
Balabanova, Z.M., Materials for Lake Bolshoi Shartash, Tr. Ural. Otd. VNIIORKh, 1949, vol. 4, pp. 75–128.
Baloch, W.A., Maeda, H., and Saisho, T., Seasonal abundance and vertical distribution of zooplankton in Lake Ikeda, Southern Japan, Microbes Environ., 1998, vol. 13, no. 1, pp. 1–8.
Barrabin, J.M., The rotifers of spanish reservoirs: ecological, systematical and zoogeographical remarks, Limnetica, 2000, vol. 19, pp. 91–167.
Bertani, I., Segers, H., and Rossetti, G., Biodiversity down by the flow: new records of monogonont rotifers for italy found in the po river, J. Limnol., 2011, vol. 70, no. 2, pp. 321–328.
Bērziņš, B. and Pejler, B., Rotifer occurrence in relation to pH, Hydrobiologia, 1987, vol. 147, pp. 107–116.
Bērziņš, B. and Pejler, B., Rotifer occurrence in relation to temperature, Hydrobiologia, 1989, vol. 175, pp. 223–231.
Bielańska-Grajner, I. and Cudak, A., Effects of salinity on species diversity of rotifers in anthropogenic water bodies, Pol. J. Environ. Stud., 2014, vol. 23, no. 1, pp. 27–34.
Bogdanov, V.D., Bogdanova, E.N., Golovatin, M.G., Dobrinskii, N.L., Korytin, N.S., Kryazhimskii, F.V., Magomedova, M.A., Mel’nichenko, I.P., Morozova, L.M., Paskhal’nyi, S.P., Sosin, V.F., and Shtro, V.G., Monitoring bioty poluostrova Yamal v svyazi s razvitiem ob’’ektov dobychi i transportirovki gaza (Monitoring of the Biota of the Yamal Peninsula in Connection with the Development of Gas Production and Transportation Facilities), Yekaterinburg: URC Aerokosmologiya, 1997.
Bogdanov, V.D., Bogdanova, E.N., Gos’kova, O.A., Mel’nichenko, I.P., Stepanov, L.N., and Yarushina, M.I., Bioresursy vodnykh ekosistem Polyarnogo Urala (Bioresources of Aquatic Ecosystems of the Polar Urals), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 2004.
Bogdanov, V.D., Bogdanova, E.N., Gavrilov, A.L., Mel’nichenko, I.P., Stepanov, L.N., and Yarushina, M.I., Ekologicheskoe sostoyanie pritokov Nizhnei Obi (reki Kharbei, Longot’’egan, Shchuch’ya) (Environmental State of the Lower Ob Tributaries (Kharbei, Longotegan, and Shchuchya Rivers)), Yekaterinburg: Ural. Univ., 2005.
Bogdanova, E.N., To the study of zooplankton of the Polar Urals (zooplankton of the Kara River basin), Biol. Resur. Polyarn. Urala, 2003, no. 3, part 2, pp. 23–29.
Bołtruszko, J.M., Epizoic communities of rotifera on freshwater bivalves, Int. J. Oceanogr. Hydrobiol., 2010, vol. 39, no. 4, pp. 75–82.
Brandl, Z., Freshwater copepods and rotifers: predators and their prey, Hydrobiologia, 2005, vol. 546, pp. 475–489.
Cannon, J.E. and Stemberger, R.S., Zooplankton (especially crustaceans and rotifers) as indicators of water quality, Transact. Am. Microsc. Soc., 1978, vol. 97, no. 1, pp. 16–35.
Cervantes-Martinez, A. and Gutierrez-Aguirre, M.A., Physicochemistry and zooplankton of two karstic sinkholes in the Yucatan Peninsula, Mexico, J. Limnol., 2015, vol. 74, no. 2, pp. 382–393.
Chittapun, S., Pholpunthin, P., and Segers, S., Diversity of rotifer fauna from five coastal peat swamps on Phuket Island, Southern Thailand, Sci. Asia, 2007, vol. 33, pp. 383–387.
Contreras, J.J., Sarma, S.S.S., Merino-Ibarra, M., and Nandini, S., Seasonal changes in the rotifer (Rotifera) diversity from a tropical high altitude reservoir (Valle de Bravo, Mexico), J. Environ. Biol., 2009, vol. 30, no. 2, pp. 191–195.
Czerniawski, R. and Domagała, J., Zooplankton communities of two lake outlets in relation to abiotic factors, Cent. Eur. J. Biol., 2010, vol. 5, no. 2, pp. 240–255.
Czerniawski, R., Pilecka-Rapacz, M., and Domagała, J., Zooplankton communities of inter-connected sections of lower River Oder (NW Poland), Cent. Eur. J. Biol., 2013, vol. 8, no. 1, pp. 18–29.
Dagne, A., Herzig, A., Jersabek, C.D., and Tadesse, Z., Abundance, species composition and spatial distribution of planktonic rotifers and crustaceans in Lake Ziway (Rift Valley, Ethiopia), Int. Rev. Gesamten Hydrobiol. Hydrogr., 2008, vol. 93, no. 2, pp. 210–226.
Devetter, M., Influence of environmental factors on the rotifer assemblage in an artificial lake, Hydrobiologia, 1998, vols. 387/388, pp. 171–178.
Dorak, Z., Zooplankton abundance in the lower Sakarya River basin (Turkey): impact of environmental variables, J. Black Sea/Mediterr. Environ., 2013, vol. 19, no. 1, pp. 1–22.
Drabkova, V.G. and Sorokin, I.N., Ozero i ego vodosbor – edinaya prirodnaya sistema (The Lake and Its Watershed Are a Single Natural System), Leningrad: Nauka, 1979.
Duggan, I.C., Green, J.D., Thompson, K., and Shiel, R.J., Rotifers in relation to littoral ecotone structure in Lake Rotomanuka, North Island, New Zealand, Hydrobiologia, 1998, vol. 387, pp. 179–197.
Eckert, B. and Walz, N., Zooplankton succession and thermal stratification in the polymictic shallow Muggelsee (Berlin, Germany): a case for the intermediate disturbance hypothesis?, Hydrobiologia, 1998, vols. 387/388, pp. 199–206.
Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates, Amsterdam: Academic, 2014, vol. 1.
Ejsmont-Karabin, J., Rotifer occurrence in relation to age, depth and trophic state of quarry lakes, Hydrobiologia, 1995, vols. 313/314, pp. 21–28.
Frutos, M.S., Neif, P.D., and Neif, J.J., Zooplankton abundance and species diversity in two lakes with different trophic state (Corrientes, Argentina), Acta Limnol. Bras., 2009, vol. 21, no. 3, pp. 367–375.
Furman, O. and Tiebo, M., Fauna of some Ural lakes: preliminary note, Tr. Ural. O-va Lyubitelei Estestvoznan., 1910, vol. 30, pp. 69–82.
Fussmann, G., Abundance, succession and morphological variation of planktonic rotifers during autumnal circulation in a hypertrophic lake (Heiligensee, Berlin), Hydrobiologia, 1993, vols. 255/256, pp. 353–360.
Ganf, G.G., Shiel, J.J., and Merrick, C.J., Parasitism: the possible cause of the collapse of a Volvox population in Mount Bold Reservoir, South Australia, Aust. J. Mar. Freshwater Res., 1983, vol. 34, no. 3, pp. 489–494.
Gastrotricha and Gnathifera: Hangbook of Zoology, Schmidt-Rhaesa, A., Ed., Göttingen: Hubert and Co. GmbH and Co. KG, 2014.
Geng, H., Xie, P., Deng, D., and Zhou, Q., Tthe rotifer assemblage in a shallow, eutrophic chinese lake and its relationships with cyanobacterial blooms and crustacean zooplankton, J. Freshwater Ecol., 2005, vol. 20, no. 1, pp. 93–100.
Gilbert, J.J., The effect of daphnia interference on a natural rotifer and ciliate community: short-term bottle experiments, Limnol. Oceanogr., 1989, vol. 34, no. 3, pp. 606–617.
Goździejewska, A. and Tucholski, S., Zooplankton of fish culture ponds periodically fed with treated wastewater, Pol. J. Environ. Stud., 2011, vol. 20, no. 1, pp. 67–79.
Green, J., Variability and instability of planktonic rotifer associations in Lesotho, southern Africa, Hydrobiologia, 2001, vol. 446, no. 1, pp. 187–194.
Green, J., Moghraby, A.I., and Ali, M.M., A faunistic reconaissance of Lakes Kundi and Keliak, western Sudan, in Limnology and Marine Biology in the Sudan, Dordrecht: Springer, 1984.
Greze, B.S., Food resources of fishes of the Yenisei River, Izv. VNIIORKh, 1957, vol. 61, pp. 3–284.
Güher, H., Diversity and abundance of Rotifera in Kadıköy Reservoir of Turkey, J. Inst. Sci. Technol., vol. 9, no. 2, pp. 636–646.
Gürbüzer, P., Buyurgan, Ő., Tekatli, C., and Altindağ, A., Species diversity and community structure of zooplankton in three different types of water body within the Sakarya River Basin, Turkey, Turk. J. Zool., 2017, vol. 41, no. 6, pp. 848–859.
Haberman, J., Dominant rotifers of Võrtsjärv (Estonia), Hydrobiologia, 1995, vols. 313/314, p. 313–317.
Haberman, J. and Kunapp, H., Mean zooplankter weight as a characteristic feature of an aquatic ecosystem, Proc. Est. Acad. Sci., Ser. Biol., Ecol., 2002, vol. 51, no. 1, pp. 26–44.
Haldna, M. and Haberman, J., Indices of zooplankton community as valuable tools in assessing the trophic state and water quality of eutrophic lakes: long term study of Lake Võrtsjärv, J. Limnol., 2014, vol. 72, no. 2, pp. 61–71.
Harney, N.V., Dhamani, A.A., and Andrew, R.J., Rotifer diversity of Malhara pond of Bhadrawati, Disthandrapur (M.S.), India, Int. J. Life Sci., 2013, vol. 1, no. 1, pp. 32–36.
Herzig, A., The analysis of planktonic rotifer populations: a plea for long-term investigations, Hydrobiologia, 1987, vol. 147, pp. 163–180.
Irfan, J., Yousuf, A.R., and Parveen, M., Species composition and habitat preference of Rotifera in Ahansar Lake, J. Comput. Eng., 2013, vol. 9, no. 1, pp. 41–48.
Iskaros, I., Bishai, R.M., and Mokhtar, F.M., Comparative study of zooplankton in Aswan Reservoir and the River Nile at Aswan, Egypt, Egypt. J. Aquat. Res., 2008, vol. 34, no. 2, pp. 260–284.
Jersabek, C.D., Distribution and ecology of rotifer communities in high-altitude alpine sites—a multivariate approach, Hydrobiologia, 1995, vols. 313/314, pp. 75–89.
Jersabek, C.D. and Bolortsetseg, E., Mongolian rotifers (Rotifera, Monogononta)—a checklist with annotations on global distribution and autecology, Proc. Acad. Natl. Sci. Philadelphia, 2010, vol. 159, pp. 119–168.
Kerentseva, N.P., Nabokikh, L.N., and Egoshin, V.V., Hydrobiology of the KaR river on the Okhansk–Galevo section, Uch. Zap. Molotov. Gos. Univ., 1946, vol. 4, no. 2, pp. 17–26.
Kim, H.-W. and Joo, G.-J., The longitudinal distribution and community dynamics of zooplankton in a regulated large river: a case study of the Nakdong River (Korea), Hydrobiologia, 2000, vol. 438, pp. 171–184.
Kirk, D.L., Volvox: A Search for the Molecular and Genetic Origins of Multicellularity and Cellular Differentiation, Cambridge: Cambridge Univ. Press, 1998.
Kozlova, I.V., Plankton of Lake Kundravinskoe, Tr. Ural. Otd. SibNIIRKh, 1966, vol. 7, pp. 77–83.
Kozlova, I.V. and Shilkova, E.V., Plankton of the Argazinsky Reservoir, Tr. Ural. Otd. SibNIIRKh, 1966, vol. 7, pp. 17–24.
Krasnovskaya, M.P., Carp of Lake Yanychkovo and its importance as the main object of the economy of Verkhnie Tavdinskie Lakes, Sverdlovsk oblast, Tr. Ural. Otd. VNIIORKh, 1949, vol. 4, pp. 213–273.
Kuczyńska-Kippen, N. and Pronin, M., Diversity and zooplankton species associated with certain hydroperiods and fish state in field ponds, Ecol. Indicators, 2018, vol. 90, pp. 171–178.
Kulikova, T.P., Zooplankton of water bodies of Petrozavodsk (Karelia), Tr. Karel. Nauchn. Tsentra Ross. Akad. Nauk, 2015, no. 2, pp. 71–88.
Kutikova, L.A., Kolovratki fauny SSSR (Rotifers of the Fauna of the USSR), Leningrad: Nauka, 1970.
Lyubimova, T.S., Production capacities of zooplankton in hatchery ponds of the Chesmenskii fish farm, Tr. Ural. Otd. SibNIIRKh, 1975, vol. 9, no. 1, pp. 201–210.
Lyubimova, T.S., Zooplankton of the mountain Lake Arakul (Southern Urals) and its products, in Gidrobiologicheskaya kharakteristika razlichnykh rybokhozyaistvennykh vodoemov Evropeiskoi chasti RSFSR (Hydrobiological Characteristics of Various Fishery Reservoirs of the European Part of the RSFSR), Sb. Nauchn. Tr. Nauchno-Issled. Inst. Ozern. Rechn. Rybn. Khoz., 1981, no. 162, pp. 56–68.
Mäemets, A., Rotifers as indicators of lake types in Estonia, Hydrobiologia, 1983, vol. 104, no. 1, рр. 357–361.
Makarov, M.M., Kucher, K.N., and Naumova, E.Yu., Vertical distribution of zooplankton after rapid change in temperature and chlorophyll concentration, Limnol. Freshwater Biol., 2019, no. 1, pp. 177–180.
Makartseva, E.S., Species composition and productivity of zooplankton, in Ekologo-produktsionnye osobennosti ozer razlichnykh landshaftov Yuzhnogo Urala (Ecological and Production Features of Lakes in Various Landscapes of the Southern Urals), Leningrad: Nauka, 1978, pp. 150–188.
Mantovano, T., Braghin, L., Schwind, L.T.F., Tiburcio, V.G., Bonecker, C.C., and Lansac-Tôha, F.A., Zooplankton communities show contrasting productivity variables thresholds in dammed and undammed systems, Limnetica, 2019, vol. 38, no. 2, pp. 669–682.
Maslennikova, L.I., Materials on the hydrobiology of Ufaleiskie lakes, Tr. Ural. Otd. VNIIORKh, 1941, vol. 3, pp. 24–36.
Matveeva, L.K., Pelagic rotifers of Lake Glubokoe from 1897 to 1984, Hydrobiologia, 1986, vol. 141, pp. 45–54.
May, L. and O’Hare, M., Changes in rotifer species composition and abundance along a trophic gradient in Loch Lomond, Scotland, UK, Hydrobiologia, 2005, vol. 546, pp. 397–404.
May, L., Bailey-Watts, A., and Kirika, A., The relationship between Trichocerca pusilla (Jennings), Aulacoseira spp. and water temperature in Loch Leven, Scotland, U.K., Hydrobiologia, 2001, vol. 446, pp. 29–34.
Miracle, M.R., Alfonso, M.T., Vicente, E., and Koste, W., Rotifers of spring pools in the coastal marshland of Albufera of Valencia Natural Park, Limnetica, 1995, vol. 11, no. 2, pp. 39–47.
Morales-Baquero, R., Cruz-Pizarro, L., and Carillo, P., Patterns in the composition of the rotifer communities from high mountain lakes and ponds in Sierra Nevada (Spain), Hydrobiologia, 1989, vols. 186/187, pp. 215–221.
Muñoz-Colmenares, M. and Sarma, S.S.S., Seasonal variations of rotifers from the high altitude Llano Reservoir (State of Mexico, Mexico), J. Environ. Biol., 2017, vol. 38, pp. 1171–1181.
Muraveiskii, S.D., Observations on the spring plankton of the Ural River and its oxbow lakes, Russ. Gidrobiol. Zh., 1923, vol. 2, pp. 14–23.
Naberezhnyi, A.I., Kolovratki vodoemov Moldavii (Rotifers of Reservoirs of Moldova), Chisinau: Shtiintsa, 1984.
Nandini, S., Ramírez-García, P., and Sarma, S.S.S., Seasonal variations in the species diversity of planktonic rotifers in lake xochimilco, mexico, J. Freshwater Ecol., 2005, vol. 20, no. 2, рр. 287–294.
Nandini, S., Merine-Ibarra, M., and Sarma, S.S.S., Seasonal variations in the species diversity of planktonic rotifers in Lake Xochimilco, Mexico, Lake Reservoir Manage., 2008, vol. 24, pp. 321–330.
Ogorodnikova, E.M., Zooplankton of the Shershnevskoe Reservoir and its role in assessing water quality, in Voprosy biogennogo zagryazneniya i regulirovaniya kachestva vod Urala (Problems of Biogenic Pollution and Regulation of the Quality of Waters in the Urals), Krasnoyarsk: Sib. Nauchno-Issled. Inst. Gidrotekh. Melior., 1977, pp. 27–39.
Oparina, N.Ya., On the rotifer fauna of the vicinities of Perm, Tr. Biol. Nauchno-Issled. Inst. Perm. Gos. Univ., 1923, vol. 1, nos. 9–10, pp. 165–175.
Pejler, B. and Bērziņš, B., On the ecology of Trichocercidae (Rotifera), Hydrobiologia, 1993, vol. 263, pp. 55–59.
Plangklang, N., Boonyanusith, C., and Athibai, S., Species richness and abundance of monogonont rotifers in relation to environmental factors in the UNESCO Sakaerat Biosphere Reserve, Thailand, J. Threat. Taxa, 2019, vol. 11, no. 9, pp. 14087–14100.
Podlesnyi, A.V. and Troitskaya, V.I., Ilmen lakes and their fishery assessment, Tr. Ural. Otd. VNIIORKh, 1941, vol. 3, pp. 121–174.
Pourriot, R., Quelques Trichocerca (Rotifères) et leurs régimes alimentaires, Ann. Hydrobiol., 1970, vol. 1, pp. 155–171.
Ramírez-García, P., Nandini, S., Sarma, S.S.S., Robles Valderrama, E., Cuesta, I., and Hurtado, M.D., Seasonal variations of zooplankton abundance in the freshwater reservoir Valle Bravo (Mexico), Hydrobiologia, 2002, vol. 467, no. 1, pp. 99–108.
Rechkalov, V.V. and Marushkina, E.V., Study of the species composition and dynamics of zooplankton of Lake Sineglazovo, Vestn. Chelyabinsk. Gos. Univ., 2005, vol. 12, no. 1, pp. 15–22.
Rogozin, A.G., Kolovratki Chelyabinskoi oblasti (Rotifers of the Chelyabinsk Oblast), Miass: Ilmen. Gos. Zapov. Ural. Otd. Ross. Akad. Nauk, 1995.
Rogozin, A.G., Zooplankton, in Ekologiya ozera Bol’shoe Miassovo (Ecology of Lake Bolshoe Miassovo), Miass: Ilmen. Gos. Zapov. Ural. Otd. Ross. Akad. Nauk, 2000, pp. 128–165.
Rogozin, A.G., Zooplankton of a hypertrophic reservoir on the example of Lake Tabankul (Southern Urals): biological diversity and biology of some species of rotifers, Izv. Chelyabinsk. Nauchn. Tsentra, 2006, no. 3, pp. 78–82.
Rogozin, A.G., On zooplankton in water bodies of the western foothills of the Southern Urals (Minyar Pond on the Sim River), Izv. Chelyabinsk. Nauchn. Tsentra, 2007, no. 3, pp. 75–79.
Rogozin, A.G., Zooplankton of Lake Uvildy, Izv. Chelyabinsk. Nauchn. Tsentra, 2009, no. 1, pp. 62–67.
Rogozin, A.G., Zooplankton of Lake Malyi Terenkul, Izv. Chelyabinsk. Nauchn. Tsentra, 2009a, no. 3, pp. 28–33.
Rogozin, A.G., Zooplankton of the Argazinskii Reservoir (South Ural) and its long-term changes, Biol. Vnutr. Vod, 2013, no. 2, pp. 25–33.
Rogozin, A.G., Zooplankton species as indicators of saprobity in the water bodies of the Urals, Voda: Khim. Ekol., 2018a, nos. 7–9, pp. 103–109.
Rogozin, A.G., On the system of bioindication of trophic conditions in water bodies, in Sbornik statei po materialam XI–XII Mezhdunar. nauchno-prakticheskoi konferentsii “Ximiya, fizika, biologiya, matematika: teoreticheskie i prikladnye issledovaniya” (Collection of Papers Based on the Materials of the XI–XII Intern. Scientific-Practical Conference “Chemistry, Physics, Biology, Mathematics: Theoretical and Applied Research”), Moscow: Internauka, 2018b, nos. 5–6 (6), pp. 10–15.
Rogozin, A.G., Materials on the fauna and ecology of Rotifers in the Urals, family Brachionidae (Rotifera, Eurotatoria, Ploima). Genus Keratella, Zool. Zh., 2019, vol. 98, no. 7, pp. 732–748.
Rogozin, A.G., Materials on the fauna and ecology of rotifers in the Ural Region: family Brachionidae (Rotifera, Eurotatoria, Ploima). Genera Anuraeopsis, Brachionus, and Notholca, Biol. Bull. (Moscow), 2019a, vol. 46, no. 8, pp. 823–833.
Rogozin, A.G., Materials on the Fauna and Ecology of Rotifers in the Urals, Family Brachionidae (Rotifera, Eurotatoria, Ploima), Genera Kellicottia, Plationus, and Platyias, Biol. Bull. (Moscow), 2021, vol. 48, no. 7, pp. 950–958.
Rogozin, A.G. and Shchetinina, O.V., Inventory of rotifers of the Ilmensky Nature Reserve, in Fauna i flora Il’menskogo zapovednika (Fauna and Flora of the Ilmensky Nature Reserve), Sverdlovsk: Ural. Otd. Akad. Nauk SSSR, 1989, pp. 42–52.
Rogozin, A.G., Snit’ko, L.V., and Timoshkin, O.A., Thermoindicator properties of zooplankton species and their measurements, Water Resour, 2015, vol. 42, no. 1, pp. 91–97.
Rosińska, J., Romanowicz-Brzozowska, W., Kozak, A., and Gołdyn, R., Zooplankton changes during bottom-up and top-down control due to sustainable restoration in a shallow urban lake, Environ. Sci. Pollut. Res. Int., 2019, vol. 26, no. 19, pp. 19575–19587.
Ruttner-Kolisko, A., Plankton Rotifers. Biology and Taxonomy, Binnengewasser, 1974, vol. 26, part 1.
Saygi, Y., Gündüz, E., Demircalp, F.Y., and Çaǧlar, S.S., Seasonal patterns of the zooplankton community in the shallow, brackish Liman Lake in Kızılırmak Delta, Turkey, Turk. J. Zool., 2011, vol. 35, no. 6, pp. 783–792.
Schmid-Araya, J.M., Rotifer communities from some Araucanian lakes of southern Chile, Hydrobiologia, 1993, vol. 255, no. 1, pp. 397–409.
Segers, H.H., A biogeographical analysis of rotifers of the genus Trichocerca Lamarck, 1801 (Trichocercidae, Monogononta, Rotifera), with notes on taxonomy, Hydrobiologia, 2003, vol. 500, pp. 103–114.
Segers, H.H., Annotated checklist of the rotifers (phylum Rotifera), with notes on nomenclature, taxonomy and distribution, Zootaxa, 2007, vol. 1564, pp. 1–104.
Shiel, R. and Koste, W., Rotifera from Australian inland waters. VIII. Trichocercidae (Rotifera: Monogononta), Trans. R. Soc. South Aust., Incorp. Records South Austr. Mus., 1992, vol. 116, no. 1, pp. 1–27.
Shitikov, V.K., Rozenberg, G.S., and Zinchenko, T.D., Kolichestvennaya gidroekologiya: metody sistemnoi identifikatsii (Quantitative Hydroecology: Methods of System Identification), Tolyatti: Inst. Ecol. Volzh. Basseina Ross. Akad. Nauk, 2003.
Sipaúba-Tavares, L.H., Anatriello, C.B., Milstein, A., Millan, R.N., and Scardoeli-Truzzi, B., Macrophyte-environment relationships during a monospecific and a multispecific massive invasion in a fishpond, Trop. Plant Res., 2017, vol. 4, no. 3, pp. 471–479.
Sládeček, V., Rotifers as indicators of water quality, Hydrobiologia, 1983, vol. 100, pp. 169–201.
De Smet, W.H., Report on rotifers from Barentsøya, Svalbard (78°30' N), Fauna Norv., Ser. A, 1993, vol. 14, pp. 1–26.
Stanachkova, M., Stefanova, M., Kozuharov, D., Raikova-Petrova, G., and Fikovska, E., Community structure of zooplankton as key factor for self-purification capacity of iskar reservoir, Ecol. Eng. Environ. Prot., 2017, vol. 9, pp. 39–46.
Steinberg, C.E.W., Ecology of Humic Substances in Freshwaters, Berlin: Springer-Verlag, 2003.
Stemberger, R.S., Cannon, J.E., and Bricker, F.J., Spatial and Seasonal Structure of Rotifer Communities in Lake Huron, Duluth: Environmental Research Laboratory Office of United States EPA, 1979.
Tauson, A.O., Hydrobiological sketch of the lakes of the upper Kama, Tr. Perm. Biol. Inst., 1934 vol. 6, nos. 1–2, pp. 103–118.
Tauson, A.O., Wild lake and its biology, Uch. Zap. Perm. Gos. Univ., 1935, vol. 1, no. 2–3, pp. 3–55.
Tauson, A.O., Zooplankton of the Kama River on the section Galevo Village–Belaya River, Izv. Estestv.-Nauchn. Inst. Molotov. Gos. Univ., 1946, vol. 12, no. 5, pp. 155–167.
Tauson, A.O., Plankton of the Upper Kama River, Uch. Zap. Molotov. Gos. Univ., 1947, vol. 4, no. 2, pp. 3–16.
The Production Ecology of Wetlands: The IBP Synthesis, New York: Cambridge Univ. Press, 2009.
Vázquez-Sánchez, A., Reyes-Vanegas, G., Nandini, S., and Sarma, S.S.S., Diversity and abundance of rotifers during an annual cycle in the reservoir Valerio Trujano, Inland Waters, 2014, vol. 4, pp. 293–302.
Vershinin, N.V., On the biology of floodplain lakes of the Kama River flooded by the Molotov Reservoir, Izv. Estestv.-Nauchn. Inst. Molotov. Gos. Univ., 1953, vol. 13, no. 7, pp. 519–564.
Viro, T. and Haberman, J., Annotated list of rotifers of Lake Võrtsjärv, Proc. Est. Acad. Sci., Ser. Biol., Ecol., 2005, vol. 54, no. 1, pp. 53–66.
Vizer, L.S., Prusevich, L.S., Vizer, A.M., Dorogin, M.A., and Matveeva, E.P., Zooplankton of the Novosibirsk Reservoir in the period of extreme water content, Vestn. Rybokhoz. Nauki, 2016, vol. 3, no. 2, pp. 92–99.
Wallace, R.L. and Starkweather, P.L., Clearance rates of sessile rotifers: in situ determinations, Hydrobiologia, 1983, vol. 104, no. 1, pp. 379–383.
Wen, X., Zhai, P., Feng, R., Yang, R., and Xi, Y., Comparative analysis of the spatio-temporal dynamics of rotifer community structure based on taxonomic indices and functional groups in two subtropical lakes, Sci. Rep., 2017, vol. 7, no. 578. https://doi.org/10.1038/s41598-017-00666-y
Xue, D., Weisong, F., Li, W., Ye, S., Liu, J., Zhang, T., and Zhongjie, L., Response of rotifer community to environmental changes in five shallow lakes in the middle reach of Changjiang River, China, Chin. J. Oceanol. Limnol., 2014, vol. 32, no. 5, pp. 1083–1091.
Yermolaeva, N.I., Some results of studying zooplankton in lakes of northern Kazakhstan, Arid. Ecosyst., 2013, vol. 3, no. 4, pp. 263–275.
Yermolaeva, N.I., Zooplankton and water quality of the Ishim River in northern Kazakhstan, Arid. Ecosyst., 2015, vol. 5, no. 3, pp. 176–187.
Yermolaeva, N.I., Species composition and spatial distribution of zooplankton in the Gulf of Ob and Gydan Bay, in Trudy III Vserossiiskoi nauchnoi konferentsii s mezhdunarodnym uchastiem “Vodnye i ekologicheskie problemy Sibiri i Tsentral’noi Azii” (Proc. III Vseross. Sci. Conf. with International Participation “Water and Ecological Problems of Siberia and Central Asia”), Inst. Vodn. Ekol. Probl. Sib. Otd. Ross. Akad. Nauk, 2017, pp. 91–99.
Yermolaeva, N.I., Zarubina, E.Yu., and Dvurechenskaya, S.Ya., Diurnal dynamics of hydrochemical indices and zooplankton in the littoral zone of the Novosibirsk Reservoir, Povolzh. Ekol. Zh., 2016, no. 2, pp. 155–166.
Yin, L., Yu, J., Yinjiang, Z., Linxuan, C., and Lijing, C., Rotifer community structure and its response to environmental factors in the Backshore Wetland of Expo Garden, Shanghai, Aquacult. Fish., 2018, vol. 3, no. 2, pp. 90–97.
Yoshida, T., Urabe, J., and Elser, J.J., Assessment of ‘top-down’ and ‘bottom-up’ forces as determinants of rotifer distribution among lakes in Ontario, Canada, Ecol. Res., 2003, vol. 18, pp. 639–650.
Yoshida, T., Ban, S., Takenouchi, T., Aono, T., Ishikawa, Y., Mikami, H., Takano, K., Imada, K., Yasutomi, R., and Takeuchi, K., Top-down control of population dynamics of the dominant rotifers in two mesotrophic lakes in Hokkaido, Japan, Arch. Hydrobiol., 2000, vol. 148, no. 4, pp. 481–498.
ACKNOWLEDGMENTS
The author is grateful to O.V. Shchetinina, the laboratory assistant of the South Urals Federal Research Center of Mineralogy and Geoecology, Ural Branch, Russian Academy of Sciences, for her invaluable assistance in the collection and processing of material and the design of illustrations.
Funding
This work was carried out within the framework of a State Assignment, project no. AAAA-A19-119101490003-1 on the planned topic of the South Federal Scientific Center of Mineralogy and Geoecology, Ural Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest. This article does not contain any studies involving animals or human participants performed by the author.
Additional information
Translated by T. Kuznetsova
Rights and permissions
About this article
Cite this article
Rogozin, A.G. The Fauna and Ecology of Rotifers in the Urals: Family Trichocercidae (Rotifera, Eurotatoria, Ploima), Genera Ascomorphella and Trichocerca. Biol Bull Russ Acad Sci 49, 763–783 (2022). https://doi.org/10.1134/S1062359022070172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359022070172