Abstract—
Information on the distribution of three genera of rotifers, Anuraeopsis, Brachionus, and Notholca of the family Brachionidae in the Ural region is summarized based on the author’s research and the analysis of published sources. Data on their localities, biology, and quantitative development in water bodies of the Ural region are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The fauna and ecology of rotifers have been poorly studied in most regions of Russia despite their important role in the plankton of water bodies. The main information is provided in a few works of hydrobiologists and are, mainly, related to fishery problems and are of applied character. Many of them were published in the middle of the past century of even earlier. To date, there is no generalized work on the fauna and ecology of the vast Ural region (except for the author’s work on a part of the Southern Ural region, Rogozin, 1995) and available publications are little-known and difficult to access. There is one more problem concerning the application of the methods of bioindication and interpretation of the results of hydrobiological studies. The information on the biology of some species is fragmentary in numerous publications, and available indicator data were obtained, as a rule, in completely different landscape and climatic conditions compared to those in the Ural region. In my opinion, now is the moment to generalize the accumulated data on the fauna and ecology of rotifers in the region based on the materials published from 1910 up to the present and my own data obtained in water bodies of the Southern Ural region.
We consider the Ural region in the broadest sense, including the Cis-Ural and Trans-Ural regions because the fauna in water bodies is a unified complex and belongs to the basins of the large Kama, Pechora, Tobol, Ob, and Ural rivers.
The individual indicator valency characterizing the features of the species biology with respect to the water temperature (Rogozin et al., 2015), saprobity, and the trophic type of a water body is presented for the species on which there was a sufficient amount of data for calculations. Obtained from local material, they best characterize the species biology namely in local geographical conditions. In cases when such information was absent, the literature data the application of which is certainly limited under these conditions was used.
Genus Anuraeopsis Lauterborn 1900. To date, a single representative of the genus is known from a single habitat in the Ural region.
Anuraeopsis fissa Gosse 1851. The first finding was recorded in Bolshoe Miassovo Lake in the foothills of the Ilmen Mountains (Southern Urals, environs of the city of Miass) in July 1991 (Rogozin, 1995). Later, the species was found in different bays in the littoral zone of the lake at depths from 0 to 2 m in 2003. The sizes were typical; the lorica length, 90–128 µm, and the width, 46–55 µm. The peak of abundance, up to 270 000 ind./m3 was recorded in August upon maximum water heating. As has been demonstrated in the course of laboratory studies, the density of the population of Anuraeopsis fissa is linearly dependent on the density of consumed algae and may reach extremely high values above two billion ind./m3 (Dumont et al., 1995). The species is typical of eutrophic water bodies (Teoreticheskie voprosy…, 1993; etc.); it is abundant in natural waters under hypertrophic conditions at high water blooms when the species switches to bacterial feeding and becomes dominant in zooplankton (Sommaruga, 1995; Hwang and Heath, 1999). The species is a thermobiont (3.0), which coincides with the data of other researchers (e.g., Jersabek and Bolortsetseg, 2010), β-mesosaprobe (1.9). It has a cosmopolitan distribution. Based on the species biology, its broader distribution in water bodies of the Ural region may be expected; however, the species has not been recorded yet in the eutrophic and hypertrophic lakes studied in the region.
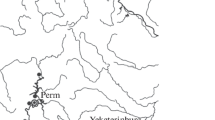
Genus Brachionus Pallas 1776. The locations of findings of the most widespread species of the genus in the Urals are shown in Fig. 1.
Brachionus angularis Gosse 1851 (Fig. 2а). Two forms of the species, the typical f. angularis Gosse 1851 and f. bidens Plate 1886, are known in the Ural region. Most researchers in the Ural region did not differentiate them, and all known forms reported in the literature concern f. bidens. The first findings of B. angularis were recorded in the Southern and Middle Cis-Ural region in the Ural and Kama rivers, respectively, and dated from the beginning of the 1920s (Muraveiskii, 1923; Oparina, 1923). Later, the species was found in many water bodies of Southern Urals from different sites in the Ural River (Akatova, 1954) to Chelyabinsk oblast (Kozlova, 1966, 1979; Lyubimova, 1971, 1975; Kovalkova et al., 1975; Rechkalov and Marushkina, 2005).
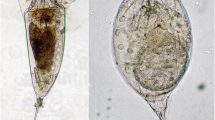
Species of the genus Brachionus from water bodies in the Ural region: (а) B. angularis f. bidens Plate 1886 from Tabankul Lake, (b) B. calyciflorus f. amphiceros Ehrenberg 1838 from Malyi Terenkul Lake, (c) B. calyciflorus f. calyciflorus Pallas 1766 from Malyi Terenkul Lake, (d) B. diversicornis f. homoceros (Wierzejski 1891) from Malyi Terenkul Lake.
There are shallow lakes and reservoirs of the steppe and forest steppe zones and often mineralized and freshwater deep lakes in the eastern foothills of the Ural Mountains. In the Middle Urals species including the form Brachionus angularis f. bidens was found in the Cis-Ural region proper in different parts of the middle and upper courses of the Kama River (Kerentseva et al., 1945; Tauson, 1946, 1947), and in the Southern Urals, it was found in lakes of the Kisegach-Miass lake system in the environs of the city of Chebarkul, Chelyabinsk oblast (my data). A typical form angularis (at present the only record in the Urals) was found in these water bodies. The loricae of the specimens had a well-expressed punctate sculpture and length of 150–153 µm, which was much smaller than the typical sizes of 168–193 µm (Kutikova, 1970). Researchers from the Ural region often mentioned the species as a mass species, but the reported density of the population was usually less than 1000 ind./m3, and it reached mass development only in fish ponds in the southern part of Chelyabinsk oblast (Lyubimova, 1971, 1975). In May 2005, we simultaneously recorded both forms in Malyi Terenkul Lake; the abundance of the typical form reached 42 500 ind./m3, and that of f. bidens reached 70 700 ind./m3; in June the species disappeared from the plankton. The studies of 2014–2015 demonstrated that B. angularis reached a high abundance in the lake already during the ice period (from 1200 ind./m3 in December to 15 300 ind./m3 in February). In neighboring Tabankul Lake, the species (f. bidens) was recorded up to 46 500 ind./m3 in May (Rogozin, 2006). Thus, the mass development of B. angularis occurs in the winter–spring period. According to our data, the species is cryophilic (0.9); it prefers weakly and moderately polluted waters, which agrees with the comparatively high saprobic valency (2.5). According to our data, it is a typical eutrophic species. The species belongs to euryhaline rotifers (Kutikova, 1970). B. angularis is the food source of many aquatic invertebrates: predatory rotifers of the genus Asplanchna, adult and late-stage larvae of copepods, predatory cladocerans, etc. It has been shown that Asplanchna has a higher grazing pressure on Brachionus than copepods (Kumar and Rao, 2001), and the latter prefer larger B. calyciflorus while avoiding small B. angularis (Rao and Kumar, 2002). The experiments have demonstrated that in the absence of predators in the competition for food resources B. angularis gradually replaces B. calyciflorus though the latter is not a detritophage but an algophage (Kumar and Rao, 2001). Despite favorable nutritional and bioproduction and hydrochemical conditions in the above-mentioned Tabankul Lake, B. angularis is second to a typical thermobiont form, Brachionus calyciflorus (see below) in the competition when water warms up in the middle of summer despite the real absence of predators during this period (Rogozin, 2006). It has been demonstrated in some studies that a key positive factor for development of the rotifer population is an increase in the water temperature; the competition for food resources and grazing by predators do not have a considerable effect on the rotifer (Wen et al., 2011). It should always be borne in mind that the species biology may differ considerably in various natural-climatic conditions. Brachionus angularis is distributed in most zoogeographical regions of the world and is known everywhere in Russia.
Brachionus asplanchnoides Charin 1947. This species was found in several, mainly mineralized shallow lakes in the Southern Trans-Ural region in the Tobol-Miass interfluve (Makartseva, 1978; Drabkova and Sorokin, 1979; Kozlova, 1988). The authors did not indicate the degree of its quantitative development, but Kozlova reported that B. asplanchnoides in Shugonyak Lake dominated in the rotifer plankton and formed, on average, 4% of the zooplankton production in the warm season. According to the published data, the species is halophilic (Jersabek and Bolortsetseg, 2010) and is distributed in the eastern Palearctic region; the species is known in the southern regions of European Russia. It is, probably, distributed in numerous mineralized lakes in the Southern Ural region, but this may be suggested only because of insufficient study of most of them.
Brachionus bennini Leissling 1924. A single find was recorded in the Ural region in its southeastern extremity (in a strict sense outside its limits), in the Ural River and its tributaries (Akatova, 1954). The species is typical of rivers and water bodies connected with them (Kutikova, 1970). It is a β-mesosaprobe (Sladeček, 1983). The species is recorded in different zoogeographical regions (Afrotropical, Nearctic, Palearctic); it occurs at relatively low latitudes, which explains its absence in more northern Ural regions. It is known everywhere on the territory of Russia.
Brachionus budapestinensis Daday 1885. It was recorded only once together with the previous species (Akatova, 1954). It is known as a thermophilic inhabitant of small eutrophic water bodies, β-mesosaprobe (Sladeček, 1983). The species is distributed in Nearctic, Neotropical, and Oriental regions, and like the previous species, it avoids northern latitudes including on the territory of Russia.
Brachionus calyciflorus Pallas 1766 (Figs. 2b, 2c) is mainly distributed in the Southern Urals; to the north it is known only from the Kama River (Graevskii and Pogankin, 1937; Tauson, 1947). Both a typical form calyciflorus and f. amphiceros Ehrenberg 1838 were recorded in the Southern Ural region. It was first found (f. amphiceros) in the Ural River in the environs of the city of Orenburg in 1923 (Muraveiskii, 1923). Later, the species was found in ponds in the southern part of Chelyabinsk oblast (Lyubimova, 1971, 1975), in Argayash, Maloe Miassovo (Makartseva, 1978; Drabkova and Sorokin, 1979), Silach (Kozlova, 1979), Tabankul (Rogozin, 2006), Malyi Terenkul (Rogozin, 2009), and Bolshoi Kisegach (unpublished data of the author) lakes in the eastern foothills of the Southern Urals and in two forest–steppe lakes, Karakulmyak (Kozlova, 1988) and Sineglazovo (Rechkalov and Marushkina, 2005) lakes in the Trans-Ural region. The specimens had the following morphometric characteristics (µm): lorica length 330–388, lorica width 186–213, length of dorsal spines 70–83, length of abdominal spines 60–68, and length of postero-lateral spines 105–120. In general, Brachionus from eutrophic lakes of the Kisegach-Miass system are larger and bear longer spines then was indicated by Kutikova (1970). This may be considered as a defense response to the pressure of Asplanchna, the main invertebrate predator in the surveyed water bodies; the length of spines on the lorica is more important for antipredator defense than the body size (Nandini et al., 2003).
As mentioned before, Brachionus calyciflorus is an algophage, a weaker competitor for food resources than B. angularis and other species of rotifers (e.g., Keratella cochlearis (Kirk, 2002); it is more actively utilized by Asplanchna. This rotifer responds negatively to blooms of some cyanobacteria despite its status as a typically eutrophic species. In particular, experimental studies on the relationships between Microcystis aeruginosa and B. calyciflorus have demonstrated that this alga is not a suitable food resource for the rotifer and has a toxic effect on it; the average lifespan of Brachionus in its presence is only 0.58 ± 0.05 day (Nandini, 2000), and they did not survive and reproduce even in the presence of only unicellar specimens of Microcystis (Nandini and Rao, 1997). Brachionus calyciflorus is resistant to the oxygen deficiency and prefers neutral waters to alkaline ones. At high temperatures it loses the competition for food resources with other algophagous rotifers, e.g., the widespread Synchaeta pectinata (Stelzer, 1998). Thus, the living conditions for B. calyciflorus in eutrophic lakes in the Southern Urals (most of which are mineralized with alkaline water) with the dominance of microcystis are far from being optimal for most of the year; in spring there is the pressure of predatory Asplanchna and cyclops and the competition with a stronger competitor, rotifers, for food resources, and in summer the competition with cladocerans and the complexity of coexistence with M. aeruginosa. That is probably why the typical form calyciflorus and the form amphiceros differ significantly in ecological properties: the first species is a thermobiont (2.3) and α-mesosaprobe (2.9), the second one is a cryophilic (1.1) β-mesosaprobic (2.4) organism (both forms are indicators of eutrophic conditions in a water body). This largely explains the features of the change of the forms of Brachionus calyciflorus during the season, which can be illustrated by the strongly eutrophicated Malyi Terenkul and Tabankul lakes connected by a channel in Chelyabinsk oblast, which we studied in 2005–2006. In May, the mass development of f. amphiceros was observed after the ice melted in Malyi Terenkul Lake; its abundance reached 12 000–27 000 ind./m3, but decreased to 4500–7000 ind./m3 by the beginning of July and to 2000–2400 ind./m3 by the end of autumn. The form calyciflorus appeared only in August during the period of the maximum water heating and biological pollution (Rogozin, 2006) and reached the density of 11 000–14 000 ind./m3. In the more heated and polluted Tabankul Lake, the form calyciflorus had an abundance an order of magnitude higher (above 100 000 ind./m3) but occurred only in the height of summer, whereas f. amphiceros occurred in spring with the abundance of more than 74 000 ind./m3 and in summer (9500 ind./m3). Brachionus c. calyciflorus provides a mass outburst in zooplankton abundance in Karakulmyak Lake at the end of summer (Kozlova, 1988). Other forms of the species (Tauson, 1947) were recorded in the Kama River, anuraeformis Brehm 1909 and dorcas Gosse 1851; data on their quantitative development are absent. Brachionus calyciflorus is known from many habitats in the Holarctic and in African, Neotropical, and Oriental regions; it occurs everywhere in Russia.
Brachionus diversicornis (Daday 1883) (Fig. 2d) is mainly distributed in the Southern Ural region. To the north it is known only from the Kama River (Tauson, 1946) and Yanychkovo Lake in its basin (Krasnovskaya, 1949). Its typical form diversicornis was mainly recorded, with several findings from the Ural River (Akatova, 1954) to the piedmont Kaslinsk and Kisegach-Miass group of lakes in the northern part of Chelyabinsk oblast (Drabkova and Sorokin, 1979; Kozlova, 1979; Rogozin, 2009). The form homoceros (Wierzejski 1891) was found in Kundravinskoe Lake (pre–forest–steppe Trans-Ural region (Makartseva, 1978)) and Malyi Terenkul Lake (Rogozin, 2009) (foothills of the Ilmen Mountains). The abundance of the species in most cases was not reported by the researchers who found them. According to our data, the species occurs in autumn (September) with a density from 300 to nearly 19 000 ind./m3 (f. diversicornis) and more than 19 000 ind./m3 (f. homoceros) in different years. Because of the small number of findings of Brachionus diversicornis, we could not determine its ecological properties (relation to temperature regime, saprobity, and trophic state of water bodies); according to the literature data, this β-mesosaprobic species prefers well-heated eutrophic waters (Sladecek, 1983; Teoreticheskie voprosy…, 1993; Jersabek and Bolortsetseg, 2010). The species is distributed in the Holarctic and Oriental regions; it extends in Russia from the west of the European territory to Eastern Siberia (Kutikova, 1970).
Brachionus plicatilis Müller 1786 (Figs. 3а, 3b) was first found in the Ural region in saline lakes of the forest–steppe Transural region in 2017: Bolshoi Shantropai (f. longicornis Fadeev 1925), Podbornoe, Solenoe, and Sladkoe lakes (the typical form). According to the literature data, it is a eurythermal (Jersabek and Bolortsetseg, 2010) β-mesosaprobic species (Sladeček, 1983). It is a halobiont but with a very wide range of tolerance to mineralization (Jersabek and Bolortsetseg, 2010; Lazareva et al., 2013); therefore, it occurs in fresh waters. It is distributed in the Holarctic and Neotropical regions and Oceania. It is known everywhere in Russia.
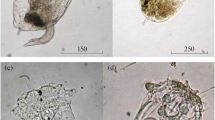
Species of the genus Brachionus from water bodies in the Ural region: (а) B. plicatilis f. longicornis Fadeev 1925 from Bolshoi Shantropai Lake, (b) B. plicatilis f. plicatilis Müller 1786 from Sladkoe Lake, (c) B. quadridentatus f. zernovi Voronkov 1907 from Tabankul Lake, (d) B. quadridentatus f. hyphalmyros Tschugunoff 1921 from Atkul Lake.
Brachionus quadridentatus Hermann 1783 (Fig. 3c) in the Southern and Middle Ural regions is presented by several forms except the typical f. quadridentatus: brevispinus Ehrenberg 1832, cluniorbicularis Skorikov 1894, hyphalmyros Tschugunoff 1921, rhenanus Lauterborn 1893, and zernovi Voronkov 1907. The first findings of Brachionus quadridentatus (f. quadridentatus) were recorded as early as at the beginning of the 20th century (Furman and Tiebo, 1910) in the South Ural forest–steppe Lakes Kozhakul and Urukul (the first lake is mineralized to 2350 mg/dm3). A typical form was found later in southern steppe regions, in the Ural River (Akatova, 1954) and water bodies in the environs of the city of Troitsk (Zinoviev, 1931) and in the pre–forest–steppe Trans-Ural region (Rechkalov and Marushkina, 2005), in the Middle Urals in the Kama River and water bodies of its basin (Oparina, 1923; Tauson, 1946, 1947; Krasnovskaya, 1949). The forms brevispinus and cluniorbicularis were found in steppe ponds in the Southern Ural region (Lyubimova, 1975), and the first form was found in steppe saline Yuzhigan Lake in the Southern Urals (my data) and in the Kama River (Tauson, 1947). We found the form hyphalmyros in the mineralized Lakes Atkul and Selezyan in the Southern Ural region (environs of the city of Etkul) in 2017. The forms rhenanus and zernowi were recorded only in the Middle Urals (Peschanoe Lake near the city of Perm, Oparina, 1923; the Kama River, Tauson, 1947), we found the latter form in the piedmont Bolshoe Miassovo Lake (environs of the city of Miass). We did not determine the ecological properties of Brachionus quadridentatus because of its few findings; according to the literature data, it is a β-mesosaprobic eurythermic euryhaline species (Kutikova, 1970; Jersabek and Bolortsetseg, 2010), an indicator of eutrophic conditions (Podshivalina and Yakovlev, 2012). Brachionus quadridentatus is a cosmopolite known in most zoogeographical regions of the world; it is spread throughout the entire territory of Russia.
Brachionus rubens Ehrenberg 1838. The first findings of the species in the Ural region were recorded in the upper and middle courses of the Kama River in the middle of the 20th century (Kerentseva et al., 1946; Tauson, 1946, 1947). In the Southern Urals, the species was first recorded in Bolshoi Kisegach and Bolshoi Ishkul lakes located in the eastern foothills of the Ilmen Mountains, but the data were not confirmed and published. Despite the fact that sampling of the material in the lakes was performed repeatedly, we never found B. rubens. The first official record was made in the forest—steppe lake Sineglazovo Lake on the southern edge of the city of Chelyabinsk (Rechkalov and Marushkina, 2005). The authors did not indicate the degree of the quantitative development of the species populations. It occurs in highly eutrophic water bodies (Kutikova, 1970) where it associates with crustaceans as a commensal and may suppress to a certain degree their development (Nandini and Rao, 1993), often being itself suppressed by filamentous colonies of cyanobacteria (Rothaupt, 1991). It is an eurythermic euryhaline (Jersabek and Bolortsetseg, 2010) α-mesosaprobe (Sladeček, 1983). Brachionusrubens is distributed throughout the Holarctic; in Russia it is spread in temperate and southern latitudes.
Brachionus sericus Rousselet 1907. The first and the only finding of the species was recorded in the Urals in the Kama River in the area of the city of Perm (Tauson, 1947). Quantitative data are not presented. According to the ecological properties, the species is an oligosaprobe inhabiting warm fresh waters (Jersabek and Bolortsetseg, 2010). It is distributed in the Palearctic, Afrotropical, and Australasian regions. In Russia it is known in the European territory and in the Caucasus.
Brachionus urceolaris Müller 1773. The first findings were recorded in the Southern and Middle Ural regions in the Ural and Kama rivers, respectively, and dated from 1923 (Muraveiskii, 1923; Oparina, 1923). One more finding in the steppe zone of the Southern Urals was recorded in 1970 (Lyubimova, 1971). B. urceolaris was repeatedly found in the Middle Ural region, in the Kama River and water bodies of its basin (Graevskii and Pogankin, 1937; Tauson, 1946, 1947; Krasnovskaya, 1949) and in the Polar Urals (Bogdanov et al., 2004). The degree of the quantitative development of the species populations is not reported. According to the literature data, it is a β‑mesosaprobic eurythermic euryhaline species living mainly in periphyton (Jersabek and Bolortsetseg, 2010). It is spread in the Holarctic and Neotropical regions.
The data indicate that despite its wide distribution the genus Brachionus is represented in the Ural region by only a few findings and its representatives seldom reach a high quantitative development. Brachionus species occur, mainly in the Southern Ural regions and in shallow, often mineralized eutrophic lakes, which is determined by the features of the biology of many species of the genus. Namely, these water bodies in the Ural region have been insufficiently studied and, taking into account the general invasion of the southern fauna to northern latitudes, a considerable broadening of the list of species and forms of the genus Brachionus in the Ural region may be expected after its thorough study.
Genus Notholca Gosse 1886. The known sites of findings of the most widespread species of the genus in the Ural region are shown in Fig. 4.
Notholca acuminata (Ehrenberg 1832) was first recorded in the Middle Urals in the Danilikha River, a tributary of the Kama River in the city of Perm (Oparina, 1923) and, later, in the Kama River (Graevskii and Pogankin, 1937). Later, the species was found in lakes in the eastern foothills of the Southern Urals (Bolshoi Kisegach, Makartseva, 1978; Bolshoe Miassovo, Rogozin, 1995; Turgoyak, Rogozin, 1998; Uvildy, Rogozin, 2009), in lakes of the forest–steppe Trans-Ural region (Sineglazovo, Rechkalov and Marushkina, 2005; Smolino, unpublished data of the author), and in an unnamed lake in the Baidaratayakha River basin on the eastern macroslope of the Polar Urals (Bogdanov et al., 2004). Other findings of N. acuminata at polar latitudes but outside the Ural region are known. The specimens found had the following sizes of the lorica: length 230–280 µm, width 90–118 µm, anterior dorsal spines 27–38 µm, intermediate spines 6–12 µm, and lateral spines 30–35 µm. The researchers who recorded N. acuminata did not report the data on the quantitative development of the species. According to our data, the species reached an abundance of 500 ind./m3 in Smolino Lake in May 2006 but later the species was not found; the species was found in Bolshoe Miassovo Lake in September, where its abundance was 172 ind./m3. The only finding in Uvildy Lake was recorded in November, where the abundance was 150 ind./m3. N. acuminata is a cryobiont (0.3) and oligosaprobe (1.0) with respect to its ecological properties. The water mineralization is, apparently, of low importance because this rotifer was found in both mineralized and freshwater bodies. This coincides with the other data on the species biology, which characterize it as a cold-water stenothermic and euryhaline organism (Jersabek and Bolortsetseg, 2010). It has a Holarctic distribution mainly in northern latitudes that follows from the features of its biology; in Russia it is known throughout the entire territory. As can be seen from the above data, the species is rather rare and not numerous in the Ural region. Unfortunately, the absence of retrospective data on the abundance of N. acuminata does not make it possible to trace the long-term dynamics; however, warming of water bodies in general (Rogozin and Gavrilkina, 2015) probably negatively affects the state of the population of this cryobiont because a temperature above 22°С is critical for it (Galkovskaja, 1987).
Notholca bipalium (Müller 1786). This species was recorded only once in the Ural River in the environs of the city of Orenburg (Muraveiskii, 1923). According to the literature data, it inhabits mineralized continental and marine waters and is distributed throughout the entire Holarctic region. Based on this, it may be suggested that new records of the species will be made when studying numerous mineralized lakes in the Southern Urals.
Notholca caudata Carlin 1943. The only locality of this species is in the Polar Ural region in Khadata-Yugan-Lor and Bolshoe Shchuchie lakes, in small piedmont lakes on the eastern macroslope, and in the channels of the river on the northern macroslope (Bogdanov et al., 2004). Quantitative development is not reported. It is considered to be a cold-water stenothermic species (Pejler and Berzins, 1989) that does not usually spread in the Holarctic below 58° N (Kutikova, 1998). There is information that reproduction of the population stops at water temperatures above 10°С (Walz et al., 1989). The species biology and modern climatic trends make findings of the species highly unlikely in the Middle and especially in the Northern Ural regions.
Notholca foliacea (Ehrenberg 1832) (Fig. 5) was first recorded in tributaries of the Tobol River, the Sinturka and Pelym rivers, in 1936 (Tauson, 1936) and, later, in the Kama River (Tauson, 1946). In the Southern Urals, it was recorded in the piedmont Lakes Bolshoe Miassovo and Turgoyak of the Ilmen Mountains. The specimens found had the following sizes of the lorica: length 170–175 µm, width 60–70 µm, length of anterior dorsal spines 22–26 µm, length of intermediate spines 18–20 µm, and length of the lateral spines 20–22 µm. The abundance varies from 1000 (Bolshoe Miassovo) to 80–230 ind./m3 (Turgoyak). The species occurs in May after the beginning of ice drift and probably inhabits the lakes in the ice period as well. It was mentioned as a spring species by many researchers (e.g., Kuczyńska-Kippen and Basińska, 2008; Shumka, 2014). It is a cryobiont (0.3) and oligosaprobe (1.3) with respect to the ecological properties. According to the literature data, it is a euryhaline (Jersabek and Bolortsetseg, 2010) and euryionic species (Gillard, 1948). It has a cosmopolite distribution but has not been yet found in the Asian region, Antarctica, or Oceania. It is known throughout the entire territory of Russia, mainly in northern and temperate latitudes.
Notholca labis Gosse 1887 is found throughout the Ural region from the Ural River near the city of Orenburg (Muraveiskii, 1923) to the polar lakes (Bogdanov, 2004). In the Southern Ural region, it is known from Turgoyak (Podlesny, 1927) and Uvildy (Rogozin, 2009) lakes in the foothills of the Ilmen Mountains (Podlesny, 1927); in the Middle Ural region it is known from Russkoe Lake in the environs of the city of Perm (Tauson, 1936). The specimens had the following sizes of the lorica: length 180–195 µm, width 90–110 µm, length of anterior dorsal spines 28 µm, length of intermediate spines 4–5 µm, and length of lateral spines 20–22 µm. The degree of the quantitative development of the species is not indicated in the literature; we found N. labis with an abundance of 357 ind./m3 in May soon after ice melting. According to the literature data, the species is similar to the previous species in biology; our own data are insufficient for more accurate characterization of it in the Ural region. The species is distributed in northern latitudes, mainly in the Palearctic (Kutikova, 1970; Jersabek and Bolortsetseg, 2010). In Russia, it is predominantly widespread in northern latitudes.
Notholca squamula (Müller 1786). The first finding of this species in the Ural region was recorded in rivers of the eastern macroslope of the Polar Urals only at the beginning of the 2000s (Bogdanov, 2004). Later, the species was found in the Southern Ural region in piedmont Lakes Turgoyak (my own data) and Uvildy (Rogozin, 2009) and the forest–steppe brackish Smolino Lake in the environs of the city of Chelyabinsk (author’s data). Only a typical form squamula was found. The specimens had the following sizes of the lorica: length 140–187 µm, width 95–115 µm, length of anterior dorsal spines 19–29 µm, length of intermediate spines 8–12 µm, and length of lateral spines 14–15 µm. According to our data, the abundance is low, 100–600 ind./m3, and the species occurs mainly at the beginning of spring including under the ice. It was recorded in Uvildy Lake in August as well. It is a well-expressed cryobiont (0.1) and oligosaprobe (0.7); the species is euryhaline according to our findings and the literature data (May 1980; Ramdani et al., 2001; Jersabek and Bolortsetseg, 2010). According to the data of May (1980), the development of N. squamula is determined by the water temperature (the optimum is below 10°С); when water is warmed up to higher temperatures, the species develops in deeper layers near the bottom layers (Stemberger et al., 1979). Therefore, it is generally typical of the winter period (Barrabin, 2000). For example, in Chudskoe Lake N. squamula reached high abundance in the littoral zone from December and in the more slowly cooling pelagic part from January to April (Virro, 2001). At the same time, N. squamula was indicated as one of the main species of zooplankton in brackish shallow lakes in the Nile delta with the maximum development in summer at temperatures above 22°С (Ramdani et al., 2001), which, apparently, confirms the existence of a specific summer race of this species (Virro, 2001). The abundance of N. squamula is greatly determined by the abundance of consumed diatoms Asterionella formosa, whereas other microalgae (Chlorella, Microcystis, etc.) are rejected (May, 1980). Notholca squamula is one of the pioneer species of zooplankton in glacial lakes formed as a result of climate warming (Kammerlander et al., 2016). Notholca squamula is distributed in all geographical regions of the world except Oceania, but mainly in northern and temperate latitudes of the Holarctic. It is distributed all over Russia.
Like Brachionus, the genus Notholca is not widespread and is comparatively rare in the regions of the Urals studied; its representatives never reach high abundance. They are mainly cryobionts with respect to the biological properties and prefer oligotrophic waters, and their development occurs during the ice period and in early spring. Because most hydrobiological studies were performed in other periods, the data on the genus Notholca may be scarce for this reason as well.
REFERENCES
Akatova, N.A., A study of zooplankton of the Ural River and some floodplain water bodies near the village Yanvartsevo, West Kazakhstan region, Trudy Zool. Inst. Akad. Nauk SSSR, 1954, vol. 16, pp. 517–531.
Barrabin, J.M., The rotifers of Spanish reservoirs: ecological, systematical and zoogeographical remarks, Limnetica, 2000, vol. 19, pp. 91–167.
Bogdanov, V.D., Bogdanova, E.N., Gavrilov, A.L., Mel’nichenko, I.P., Stepanov, L.N., and Yarushina, M.I., Bioresursy vodnykh ekosistem Polyarnogo Urala (Bioresources of Aquatic Ecosystems of Polar Urals), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 2004.
Drabkova, V.G. and Sorokin, I.N., Ozero i ego vodosbor – edinaya prirodnaya sistema (A Lake and Its Watershed Are a Single Natural System), Leningrad: Nauka, 1979.
Dumont, H., Sarma, S.S.S., and Jawahar, A., Laboratory studies on the population dynamics of Anuraeopsis fissa (Rotifera) in relation to food density, Freshwater Biol., 1995, vol. 33, no. 1, pp. 39–46.
Furman, O. and Tiebo, M., The fauna of some lakes of the Urals: preliminary notes, Tr. Ural. O-va Lyubit.Estestvoznan., 1910, vol. 30, pp. 69–82.
Galkovskaja, G.A., Planktonic rotifers and temperature, Hydrobiologia, 1987, vol. 147, pp. 307–317.
Gillard, A., De Brachionidae van Belgie met Beschouwingen over de Taxonomie van de Familie, Natuurwet. tijdschr., 1948, vol. 30, nos. 6–7, pp. 159–208.
Graevskii, E.Ya. and Pogankin, M.V., Materials on the hydrofauna of the Kama River and its floodplain near Solikamsk (Chusovaya River), Izv. Biol. Nauchno-Issled. Inst. Perm.Gos. Univ., 1937, vol. 11, nos. 3–4, pp. 79–132.
Hwang, S.-J. and Heath, R.T., Zooplankton bacterivory at coastal and offshore sites of Lake Erie, J. Plankton Res., 1999, vol. 21, no. 4, pp. 699–719.
Jersabek, C.D. and Bolortsetseg, E., Mongolian rotifers (Rotifera, Monogononta)—a checklist with annotations on global distribution and autecology, Proc. Acad. Nat. Sci. Philadelphia, 2010, vol. 159, pp. 119–168.
Kammerlander, B., Koinig, K.A., Rott, E., Sommaruga, R., Tartarotti, B., Trattner, F., and Sonntag, B., Ciliate community structure and interactions within the planktonic food web in two alpine lakes of contrasting transparency, Freshwater Biol., 2016, vol. 61, pp. 1950–1965.
Kerentseva, N.P., Nabokikh, L.N., and Egoshin, V.V., Hydrobiology of the Kama River in the Okhansk–Galevo section, Uch. Zap. Molotov. Gos. Univ., 1946, vol. 4, no. 2, pp. 17–26.
Kirk, K.L., Competition in variable environments: experiments with planktonic rotifers, Freshwater Biol., 2002, vol. 47, no. 6, pp. 1089–1096.
Koval’kova, M.P., Kozlova, I.V., and Shilkova, E.V., Food resources of Lake Sugoyak and their use by fishes, Tr. Ural. Otd.SibNIIRKh, 1975, vol. 9, no. 2, pp. 3–10.
Kozlova, I.V., Plankton of Lake Kundravinskoe, Tr. Ural. Otd.SibNIIRKh, 1966, vol. 7, pp. 77–83.
Kozlova, I.V., Zooplankton of the Kaslinskie group of lakes and its production, Sb. Nauch. Tr. Nauchno-Issled. Inst. Ozern. Rechn. Rybn. Khoz., 1979, no. 10, pp. 118–124.
Kozlova, I.V., Changes in zooplankton communities of shallow fishless and carp-rich lakes of the Urals during rearing juvenile whitefish in them, Ekologiya, 1988, no. 2, pp. 25–29.
Krasnovskaya, M.P., Carp of Lake Yanychkovo and its importance as the main harvested object of Verkhne-Tavdinskie lakes, Sverdlovsk oblast, Tr. Ural. Otd. VNIIORKh, vol. 4, pp. 213–273.
Kuczyńska-Kippen, N. and Basińska, A., Spatio-temporal distribution of zooplankton between macrophyte and open water zones of Lake Wasowskie, Polska Akademia Nauk Oddział w Lublinie, 2008, vol. 5, pp. 75–84.
Kumar, R.R. and Rao, T.R., Effect of the cyclopoid copepod Mesocyclops thermocylopoides on the interactions between the predatory rotifer Asplanchna intermedia and its prey Brachionus calyciflorus and B. angularis,Hydrobiologia, 2001, vol. 453, nos. 1–3, pp. 261–268.
Kutikova, L.A., Kolovratki fauny SSSR (Rotatoria) (Rotifers of the Fauna of the USSR (Rotatoria)), Leningrad: Nauka, 1970.
Kutikova, L.A., Remarks on the rotifer fauna of the north and north-west waterbodies of Russia, Hydrobiologia, 1998, vols. 387/388, pp. 79–82.
Lazareva, V.I., Gusakov, V.A., Zinchenko, T.D., and Golovatyuk, L.V., Zooplankton of brakish rivers of the arid zone of southern Russia (Elton Lake basin), Zool. Zh., 2013, vol. 92, no. 8, pp. 882–892.
Lyubimova, T.S., Zooplankton of nursery ponds of the Chesmensky Fish Farm, Chelyabinsk oblast, Tr. Ural. Otd.SibNIIRKh, 1971, vol. 8, pp. 253–258.
Lyubimova, T.S Productional capacity of zooplankton of nursery ponds of the Chesmensky Fish Farm, Tr. Ural. Otd.SibNIIRKh, 1975, vol. 9, no. 1, pp. 201–210.
Makartseva, E.S., Species composition and productivity of zooplankton, in Ekologo-produktsionnye osobennosti ozer razlichnykh landshaftov Yuzhnogo Urala (Ecological and Productional Characteristics of Lakes of Different Landscapes of the Southern Urals), Leningrad: Nauka, 1978, pp. 150–188.
May, L., On the ecology of Notholca squamula Müller in Loch Leven, Kinross, Scotland, Hydrobiologia, 1980, vol. 73, pp. 177–180.
Muraveiskii, S.D., Observations on the spring plankton of the Ural River and its oxbows, Russ. Gidrobiol. Zh., 1923, vol. 2, pp. 14–23.
Nandini, S., Responses of rotifers and cladocerans to Microcystis aeruginosa (Cyanophyceae): a demographic study, Aquat. Ecol., 2000, vol. 34, no. 3, pp. 227–242.
Nandini, I. and Rao, T.R., Effect of the epizoic rotifer Brachionus rubens on the population growth of three cladoceran species, Hydrobiologia, 1993, vols. 255/256, pp. 325–332.
Nandini, S. and Rao, T.R., Somatic and population growth in selected cladoceran and rotifer species offered the cyanobacterium Microcystis aeruginosa as food, Aquat. Ecol., 1997, vol. 31, no. 3, pp. 283–298.
Nandini, S., Rerez-Chavez, R., and Sarma, S.S.S., The effect of prey morphology on the feeding behaviour and population growth of the predatory rotifer Asplanchna sieboldi: a case study using five species of Brachionus (Rotifera), Freshwater Biol., 2003, vol. 48, no. 12, pp. 2131–2140.
Oparina, N.Ya., The rotifer fauna in the vicinities of Perm, Tr. Biol. Nauchno-Issled. Inst. Perm.Gos. Univ., 1923, vol. 1, nos. 9–10, pp. 165–175.
Pejler, B. and Berzins, B., On choice of substrate and habitat in brachionid rotifers, Hydrobiologia, 1989, vols. 186/187, pp. 137–143.
Podlesnyi, A.V., Lake Turgoyak, Rab. Sib. Ikhtiol. Lab., 1927, vol. 2, no. 5, pp. 60–87.
Podshivalina, V.I. and Yakovlev, V.A., Monitoring the status of small and medium-size rivers of the forest–steppe Trans-Volga region by zooplankton, Voda: Khim. Ekol., 2012, no. 1, pp. 56–60.
Ramdani, M., Elkhiati, N., Flower, R.J., Birks, H.H., Kraiem, M.M., Fathi, A.A., and Patrick, S.T., Open water zooplankton communities in North African wetland lakes: the CASSARINA project, Aquat. Ecol., 2001, vol. 35, pp. 319–333.
Rao, T.R. and Kumar, R., Patterns of prey selectivity in the cyclopoid copepod Mesocyclops thermocylopoides,Aquat. Ecol., 2002, vol. 36, no. 3, pp. 411–424.
Rechkalov, V.V. and Marushkina, E.V., A study of the species composition and dynamics of zooplankton of Lake Sineglazovo, Vestn. Chelyab. Gos. Univ., 2005, vol. 12, no. 1, pp. 15–22.
Rogozin, A.G., Kolovratki Chelyabinskoi oblasti (Rotifers of Chelyabinsk oblast), Miass: Ilmen. Gos. Zapov., Ural. Otd., Ross. Akad. Nauk, 1995.
Rogozin, A.G., Ecology of zooplankton, in Ekologiya ozera Turgoyak (Ecology of Lake Turgoyak), Miass: Ilmen. Gos. Zapov., Ural. Otd., Ross. Akad. Nauk, 1998, pp. 84–113.
Rogozin, A.G., Comparative saprobiological characteristics of Turgoyak and Bol’shoe Miassovo lakes, Izv. Chelyab. Nauchn. Tsentra, 1998a, no. 1, pp. 81–85.
Rogozin, A.G., Zooplankton of a hypertrophic reservoir as exemplified by Lake Tabankul (Southern Urals): biological diversity and biology of some species of rotifers, Izv. Chelyab. Nauchn. Tsentra, 2006, no. 3, pp. 78–82.
Rogozin, A.G., Zooplankton of Lake Uvil’dy, Izv. Chelyab. Nauchn. Tsentra, 2009, no. 1, pp. 62–67.
Rogozin, A.G. and Gavrilkina, S.V., Long-term changes in the thermal regime of Bol’shoe Miassovo Lake (Southern Urals) as a result of climate warming, Meteorol. Gidrol., 2015, no. 8, pp. 98–102.
Rogozin, A.G., Snit’ko, L.V., and Timoshkin, O.A., Thermoindicator properties of zooplankton species and their measurements, Water Resour., 2015, vol. 42, no. 1, pp. 91–97.
Rothaupt, K.O., The influence of toxic and filamentous blue-green algae on feeding and population growth of the rotifer Brachionus rubens,Internationale Revue der Gesamten Hydrobiologie, 1991, vol. 76, no. 1, pp. 67–72.
Shumka, S., Rotifers in the littoral zone of Lake Shkodra/Skadar (Albania–Montenegro) as a tool for determining water quality, Int. Res. J. Biol. Sci., 2014, vol. 3, no. 3, pp. 71–77.
Sladeček, V., Rotifers as indicators of water quality, Hydrobiologia, 1983, vol. 100, pp. 169–201.
Sommaruga, R., Microbial and classical food webs: a visit to a hypertrophic lake, FEMS Microbiol. Ecol., 1995, vol. 17, pp. 257–270.
Stelzer, C.-P., Population growth in planktonic rotifers. Does temperature shift the competitive advantage for different species?, Hydrobiologia, 1998, vols. 387/388, pp. 349–353.
Stemberger, R.S., Cannon, J.E., and Bricker, F.J., Spatial and Seasonal Structure of Rotifer Communities in Lake Huron, Duluth: Environmental Research Laboratory Office of United States EPA, 1979.
Tauson, A.O., Hydrobiological essay on lakes and rivers of the Garinskii district of Sverdlovsk oblast and their fishery assessment, Uch. Zap. Perm.Gos. Univ., 1936, vol. 2, no. 1, pp. 85–165.
Tauson, A.O., Zooplankton of the Kama River on the Galevo–Belaya River section, Izv. Estestv.-Nauch. Inst. Molotov. Gos. Univ., 1946, vol. 12, no. 5, pp. 155–167.
Tauson, A.O., Plankton of the upper Kama River, Uch. Zap. Molotov. Gos. Univ., 1947, vol. 4, no. 2, pp. 3–16.
Teoreticheskie voprosy klassifikatsii ozer (Theoretical Problems of Lake Classification), St. Petersburg: Nauka, 1993.
Virro, T., Life cycle patterns of rotifers in Lake Peipsi, Hydrobiologia, 2001, vols. 446–447, no. 1, pp. 85–93.
Walz, N., Gschloessl, T., and Hartmann, U., Temperature aspects of ecological bioenergetics in Brachionas angularis (Rotatoria), Hydrobiologia, 1989, vols. 186/187, pp. 363–369.
Wen, X.-L., Xi, Y.-L., Yang, Y.-F., Zhang, X.-A., and Zhang, G., Temperature is the key factor controlling population dynamics of Brachionus angularis in Lake Jinghu during summer and autumn, J. Freshwater Ecol., 2011, vol. 26, no. 2, pp. 277–286.
Zinov’ev, A.P., Water bodies of the Troitskii Forest–Steppe Reserve and their fauna (Copepoda and Phillopoda), Tr. Biol. Nauchno-Issled. Inst. Perm.Gos. Univ., 1931, vol. 3, no. 4, pp. 281–367.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by N. Ruban
Rights and permissions
About this article
Cite this article
Rogozin, A.G. Materials on the Fauna and Ecology of Rotifers in the Ural Region: Family Brachionidae (Rotifera, Eurotatoria, Ploima). Genera Anuraeopsis, Brachionus, and Notholca. Biol Bull Russ Acad Sci 46, 823–833 (2019). https://doi.org/10.1134/S1062359019080132
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359019080132