Abstract
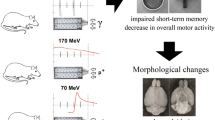
The early delayed effects of accelerated carbon ions and protons on the cognitive functions of mice using tests of the total activity, spatial learning, and long-term and short-term hippocampal-dependent memory were studied. The obtained results showed that irradiated animals do not develop an altered behavioral pattern: the level of anxiety is not increased, the exploratory model of behavior is clearly pronounced, and there is no deficiency of hippocampal-dependent memory. However, the long-term memory test revealed fewer errors in finding an escape box in a group of animals irradiated with protons compared to the control animals and mice irradiated with carbon ions. The results may indicate a better preservation of memory traces under these conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
For over a century, radiation therapy has been one of the most effective treatments for cancer. Each year, more than 200 000 patients are exposed to brain irradiation, during which the healthy tissues surrounding tumors are also exposed with the possibility of the development of various neurological disorders in patients. We are talking about both acute and chronic radiation-induced brain injury, as well as radiation encephalopathy. Such disorders are clinically manifested as focal neurological deficits, secondary epilepsy, mental and behavioral disorders, increased intracranial pressure, and progressive disorders of hippocampal-mediated learning and memory, which significantly impairs the quality of patients’ extended life [1]. Depending on the duration of clinical symptoms after a course of radiation therapy, the effects are classified as acute, early delayed, and late delayed damage [2]. Acute brain damage occurs during and/or within a few days after exposure. Early delayed damage occurs 1.5–3 months after irradiation; however, some researchers believe that this time should be increased to six months [3]. Although both types of these injuries can lead to serious consequences for the patient’s health, they most often go away after short-term targeted treatment. In contrast, late delayed brain damage usually develops after six months after irradiation and is considered irreversible, with progressive pathogenesis [4].
In the past decade, proton and ionic therapy of tumors has actively developed and become a priority among radiation methods of fighting cancer. Due to the unique properties of heavy ions, which make it possible to unload the dose in a given volume with high accuracy, it is possible to suppress effectively the growth of tumors located deeply or in close proximity to the anatomical structures that are sensitive to radiation, while minimizing the load on surrounding healthy tissues [5]. Despite the fact that most patients, in particular those with tumors in the head and neck, undergo radiation therapy using X-rays or γ-rays, to date, a large amount of information has accumulated on the benefits of hadron therapy. In this regard, more and more radiological centers are trying to switch to the use of intensively modulated radiation therapy based on accelerators with the extraction of proton or carbon ion beams in order to deliver more safely and efficiently accurate radiation doses to the tumor while minimizing the burden on the surrounding healthy tissues [6]. Currently, the proton therapy complex “Prometheus”, specializing in the treatment of tumors in the head and neck of humans, and at the same time allowing experiments on animals, is currently operating at the Physical Technical Center of Lebedev Physical Institute (Protvino). The Center for Collective Use “Radiobiological Stand on a Carbon Beam U-70” on the basis of the Institute for High Energy Physics named by A.A. Logunov of National Research Centre “Kurchatov Institute” (Protvino) is intensively studying beams of accelerated carbon ions with an energy of 450 MeV/nucleon.
It should be noted that there is a lack of systematic experimental data on the bioeffects of protons and accelerated carbon ions with different characteristics. As a result, there are no fundamental foundations for the specific action of accelerated particles on critical structures and processes in various organs and tissues during total or local irradiation in doses characteristic of outer space, as well as in conventional radiation therapy. One of the problems of generalizing the data obtained from different accelerators is the sharply differing technical capabilities of the equipment that forms particle beams with different physical characteristics, as well as the different methods of dose delivery. This requires experimental studies on a specific installation under different irradiation conditions in vivo, which would allow in the future to adapt the therapeutic settings quickly for practical purposes and vary approaches in the treatment of tumors of different types and locations. Published data on the effect of different types of densely ionizing radiation on the cognitive functions and neurogenesis of laboratory animals irradiated by the radiotherapy scheme or when modeling the conditions of long-term space flights are rather scarce, contradictory, and depend on the type of object, dose, irradiation mode, applied methods, and timing of testing [7]. In the course of a comprehensive radiobiological study of carbon ions of the U-70 accelerator complex and a thin scanning proton beam at the Prometeus facility, it became possible to assess the effect on the behavior of mice of these types of radiation in absorbed doses used in radiotherapy and simulating radiation effects on the central nervous system during prolonged space flights.
The aim of this work is to evaluate the early delayed effects of accelerated carbon ions and protons on the cognitive functions of mice using tests for general exploratory activity, spatial learning, and short-term and long-term hippocampus-dependent memory.
MATERIALS AND METHODS
The experiments were carried out on two-month-old male white outbred mice of the SHK colony (28–34 g). Animals were kept in cages of ten individuals under standard conditions of the vivarium of the Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences (ITEB RAS) and were divided into three groups: unirradiated control, irradiated with protons, and irradiated with accelerated carbon ions.
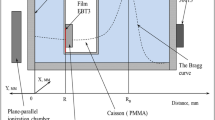
Animals were anesthetized with a xylazine–zoletilic mixture and placed in special well-aerated plexiglas box. To calculate the irradiation plan using a cone beam computed tomograph, three-dimensional images of the cell with the animal were obtained (Figs. 1a, 1b). The head of the mice was irradiated with a dose of 1.8 Gy on a proton synchrotron (Proton therapy complex Prometeus, PTC LPI RAS, Protvino) with a thin proton beam from one direction by scanning along a given target volume in a modified Bragg peak with proton energy at the output of the accelerator of 85–100 MeV and a Sigma beam of 2.8–3.6 mm. The irradiation mode is pulsed with a duration of 200 ms and a cycle of 2 s. In such a cyclic mode of operation of the setup, each cycle of protons is injected into the channel, accelerated to a given energy, and then released into the given target. The dose rate was 1.5 Gy/min. Dose control was carried out by a clinical dosimeter based on a diamond detector (IFTP, Russia) and a dosimetric film (Gafchromic radiotherapy film EBT2, United States).
Another group of animals was subjected to total exposure to accelerated carbon ions in a modified Bragg peak at a dose of 1.5 Gy on the premises of the temporary radiobiological stand of the U-70 accelerator complex (NSC IHEP, Protvino). The beam energy at the accelerator output was 450 MeV/nucleon. The experimental setup brought up to 1.0 × 109 carbon nuclei in the slow withdrawal mode with a cycle of 8 s and a duration of the withdrawal of 0.6 s. The dose rate was 1.6 Gy/min. Irradiation was carried out in a uniform beam formed by a “wobbler” magnet. The exposure session was accompanied by an EBT3 dosimetric film (Gafchromic® film), mounted on the end of the container with mice from the side of the incident beam, and was controlled using a neutron monitor—a sphere made of polyethylene with a thermal neutron detector inside. The control mice were also anesthetized and subjected to “false” irradiation with the contents under the same conditions as the experimental groups.
Three months after irradiation, which corresponds to the terms of early delayed damage, the following set of methods was used to evaluate the total activity, spatial learning, and short-term and long-term hippocampus-dependent memory: the open field test, the test for recognizing a new object, and the Barnes maze. The “open field” setup is used to determine the level of motor, orientational-exploratory activity, and the ratio of active and passive defensive reactions in a moderately stressful environment [8]. This test, proposed by C.S. Hall in 1936, makes it possible to assess the severity of elementary behavioral acts in rodents under stressful conditions that arise in response to placing a laboratory animal in an installation that has a larger area and intensity of illumination than a cage for its daily maintenance. A large number of “runs” to the center of the open field testifies to the predominance of tentative exploratory behavior over low stress levels in animals. The emotional status is assessed by criteria such as the frequency and duration of grooming, as well as the number of urinations and bowel movements. The observation time in the open field test took four minutes. The Barnes Maze is used to evaluate spatial learning and memory. This test consists of two phases: training for three days and testing on the third and nineth days after training. The observation time took three minutes. The distance moved, speed, and time spent both in the correct sector and in general during the session are recorded, as well as the time delay before finding shelter. Moderate negative reinforcements (bright light, fan) provide motivation for the animal to seek refuge. Being less stressful, it serves as a good alternative to the Morris test. To study disturbance of the functions of nonspatial hippocameral short-term memory, a new object recognition test was applied, which is based on memorization of familiar objects and the natural preference for rodents for novelty, which makes it possible to reveal the selective effect on attention and episodic memory. During the experiment, at the stages of training and testing, the cumulative time of study of the familiar and new objects is recorded and the discrimination coefficient is calculated (KD). Testing was carried out in the “open field” installation, with which the animals were already familiar. The observation time took five minutes with an interval between steps of 15–20 minutes. All behavioral tests were accompanied by automatic video tracking of mice using special software.
The analysis of the significance of differences between groups was performed using the Mann–Whitney U-test (significance level p < 0.05). Statistical comparisons of learning curves were performed using ANOVA analysis of variance in the IgorPro 8 statistical analysis software package.
RESULTS
As can be seen from the histograms in Figs. 2a and 2b, mice irradiated with both protons and carbon ions were more active (moving speed and total distance moved higher), they often went to the center of the open field and spent more time there compared to unirradiated control animals (Figs. 2c, 2d). In addition to assess exploratory activity, vertical activity was analyzed by the number of racks; and to estimate the emotional state of animals, the number of acts of grooming, bowel movements, and urination was analyzed. Mice after exposure to accelerated carbon ions showed a higher level of vertical activity (p < 0.05), while the level of anxiety in the irradiated animals of both groups did not differ from the control (data not illustrated). The total observations can indicate not only an increase in locomotor activity in irradiated animals, but also the predominance of exploratory behavior over the defensive reaction in a group of mice irradiated with accelerated carbon ions.
Figure 3a shows the learning curves that demonstrate a decrease in the time taken to finding the target hole and a decrease in the number of errors (the number of hole pokes studied) with an increase in the number of trials (nine sessions). All animal groups showed good training for three days in the Barnes maze. The analysis of variance of the learning curves by the criterion of the time of the search for target hole revealed significant differences in the test for carbon-irradiated mice. Mice from this group were faster than others in finding shelter in the first days of testing; however, the dynamics of performance by mice in locating the target hole was slower compared to the control and proton-irradiated groups, with a longer search at the beginning of training. It should be noted that when analyzing the number of errors, i.e., incorrect hole pokes (without shelter), no differences were found between the experimental groups (Fig. 3b). All mice made the same number of errors at each stage of training. The faster passage of the maze by a group of mice after irradiation with accelerated carbon ions is probably not associated with improved learning and long-term memory, but rather with more pronounced exploratory activity and a high speed of movement through the maze. The delay before finding the target hole at the end of training in all groups was the same, which indicates the same level of acquired skill before the final test.
As follows from Fig. 4, when conducting the test for long-term memory, animals irradiated with protons showed a smaller number of errors in finding the target hole compared with the control group and animals irradiated with accelerated carbon ions.
When conducting the test for recognizing a new object, it was revealed that when replacing an old object with a new frequency of approaches and the time spent in a new object is most pronounced in an unirradiated group of mice. An analysis of the preference for novelty in testing showed that the discrimination rate (KD) for control mice, as well as groups irradiated with accelerated carbon ions and protons, three months after irradiation was 0.270, 0.167, and 0.032, respectively. Therefore, three months after irradiation in all the groups studied, there were no destructions of nonspatial hippocampal mediated short-term memory: the animals spent much more time exploring a new object than making acquaintance at the testing stage, while the preference for novelty in mice was least pronounced in the group irradiated with protons (Fig. 5).
The effect of accelerated carbon ions and protons on nonspatial short-term memory in mice: average values of the discrimination coefficient using the novel objects recognition test. KD = t(A2) – t(A1) /t(A2) + t(A1), where t(A2) is the total exploration time for a new object, and t(A1) is the total exploration time for an old object. The values of KD > 0.1 mean that the animal distinguishes new and old objects.
DISCUSSION
Radiation therapy is widely used to treat disseminated primary and metastatic brain cancer, and in many patients who have lived for more than six months after treatment, subsequently chronic radiation-induced disorders of the central nervous system develop. This is especially true for radiation therapy of brain tumors in children, after which, against the background of an increase in life expectancy, its quality often significantly deteriorates due to cognitive impairment. Although the pathogenesis of these disorders is still unknown, one of the main mechanisms may be a decrease in neurogenesis in the hippocampus. There are very few clinical and experimental data on the effect of irradiation with protons and carbon ions in therapeutic doses on various cognitive functions and their relationship with neurochemical processes in different parts of the brain, especially in the early long-term after exposure, compared with a large number of studies on the effect of X-ray radiation. Thus, in [9], the brain of a male C57BL/J6 mouse at the age of 21 days was irradiated in the dose range of 2–10 Gy of X-ray radiation; 48 hours later, an immunohistochemical analysis was performed. It was shown that the number of proliferating cells of the dentate subgranular zone of the hippocampus and their offspring, immature neurons, decreased in a dose-dependent manner. After irradiation of mice at a dose of 5 Gy and staining of proliferating BrdU cells after one and three months, it was found that irradiation significantly reduced the production of new neurons, but did not alter the glial components of the brain. It was noted that reduced neurogenesis is associated with a chronic inflammatory reaction that, three months after irradiation, became clinically accompanied by a spatial memory deficit. These data confirmed that irradiation of young animals causes long-term deterioration of neurogenesis, which subsequently causes hippocampus-dependent memory deficiency.
In our work, the irradiation of male mice at the age of two months was carried out with accelerated carbon ions totally and with a thin scanning proton beam cranially. It was revealed that, three months after exposure, the animal behavior pattern does not change: the level of anxiety is not increased, animals show high locomotor activity with a likely increase in the exploratory behavior, and there is no deficit of short-term and long-term hippocampus-dependent memory. Interestingly, in the estimation of long-term memory using the Barnes test the group of animals local head irradiated with protons makes significantly fewer errors in finding a target hole compared to control mice and a group of animals whole irradiated with accelerated carbon ions. This may indicate a better preservation of memory traces, as well as a decrease in afferent generalization, when animals are more focused on conditioned stimuli, while more quickly solving the problem [10]. In addition, in the works of Rabin et al., it was shown that irradiation of the whole organism causes more significant disturbances in the central nervous system compared with local head irradiation in equivalent dose values [11]. Similar results were obtained when rats were irradiated with protons with an energy of 165 MeV at a dose of 1.5 Gy, which caused an improvement in the working memory of animals, but this effect was inverted when the dose was increased to 3 Gy [12]. In addition, in model experiments on monkeys irradiated with high-energy protons and carbon ions, it was demonstrated that irradiation of the head with protons at a dose of 3 Gy did not cause any significant changes either in the animal’s cognitive functions or in the concentrations of monoamines and their metabolites in the peripheral blood. Moreover, exposure to carbon ions at a dose of 1 Gy led to a significant deterioration in cognitive functions and a significant decrease in the concentration of serotonin metabolites in monkey blood [13]. In [14], the effect of proton irradiation at doses of 1 and 2 Gy in the Bragg peak with an energy of 170 MeV on the learning, reproduction of skills, and the concentration of monoamines and their metabolites in the hippocampus and other brain structures of Wistar rats was studied. When irradiated at a dose of 2 Gy, there was a tendency toward progression of disturbances in the functioning of long-term working memory already formed at the time of irradiation. At the same time, irradiation at a dose of 1 Gy led to a less significant deterioration and the decrease in the learning coefficient did not reach a significant level. Irradiation with protons in both doses did not affect the production and reproduction of the passive avoidance reflex. The authors note a decrease in the concentration of catecholamines in the prefrontal cortex and a concentration of 3-MT and a dopamine metabolite in the striatum, while no significant changes were found in all other structures, including the hypothalamus. When studying the total effect of accelerated carbon ions with an energy of 500 MeV at a dose of 1 Gy, it was shown that, 30 and 90 days after irradiation, changes in the functioning of noradrenaline, dopamine, and serotonergic systems occur in the rat brain. The greatest differences were observed in the prefrontal cortex and the hypothalamus, which indicates the important role of these parts of the brain in the implementation of the late effects of radiation on the functions of the central nervous system. The authors believe that some time after exposure, compensatory-restoration mechanisms are actively implemented, which, at relatively low linear energy transfer (LET) values, can ultimately lead to the restoration of some cognitive functions [15].
The use of a single dose in our work, equivalent in size to one fraction of the course of radiotherapy, was due to the fact that the most promising direction in proton and ion therapy is currently hypofractionation, i.e., an increase in a single dose and a decrease in the number of fractions in the course. In this regard, we plan to test higher doses than those approved in conventional radiotherapy. Earlier, when studying the hypofractionated regime of irradiation of the solid form of Ehrlich ascites carcinoma, not only was the possibility of complete suppression of tumor growth shown, so was a decrease in the long-term radiation reactions of the skin, recurrence, and the effect on the average life span of animals [16]. It is difficult to imagine how carbon ions with a higher RBE compared to protons, which was found on other objects and test systems, will affect cognitive functions in high-precision irradiation of head tumors. For example, it was shown that fractional exposure of the head of 6- to 9-year-old primates at a dose of 40 Gy of X-rays (5 Gy twice a week for four weeks) results, six months after irradiation, in a significant disturbance of the recognition test of a new object, which is closely related to the work of the periarchinal cortex [17].
The nature of the effects obtained in mice in the early long-term period when exposed to a thin scanning beam of protons and accelerated carbon ions allows us to conclude that there is no negative effect on the cognitive abilities of laboratory animals. It will open up prospects for the next step in the development of hadron therapy—the possibility of increasing a single dose of radiation and developing hypofraction schemes in the treatment of cancer.
REFERENCES
Bramlett, H.M. and Dietrich, W.D., Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes, J. Neurotrauma, 2015, vol. 32, pp. 1834–1848.
Sheline, G.E., Radiation therapy of brain tumors, Cancer, 1977, vol. 39, suppl. 2, pp. 873–881.
Yan, L., Xi, Z., and Drettner, B., Epidemiological studies of nasopharyngeal cancer in the Guangzhou area, China. Preliminary report, Acta Otolaryngol., 1989, vol. 107, nos. 5–6, pp. 424–427.
Brown, W.R., Blair, R.M., Moody, D.M., et al., Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia, J. Neurol. Sci., 2007, vol. 257, nos. 1–2, pp. 67–71.
Khmelevskii, E.V., Radiation therapy for prostate cancer: photons, protons, or heavy ions, Radiats. Onkol. Yad. Med., 2013, no. 1, pp. 28–33.
Cheung, K.Y., Intensity modulated radiotherapy: advantages, limitations and future developments, Biomed. Imaging Interv. J., 2006, vol. 2, no. 1, p. e19.
Kiffer, F., Boerma, M., and Allen, A., Behavioral effects of space radiation: a comprehensive review of animal studies, Life Sci. Space Res., 2019, vol. 21, pp. 1–21.
Whimbey, A.E. and Denenberg, V.H., Two independent behavioral dimensions in open-field performance, J. Comp. Physiol. Psychol., 1967, vol. 63, no. 3, pp. 500–504.
Rola, R., Raber, J., Rizk, A., et al., Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice, Exp. Neurol., 2004, vol. 188, pp. 316–330.
Ushakov, I.B., Shtemberg, A.S., and Shafirkin, A.V., Reaktivnost’ i rezistentnost’ organizma mlekopitayuschikh. Printsipy formirovaniya, regulyatsii i prognozirovaniya (The Reactivity and Resistance of the Mammalian Organism. The Principles of Formation, Regulation, and Forecasting), Moscow: Nauka, 2007.
Rabin, B.M., Shukitt-Hale, B., Carrihill-Knoll, K.L., and Gomes, S.M., Comparison of the effects of partial- or whole-body exposures to 16O particles on cognitive performance in rats, Radiat. Res., 2014, vol. 3, no. 181, pp. 251–257.
Shtemberg, A.S., Bazyan, A.S., Lebedeva-Georgievskaya, K.D., et al., Effect of high-energy proton irradiation on the behavior of rats and its neurochemical mechanisms, Aviakosm. Ekol. Med., 2013, vol. 47, no. 6, pp. 54–60.
Belyaeva, A.G., Shtemberg, A.S., Nosovskii, A.M., et al., Effect of high-energy protons and 12C carbon ions on cognitive functions of monkeys and the content of monoamines and their metabolites in peripheral blood, Neurochem. J., 2017, vol. 34, no. 1, pp. 1–9.
Shtemberg, A.S., Kokhan, V.S., Kudrin, V.S., et al., The effect of high-energy protons in the Bragg peak on the behavior of rats and the exchange of monoamines in some brain structures, Neurochem. J., 2015, vol. 32, no. 1, pp. 78–85.
Belokopytova, K.V., Belov, O.V., Kudrin, V.S., et al., Dynamics of metabolism of monoamines in the structures of the rat brain at a later time after irradiation with accelerated carbon ions, Neurochem. J., 2016, vol. 33, no. 2, pp. 147–155.
Balakin, V.E., Shemyakov, A.E., Zaichkina, S.I., et al., Long-term radiation effects after hypofractionated irradiation with protons of solid Ehrlich carcinoma in mice, Biophysics (Moscow), 2017, vol. 62, no. 1, pp. 138–142.
Robbins, M.E., Payne, V., Tommasi, E., et al., The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment, Int. J. Radiat. Oncol. Biol. Phys., 2009, vol. 73, pp. 499–505.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. The study was approved by the Biosafety and Bioethics Commission of the ITEB RAS (protocol no. 23). The experiments were carried out in accordance with the requirements of the Federation of European Scientific Associations for the maintenance and use of laboratory animals in scientific research (FELASA).
Rights and permissions
About this article
Cite this article
Sorokina, S.S., Zaichkina, S.I., Rozanova, O.M. et al. The Early Delayed Effect of Accelerated Carbon Ions and Protons on the Cognitive Functions of Mice. Biol Bull Russ Acad Sci 47, 1651–1658 (2020). https://doi.org/10.1134/S1062359020120109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359020120109