Abstract
People often encounter various sources of ionizing radiation, both in modern medicine and under various environmental conditions, such as space travel, nuclear power plants or in conditions of man-made disasters that may lead to long-term cognitive impairment. Whilst the effect of exposure to low and high doses of gamma and X-radiation on the central nervous system (CNS) has been well investigated, the consequences of protons and heavy ions irradiation are quite different and poorly understood. As for the assessment of long-term effects of carbon ions on cognitive abilities and neurodegeneration, very few data appeared in the literature. The main object of the research is to investigate the effects of accelerated carbon ions on the cognitive function. Experiments were performed on male SHK mice at an age of two months. Mice were irradiated with a dose of 0.7 Gy of accelerated carbon ions with an energy of 450 meV/n in spread-out Bragg peak (SOBP) on a U-70 particle accelerator (Protvino, Russia). Two months after the irradiation, mice were tested for total activity, spatial learning, as well as long- and short-term hippocampus-dependent memory. One month after the evaluation of cognitive activity, histological analysis of dorsal hippocampus was carried out to assess its morphological state and to reveal late neuronal degeneration. It was found that the mice irradiated with accelerated carbon ions develop an altered behavioral pattern characterized by anxiety and a shortage in hippocampal-dependent memory retention, but not in episodic memory. Nissl staining revealed a reduction in the number of cells in the dorsal hippocampus of irradiated mice, with the most pronounced reduction in cell density observed in the dentate gyrus (DG) hilus. Also, the length of the CA3 field of the dorsal hippocampus was significantly reduced, and the number of cells in it was moderately decreased. Experiments with the use of Fluoro-Jade B (FJB) staining revealed no FJB-positive regions in the dorsal hippocampus of irradiated and control animals 3 months after the irradiation. Thus, no morbid cells were detected in irradiated and control groups. The results obtained indicate that total irradiation with a low dose of carbon ions can produce a cognitive deficit in adult mice without evidence of neurodegenerative pathologic changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People often encounter various sources of ionizing radiation, both in modern medicine and under various environmental conditions, such as space travel, working at nuclear power plants or in conditions of man-made disasters. A large number of studies have highlighted the potential radiation hazard of space exposure (Rabin et al. 2003; Casadesus et al. 2004; Barker et al. 2007). To limit the negative impact of radiation on human performance and resistance to diseases such as Alzheimer’s, cancer, stroke, and many others, it is necessary to assess the risk level for the central nervous system. Cosmic rays are composed of high-energy nuclei of all stable elements, including oxygen, helium, hydrogen, iron, and carbon. Shukitt-Hale et al. (2000) ; Higuchi et al. (2002) with colleagues have shown that whole-body 56Fe-particle irradiations can induce specific hippocampus-dependent cognitive impairment in rats. It was assumed that therapeutic irradiation of the brain and the spinal cord is associated with cognitive impairment. In works of Fike and Gobbel (1991) it was shown that relatively high radiation doses can cause not only functional but also structural injuries. A few years later, it was demonstrated that different doses of radiation affect brain function, but not morphology, inducing cognitive deficit in both adults and young patients (Roman and Sperduto 1995; Abayomi 1996). These cognitive disorders are hippocampus-dependent and affect processes such as learning, memory, and spatial information processing (Meyers et al. 2000).
About 50–90% of patients who have undergone radiation therapy for primary and metastatic brain cancer show a cognitive deficit that reduces their quality of life (Makale et al. 2017). In the last few years, carbon ions have become increasingly used in radiotherapy of tumors (Cui et al. 2010). The ability to selectively target the tumor tissue, protecting nearby healthy tissue, makes carbon ions a more promising radiotherapy tool compared to X-radiation (Rabin et al. 2003; Kim et al. 2008; Marty et al. 2014), while the data on the impact of carbon ions on cognitive processes and neuronal proliferation are limited.
The mechanisms of radiation-induced cognitive decline are still unknown. It is believed that it may be accompanied by changes in the microenvironment of the CNS (Greene-Schloesser et al. 2012; Makale et al. 2017), vascular neuroinflammation and impaired function of neuron progenitor cells in the DG of the hippocampus (Monje et al. 2002), as well as changes in neurogenesis. Due to the fact that hippocampal neurogenesis mediates cognitive functions (Gould et al. 1999; Kempermann 2002), it was hypothesized that after high-LET particle exposure there is a decrease in the production of neurons, which in turn plays an important role in radiation-induced cognitive disorders (Rola et al. 2004). In addition, there is currently abundant evidence that doses of ionizing radiation higher than certain thresholds can induce microglia activation and pro-inflammatory derived factors that contribute to radiation-induced brain damage (Monje et al. 2002; Hwang et al. 2006).
Earlier we have investigated the biological effects induced by accelerated carbon ions with an energy of 450 meV/n in the SOBP (0.1–1.5 Gy) in mice: dose dependence of cytogenetic damage to the bone marrow, weight index for the thymus and the spleen, and induction of reactive oxygen species in whole blood. In the case of carbon ions characterized by a larger relative biological effectiveness, we observed a higher level of cytogenetic damage to the bone marrow and spontaneous reactive oxygen species production in blood cells, as well as a lower weight index for the lymphoid organs compared with X-ray-irradiated mice (Sorokina et al. 2017). The significant physiological impact of these ions suggests higher health risks, including a possible cognitive deficit, for people exposed to high-LET radiation during space travel and radiotherapy. Several studies have reported cognitive impairment in animals irradiated with HZE (heavy ions) doses of 0.01–0.2 Gy, suggesting that the doses related to NASA’s planned mission to Mars pose significant health risks, and even lower doses are currently required to be investigated (Rabin et al. 2015; Wyrobek and Britten 2016; Britten et al. 2018).
Whilst the effects of exposure to low- and high doses of gamma and X-radiation on the CNS have been well investigated, the consequences of proton and heavy ion irradiation are quite different and poorly understood. As for the assessment of long-term effects of carbon ions on cognitive abilities and neurodegeneration, very limited literature data have appeared.
The main objective of the research presented here is to investigate the effects of a comparatively low dose (0.7 Gy) of accelerated carbon ions on the cognitive function in mice.
Materials and methods
Irradiation procedure
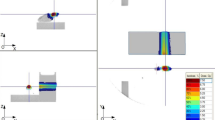
Experiments were performed on 2-month-old outbred albino SHK male mice. The experiments conformed to the regulations and legal acts concerning the procedures of animal experiments and the humane treatment of animals carried out following the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. Ten mice per cage were housed in a vivarium on a 12 h light–12 h dark cycle regime with free access to food and water (ITEB RAS, Russia). Unanesthetized animals were used as a control. Mice were placed in a rectangular plexiglass block (two mice in a container) immediately before the irradiation. Mice (n = 10) were irradiated with a dose of 0.7 Gy of accelerated carbon ions with an energy of 450 meV/n on a U-70 accelerator (NRC «Kurchatov Institute»—IHEP, Russia). To ensure uniform biological dose distribution within the mice body volume, the irradiation was carried out in SOBP. The Bragg peak was modified with the use of a ridge filter, which expanded the zone of maximum energy release by ions up to 10 mm. The estimated LET for carbon ions in the modified Bragg peak was 100 keV/μm. The dose rate was 1.6 Gy/min. Mice were deeply anesthetized during irradiation and placed in a caisson in such a way that the body of the mouse was perpendicular to the beam (Fig. 1). The coordinates of the caisson were chosen so that the body of the animal is located at the zone of the uniform transverse irradiation field. The verification of the carbon beam profile and dose control was carried out using a neutron monitor and the gafchromic EBT3 films (USA) and the mosaic plane-parallel ionization chamber. Unirradiated animals (n = 10) were subjected to the same handling procedures, but with the switched off setup.
Two months after irradiation, mice were tested for total activity, spatial learning, and long- and short-term hippocampus-dependent memory (open field, Barnes maze, novel object recognition). One month after the evaluation of cognitive activity, histological analysis of the dorsal hippocampus was carried out to assess its morphological state by staining with Nissl and to reveal late neuronal degeneration by staining with Fluoro Jade-B.
Open field
Open field tests were performed as described (Christmas and Maxwell 1970). An open field was represented as a white square box with a size of 60 × 40 cm, with a video camera set up at a height of 1.5 m to record the trials. Animals were individually placed in the center of the open field space, exacerbate the uncomfortable conditions with bright fluorescent light and a fan. A behavioral and movement analyses were performed with a custom-made software. Animal activity was recorded for 4 min at 10:00 am. The following behavioral parameters were studied: the frequency of entry and duration of stay in the center and corners of the box, the speed and distance that the animal moves during the tests.
Barnes maze
The Barnes circular maze (110 cm in diameter, 20 holes) has been described (Barnes 1994). Mice were trained to enter the escape box by placing them outside the escape box in the Barnes maze for up to 3 min after which mice were left in the escape box for 2 min. The following day, mice were placed in the center of the maze and given 3 min to locate the escape box. The number of mistakes (incorrect hole pokes), latency to finding and entering the escape box, and the navigation patterns were recorded. Mice that failed to enter the escape box within 3 min were guided to the box and remained there for 2 min prior to return to their home cage. Mice were subjected to three trials per day at 15–20-min intervals for 3 days. At the beginning of a trial, each mouse was placed at the center of the maze and bright fluorescent light and fan were activated to motivate escape behavior. Each trial ended when the mouse entered the escape box or 2 min after the start of recording. To determine how well an animal learned the location of the goal box, two tests without the escape box were performed, on the second and ninth days after training trials. All trials, the path length and movement velocity were recorded by the custom-made software.
Novel object recognition test (NOR)
The NOR test can be applied to study such problems as learning, memory, novelty preference; moreover, it can show the involvement of different regions of the brain in recognition process. In comfortable conditions at the first stage of acquaintance with a new environment, mice touch two objects placed in the arena with equal frequency, but replacing one object with a new one, they approach it more often and explore it longer than the already familiar object. With the formula DI = (TN−TF)/(TN + TF), we can calculate the index of stimulus recognition or discrimination index (DI) (Baxter 2010), where TN is the exploration time devoted to a novel object, and TF is the time devoted to the familiar object. The index can vary between + 1 and − 1, where a positive score indicates that the animal spent more time with the novel object, a negative score indicates that the animal spent more time with the familiar object, and a zero score indicates a null preference (Barker et al. 2007; Oliveira et al. 2010). Mice were tested in two consecutive trials with a 15-min interval. For the testing sessions, two identical plastic objects were placed in the open field, and each mouse was allowed to explore them for 5 min. Twenty-five minutes after the first trial (familiarization phase), each animal was tested in a trial with one of the familiar objects was replaced by a novel object. The working memory was assessed using the DI.
Histology
Three months after the irradiation, animals were decapitated; brain was rapidly removed from the skull and placed in cold 4% paraformaldehyde (in PBS, at 4 °C for 48 h). After cryoprotection in a gradient of sucrose (10% and 20% sucrose in PBS at 4 °C for 24 h each), brains were rapidly frozen in the vapor phase of liquid nitrogen. Coronal Sects. (15 μm) were cut with a cryostat at − 19 °C (Thermo Shandon Cryotome E, Thermo Scientific, USA) and collected on poly-l-lysine coated slides for subsequent Nissl and Fluoro-Jade B staining. Nissl staining was used to detect cell injury and Fluoro-Jade B staining was used to visualize degenerative neurons in the dorsal hippocampus. Slide-mounted sections were dried at room temperature overnight, stained with 0.1% cresyl violet for 5–8 min until the desired depth of staining was achieved. After a quick rinse in tap water to remove excess stain, stained slides were dehydrated through graded ethanol, cleared in xylene, and cover slipped with Eukitt (Fluka, Germany) mounting medium. All tissue sections were photographed under identical conditions. In Nissl- and Fluoro-Jade B stained sections, neuronal quantification was carried out in the dentate hilus (counting frame 300 × 300 μm) and hippocampal pyramidal cell layers (fields CA3a, CA3b and CAl; counting frame 500 × 500 μm). Counts were performed in the right hippocampus at levels corresponding to AP = − 1.7–1.94 of the Paxinos and Franklin (2001) atlas under a Leica DM6000B fluorescent microscope (Leica Microsystems, Germany). At least five different sections from each animal were evaluated. All analyses were carried out using the ImageJ software (1.43u, USA) by an investigator who was blind to the experimental group.
Statistical analysis
Data shown represent the mean ± SEM for individual animals obtained from N experiments. Statistical significance was analyzed by a two-tailed unpaired t test (the Student’s t test; Wilcoxon one-sample test). Differences were considered statistically significant at p < 0.05. The statistical analysis in the examination of histological sections was performed using the nonparametric Mann–Whitney U test. In all cases, two-sided alternative hypotheses were used. The results were processed with the program SPSS (version 21, IBM Corp., USA). Statistical analysis of the learning rate of mice was performed in program R using standard statistical packages.
Results
Open field
Investigating the motional behavior based on parameters such as frequency of access, the total duration of stay and distance moved, obtained for different zones (center, corners, and walls) specified in the custom-made software, in mice two months after exposure to accelerated carbon ions, we can evaluate the anxiety of the animal. Figure 2 shows that the total distance moved (Fig. 2a) decreased in irradiated mice compared to control animals (p = 0.0406), while the mean velocity did not differ significantly (p = 0.1413, Fig. 2b). The frequency of access to the center as well as the total duration of the stay in the center of the open field in 12C-irradiated mice decreased too (p = 0.005 and p = 0.033, Fig. 2c, d), while the total duration of the stay in the corners and the frequency of access to the borders were similar to those in unirradiated mice (data not shown). The decrease in the time spent in the central zone of the open field and the total activity in irradiated mice are considered as indicators of anxiety.
Changes in exploratory behavior in the open field test in mice exposed to accelerated carbon ions. a velocity, cm/s; b distance moved, cm; c frequency of access to the center of the open field; d total (cumulative) duration of the stay in the center of the open field. Data are shown as the mean ± SEM (n = 10 animals per group). White and gray bars correspond to control and irradiated animals
Barnes maze
Reduction of time to find the goal box and the decline in the number of mistakes (incorrect hole pokes) during repeated test trials in the Barnes maze indicates improvement in locating the target hole.
All animals showed good acquisition, as demonstrated by a reduced number of mistakes in finding the target hole over three training days (nine trials). In addition, we have calculated the learning rate of mice and found out the slope ratio using a linear approximation. The figure shows that the control animals differ from the group of irradiated mice (p = 0.0021), and in the process, the rate of learning speed in the group of irradiated mice is positive, which corresponds to the extinction of the acquired skill over time (Fig. 3a).
Effect of carbon ions on the mice learning rate (a) and spatial memory (b) in the Barnes maze. a Mice were pre-trained to enter the escape box by placing them outside the escape box in the Barnes maze for up to 3 min, after which mice were left in the escape box for 2 min. Animals were subjected to three trials per day at 15–20-min intervals during three training days. Based on the time of search of the goal box, a linear approximation of the learning of each mouse in the group was analyzed, and the slope coefficient was determined. b Test 1—testing animals to finding the goal box (s) in the maze on the 2nd day after learning; test 2—testing animals in the maze on 9th day after learning. Data are shown as the mean ± SEM (n = 10 animals per group). White and gray bars correspond to control and irradiated animals. Irradiated mice showed a deficit in hippocampal-dependent memory retention.
To assess whether the acquired skill (learning the location of the goal box) is fixed by animal, two pilot trials without the escape box were performed, on the second (test 1) and 9th (test 2) days after training trials. As can be seen in Fig. 3b, during the first probe trial, the total number of hole visits by control and irradiated mice was similar (p = 0.4598). During the second probe trial, control mice showed a higher target hole preference index (i.e., target hole visits/average non-target visits) than control animals, demonstrating spatial learning (p = 0.0382). Thus, in the long-term memory test, control animals showed less mistakes in locating the escape box compared to the irradiated group.
Novel object recognition
Nowadays, the NOR test is considered as a translation model of episodic memory (Barker et al. 2007). It is assumed that information about a familiar object is in the animal memory and there is a preference for a new object (Ennaceur 2010). Recognition of new objects requires the usage of cognitive skill sets, in particular, the capacity to solve tasks is associated with the study of new environments or individual new objects (Silvers et al. 2007). We did not reveal intergroup differences in the total time of exploration of objects (cumulative duration and frequency of nose touching objects) at the stage of familiarization (Fig. 4a, b). The impairments in the NOR test revealed changes in the functional relationship between the hippocampus and medial prefrontal cortex, although these changes may be caused by different factors. These changes alter the ability of animals to distinguish new objects from familiar ones. In the test phase, the animals of both groups spent much more time exploring the new object than the familiar object. Control animals showed increased interest in both objects, while irradiated mice principally explored novel object 2. An analysis of this preference for novelty indicated that the DI for control mice and irradiated animals was 0.255 and 0.636, respectively. Thus, the NOR test shows that the recognition memory remained intact in mice 3 months following 0.7 Gy 12C particle irradiation.
Effect of carbon ions on the mice episodic memory retention in the novel object recognition task: a Frequency of the episodes of exploratory behavior (nose touches). b Cumulative duration of exploratory behavior (% from trial duration). White and gray bars correspond to objects locations. At familiarization phase, similar objects were presented; at test phase, object 2 was changed for novel. At the stage of familiarization, intergroup differences are not revealed. In the test phase, the animals of both groups spent more time exploring the new object than the familiar object. The DI value for control mice and irradiated animals was 0.255 and 0.636, respectively. Data are shown as the mean ± SEM (n = 10 animals per group)
Histology
Experimental data raise the question of whether late effects of ionizing radiation on cognition are a mark of developing neurodegeneration or whether dementia is a consequence of acute neuroinflammation and radionecrosis. The probable answer is that the neurological outcome and dominant mechanisms of damage are dose-dependent for any specific model (Betlazar et al. 2016).To address this issue in our model, we performed histological analysis of the hippocampus of irradiated mice to assess its morphological state (Nissl staining) and to reveal late neuronal degeneration (Fluoro Jade-B staining). Some reports have shown that Fluoro-Jade can also be useful for the detection of the glial cell death (Damjanac et al. 2007).
Histological analysis of the dorsal hippocampus was carried out 1 month after the evaluation of cognitive activity (two months after irradiation). In Nissl-stained sections, quantification of neurons was carried out in the dentate hilus (counting frame 300 × 300 μm) and hippocampal pyramidal cell layers (fields CA3a, CA3b and CAl; counting frame 500 × 500 μm). Calculations were performed in the right hippocampus at levels corresponding to AP = − 1.7–1.94 of the Paxinos and Franklin (2001) atlas. At least five different sections were evaluated from each animal. It was found with the use of Nissl staining that the number of cells in the dorsal hippocampus decreased in the group of irradiated animals compared with the unirradiated control group (Fig. 5a). The most pronounced decrease in cell density was observed in the DG of the irradiated group (631.68 ± 137.39 cells/mm2 in the irradiated group, n = 34, compared to 726.31 ± 148.11 cells/mm2 in the control group, n = 33, Mann–Whitney test U = 349.5, p = 0.007) (Fig. 5b). In addition, the length of the CA3c field of the dorsal hippocampus in the group of irradiated mice significantly decreased (0.19 ± 0.03 mm, n = 34, compared to 0.16 ± 0.02 mm in the control group, n = 33, the Mann–Whitney criterion U = 341.5, p = 0.005), and the number of cells in this field was reduced (57.7 ± 10.5 cells, n = 34, as compared with 65.4 ± 14.2 cells in the control group, n = 33, the Mann–Whitney test U = 395.5, p = 0.038). The number of samples (n) represented the total number of brain slices used to quantify cells.
Histological analysis of the dorsal hippocampus 3 months after the carbon ions irradiation of mice: a Nissl-stained sections: neuronal quantification in the dentate hilus and hippocampal pyramidal cell layers (fields CA3a, CA3b and CAl). b Changes in dentate gyrus cell numbers and cell layer CA3c of hippocampus. Low dose of carbon ions reduced the number of cells in the DG as well as the length of the CA3c field of the dorsal hippocampus and the number of cells in this field. Data are shown as the mean ± SEM (n = 5 animals per group)
Degenerative cells in the dorsal hippocampus were visualized using FJB staining. Experiments with the use of FJB-staining showed no FJB-positive staining in the fields CA1, CA3a, b, c and DG hilus of the dorsal hippocampus in irradiated and control animals 3 months after irradiation. Thus, neither morbid neurons nor glial cells were detected in irradiated nor in control groups (Fig. 6).
Discussion and conclusions
It is known that impaired hippocampal-dependent functions such as spatial learning and memory reflect developing radiation-induced cognitive changes (Roman and Sperduto 1995; Abayomi 1996). Such cognitive impairments and anxiety-like symptoms like decreased operant responses in laboratory animals and accelerated aging are associated with HZE exposure (Christmas and Maxwell 1970; Snyder et al. 2005). All of them can be associated with the decrease of neurogenesis in the DG, radiation-induced alterations in vascular and neuroinflammatory glial cell clonogenic populations, oxidative stress and acute cell death in irradiated region of brain (Greene-Schloesser et al. 2012; Fike 2011; Sona et al. 2015; Hladik and Tapio 2016). Cognitive impairments after the treatment of brain tumors by radiation of adults and children as well as health risks to cosmic ray-exposed astronauts have been directly connected to the disorder of DG neurogenesis, but despite the acceptance of this fact, there are extremely few studies of the impact of 12C on neurogenesis (Silvers et al. 2007; Cacao and Cucinotta 2016). To investigate changes in neurogenesis induced by HZE radiation, different rodent models were used. However, it is worth pointing out that these studies have tested limited number of doses, animal characteristics (sex, age), times after exposure and biological endpoints. Sufficient body of evidence was accumulated suggesting specific vulnerability of hippocampal neurogenesis, since proliferating cells were reactive to lower doses at an early age on multiple animal models (Jenrow et al. 2013; Blomstrand et al. 2014). It was shown that proliferating precursor cells of the subgranular zone dentate gyrus and their progeny were exposed to apoptosis after irradiation in adult rodents, while over months after exposure the consequential reduction in the production of new neurons is still observed (Tada et al. 2000; Mizumatsu et al. 2003). In rats, the cranial X-rays irradiation with a single high dose of 10 Gy almost completely stops the production of new neurons, while the surviving progenitor cells take the glial phenotype (Monje et al. 2002). Besides, data of hippocampal structure and function after irradiation in prenatal or neonatal rodents were accumulated (Sienkiewicz et al. 1992; Moreira et al. 2001).
Despite the noticeable impact on hippocampal neurogenesis, behavioral changes were undetectable with doses lower than 5 Gy (Sona et al. 2015). Most data on neuronal damage were acquired after irradiation with therapeutically relevant doses (5–50 Gy) of X-ray or gamma radiation while other sources and lower dose ranges remained less studied. The work performed by Parihar and Limoli (2013) documented dose-dependent (0.1–1 Gy and 1–10 Gy) and persistent reduction in dendritic complexity of hippocampal neurons in mice 10- and 30-days post-radiation. Dendrite branching, length, and area were dose-dependently reduced compared to sham-irradiated controls. Other studies with < 2 Gy whole-brain radiation showed that ionizing radiation may stimulate defenses against neuroinflammation and attenuate oxidative stress, which crucially influences cell proliferation, cell functioning and ultimately cell survival in the central nervous system (Betlazar et al. 2016). Concerning accelerated carbon, there are scarce data showing that 4 Gy single exposure results in metabolic and behavioral alterations on the background of neuronal death (Liu et al. 2018).
Based on the mentioned works and sets of data that irradiation-induced loss of neural precursor cells affects hippocampal function (Tofilon and Fike 2000; Wong and Van der Kogel 2000), we investigated whether the exposure to accelerated carbon ions leads to cognitive impairment 2 months after irradiation of mice and hippocampal neurodegeneration 3 months after irradiation.
Our results demonstrate that mice, irradiated with accelerated carbon ions in the Bragg peak at a dose of 0.7 Gy, develop an altered behavioral pattern characterized by anxiety and deficit in hippocampus-dependent memory retention but not in episodic memory. Similar results were obtained by Casadesus et al. (2004). It has been demonstrated that in rats irradiated with 1.5 Gy of 56Fe particles, the latency in entering the open field centre was longer, the frequency of visiting these parts was lesser, and the duration of stay there was lower as well, independent of the total activity. In the work of Philpott et al. (1985) on cranial irradiation of C57BL/6 mice with 0.5 Gy of 56Fe and 40Ar particles, the changes of morphology and synaptic density in the hippocampus as well as a progressive decline in motor activity were observed. It has been shown that a similar decrease in spontaneous motor activity by 24 h after irradiation is associated with increased cerebellum oxidative stress. Rabin et al. (2003) have demonstrated earlier that exposing rats to total body irradiation with a dose of 1 Gy of 56Fe-particle ion induces a significant reduction in working and reference memory nine months after irradiation. Shukitt-Hale with colleagues (2000) revealed that during the first month after the irradiation with 1.5 Gy of 56Fe-particles, the spatial working memory was disrupted in rats. These data indicated that exposure to heavy charged particles can cause significant behavior disorders and affect the integration of new neurons and glia cells into the dentate granule cell layer. Moreover, changes in the populations of neurons and astrocytes were found in hippocampus and cortex. It was also shown that 56Fe-particle radiation induced deficits in spatial learning and reference memory that are mediated by synaptic neurotransmitter release (Marty et al. 2014). But in another work Kim et al. (2008) demonstrated that 1, 3 and 7 days after acute whole-body irradiation of mice with 60Co-gamma rays with a dose of 2 Gy basal locomotor activity was not altered when compared to sham control animals.
In our work in mice irradiated with accelerated carbon ions at a dose of 0.7 Gy, no signs of degenerating neurons were observed in the hippocampus three months after the exposure, although the cell density was decreased. It is interesting to note that the decrease in the number of cells was more prominent in the hilus of the DG, whose subgranular zone is the known site of adult neurogenesis and critical structure to the spatial memory formation. At the same time, an active neurodegeneration in this area was not detected. One possible speculative explanation of these results could be the suggestion that irradiation could affect neurogenesis or new cells integrated into the network. Recently Liu et al. (2018) have shown that impaired cognitive performance, neurodegeneration and neuronal cell death occurred in mice one month after 4 Gy carbon ion exposure. With respect to the histopathology evaluation, high-LET carbon ions led to the injury of the hippocampus characterized by a decrease in the number of Nissl-stained dark neurons, especially at the edge of the DG and CA1 regions. These data suggest that carbon-ion-induced cognitive changes were mainly manifested as hippocampus-mediated learning and memory deficits. However, much about the influence of carbon ions on neurogenesis is still unknown, e.g. the timing of its effect on neurogenesis. In recent work (Zanni et al. 2018), a reduced proliferation was found (by Ki67 and BrdU staining), and decreased numbers of immature neurons 2 h after irradiation of mice with 1 Gy of 12C ions, as compared with control mice. Three months later, the same level of Ki67 + and DCX + cells was seen in irradiated and sham mice, indicating the mice brain capacity for recovery of proliferation and increasing of immature neuron numbers. In other work (Rola et al. 2004), irradiated C57BL/6 mice at 2 months after total body irradiation with 1, 2 and 3 Gy of 56Fe ions showed dose-dependent progressively fewer BrdU-positive cells. These data were verified by Ki-67 immunostaining in the subgranular zone dentate gyrus where the number of Ki-67-positive cells also decreased in a dose-dependent manner. It was found that in irradiated animals the number of immature neurons was significantly decreased (34% after 1 Gy and 71% after 3 Gy). Histopathological analysis revealed that decline of the neuronal cell numbers in the subgranular zone has been accompanied by chronic and diffuse astrocytosis and changes in pyramidal neurons inside and around the hippocampal formation.
Neuroinflammation and microglial activation are determinative factors for cell death in many pathological conditions. It was shown that the induction of activated microglia and neuroinflammation occur a few hours after irradiation (Ben Abdallah et al. 2007; Kalm et al. 2009; Veeraraghavan et al. 2011; Tseng et al. 2014). Depending on the degree of initial inflammatory response, microglial activation and neuronal death could became viciously related, inducing neurodegenerative pathology (Chen et al. 2016; Kempuraj et al. 2016). Krukowski et al. (2018) find out that forehanded temporary microglia depletion, 1 week after helium radiation < 1 Gy, prevents the development of long-term memory deficits. On the other hand, acute neuroinflammation could be beneficial to the central nervous system, minimizing the injury by activating the innate immune system (Crutcher et al. 2006). Studies with < 2 Gy whole-brain radiation showed that ionizing radiation may stimulate defenses against neuroinflammation and attenuate oxidative stress, which crucially influences cell proliferation, cell functioning and ultimately cell survival in the central nervous system (Betlazar et al. 2016). Similarly, human neural stem cell cultures irradiated with charged particles at 0.05–0.25 Gy showed increased levels of ATP, and decreased ROS/RNS levels, which contributed to increased cell survival, as opposed to cells irradiated at higher doses of 1 Gy (Baulch et al. 2015). Based on known literature data, we assumed that chronic microglia activation either does not occur (Ben Abdallah et al. 2007; Sweet et al. 2014; Acharya et al. 2015) or occurs at HZE doses significantly higher than 0.7 Gy (Raber et al. 2016; Monje et al. 2002; Greene-Schloesser et al. 2012; Mizumatsu et al. 2003; Morganti et al. 2014; Estable-Puig et al. 1964). This is implicitly confirmed by the fact that we did not observed any sign of active neurodegeneration in our experiments. Acute neuroinflammation still could be responsible for neuronal cell loss revealed by Nissl staining.
Thus, to date, the effect of HZE radiation on the central nervous system remains poorly understood and contradictory. Obtained results indicate that total irradiation with a rather low dose of carbon ions could produce a cognitive deficit in adult mice without evidence of neurodegenerative pathologic changes.
References
Abayomi OK (1996) Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol 35:659–663
Acharya MM, Patel NH, Craver BM, Tran KK, Giedzinski E, Tseng BP, Parihar VK, Limoli CL (2015) Consequences of low dose ionizing radiation exposure on the hippocampal microenvironment. PLoS ONE 10:e0128316
Barker GRJ, Bird F, Alexander V, Warburton EC (2007) Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27:2948–2957
Barnes D (1994) Stimulus equivalence and relational frame theory. Psychol Rec 44:91–124
Baulch JE, Craver BM, Tran KK, Yu L, Chmielewski N, Allen BD, Limoli CL (2015) Persistent oxidative stress in human neural stem cells exposed to low fluences of charged particles. Redox Biol 5:24–32
Baxter MG (2010) “I’ve seen it all before”: explaining age-related impairments in object recognition. Behav Neurosci 124:706–709
Ben Abdallah NM, Slomianka L, Lipp HP (2007) Reversible effect of X-irradiation on proliferation, neurogenesis, and cell death in the dentate gyrus of adult mice. Hippocampus 17:1230–1240
Betlazar C, Middleton RJ, Banati RB, Liu G-J (2016) The impact of high and low dose ionising radiation on the central nervous system. Redox Biol 9:144–156
Blomstrand M, Kalm M, Grandér R, Björk-Eriksson T, Blomgren K (2014) Different reactions to irradiation in the juvenile and adult hippocampus. Int J Radiat Biol 90(9):807–815
Britten RA, Jewell JS, Duncan VD, Hadley MM, Macadat E, Musto AE, La Tessa C (2018) Impaired attentional set-shifting performance after exposure to 5 cGy of 600 MeV/n 28Si particles. Radiat Res 189(3):273–282
Cacao E, Cucinotta FA (2016) Modeling impaired hippocampal neurogenesis after radiation exposure. Radiat Res 185(3):319–331. https://doi.org/10.1667/RR14289.S1
Casadesus G, Shukitt-Hale B, Cantuti-Castelvetri I, Rabin BM, Joseph JA (2004) The effects of heavy particle irradiation on exploration and response to environmental change. Adv Space Res 33:1340–1346
Chen WW, Zhang X, Huang WJ (2016) Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep 13(4):3391–3396
Christmas AJ, Maxwell DR (1970) A comparison of the effects of some benzodiazepines and other drugs on aggressive and exploratory behaviour in mice and rats. Neuropharmacology 9(1):17–29
Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry VH, Popovich PG, Weaver LC (2006) Debate: “is increasing neuroinflammation beneficial for neural repair?” J Neuroimmune Pharmacol 1:195–211
Cui L, Pierce D, Light KE, Melchert RB, Fu Q, Kumar KS, Hauer-Jensen M (2010) Sublethal total body irradiation leads to early cerebellar damage and oxidative stress. Curr Neurovasc Res 7(2):125–135
Damjanaca M, Bilan AR, Barrier L, Pontcharraud R, Annec C, Hugona J, Page G (2007) Fluoro-Jade B staining as useful tool to identify activated microglia and astrocytes in a mouse transgenic model of Alzheimer’s disease. Brain Res 1128:40–49
Ennaceur A (2010) One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215:244–254
Estable-Puig JF, Estable RF, Tobias C, Haymaker W (1964) Degeneration and regeneration of myelinated fibers in the cerebral and cerebellar cortex following damage from ionizing particle radiation. Acta Neuropathol 4:175–190
Fike JR (2011) Physiopathology of radiation-induced neurotoxicity. Rev Neurol 167(10):746–750
Fike JR and Gobbel GT (1991) Central nervous system radiation injury in large animal models. In: Radiation injury to the nervous system, Gutin PH, Leibel SA and Sheline GE (eds), Publisher, p. 113–135
Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2:260–265
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD (2012) Radiation-induced brain injury: a review. Front Oncol 2:73
Higuchi Y, Nelson GA, Vazquez M, Laskowitz DT, Slater JM, Pearlstein RD (2002) Apolipoprotein E expression and behavioral toxicity of high charge, high energy (HZE) particle radiation. J Radiat Res 43(Suppl):219–224
Hladik D, Tapio S (2016) Effects of ionizing radiation on the mammalian brain. Mut Res Rev Mut Res 770(Part B):219–230
Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO (2006) Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis 21:457–467
Jenrow KA, Brown SL, Lapanowski K, Naei H, Kolozsvary A, Kim JH (2013) Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. J Radiat Res 179(5):549–556
Kalm M, Fukuda A, Fukuda H, Ohrfelt A, Lannering B, Björk-Eriksson T, Blennow K, Márky I, Blomgren K (2009) Transient inflammation in neurogenic regions after irradiation of the developing brain. Radiat Res 171:66–76
Kempermann G (2002) Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 22:635–638
Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A (2016) Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine 1(1):1003
Kim J-S, Lee H-J, Kim JC, Kang SS, Bae C-S, Shin T, Jin J-K, Kim SH, Wang H, Moon C (2008) Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with Inhibition of hippocampal neurogenesis. J Radiat Res 49(5):517–526
Krukowski K, Feng X, Paladini MS, Chou A, Sacramento K, Grue K, Riparip L-K, Jones T, Campbell-Beachler M, Nelson G, Rosi S (2018) Temporary microglia-depletion after cosmic radiation modifies phagocytic activity and prevents cognitive deficits. Sci Rep 8:7857
Liu Y, Yan J, Sun C, Li G, Li S, Zhang L, Di C, Gan L, Wang Y, Zhou R, Si J, Zhang H (2018) Ameliorating mitochondrial dysfunction restores carbon ion-induced cognitive deficits via co-activation of NRF2 and PINK1 signaling pathway. Redox Biol 17:143–157
Makale MT, McDonald CR, Hattangadi-Gluth J, Kesari S (2017) Brain irradiation and long-term cognitive disability: current concepts. Nat Rev Neurol 13(1):52–64
Marty VN, Vlkolinsky R, Minassian N, Cohen T, Nelsonb GA, Spigelmana I (2014) Radiation-induced alterations in synaptic neurotransmission of dentate granule cells depend on the dose and species of charged particles. Radiat Res 182:653–665
Meyers CA, Geara F, Wong PF, Morrison WH (2000) Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys 46(1):51–55
Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR (2003) Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res 63:4021–4027
Monje ML, Mizumatsu S, Fike JR, Palmer TD (2002) Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955–962
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23(5):489–495
Morganti JM, Jopson TD, Liu S, Gupta N, Rosi S (2014) Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PLoS ONE 9:e93650
Oliveira AMM, Hawk JD, Abel T, Havekes R (2010) Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem 17:155–160
Parihar VK, Limoli CL (2013) Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A 110:12822–12827
Paxinos G, Franklin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates, 2nd edn. Academic Press, San Diego
Philpott DE, Sapp W, Miquel J, Kato K, Corbett R, Stevenson J, Black S, Lindseth KA, Benton EV (1985) The effect of high energy (HZE) particle radiation (40Ar) on aging parameters of mouse hippocampus and retina. Scan Electron Microsc 3:1177–1182
Raber J, Allen AR, Sharma S, Allen B, Rosi S, Olsen RH, Davis MJ, Eiwaz M, Fike JR, Nelson GA (2016) Effects of proton and combined proton and 56Fe radiation on the hippocampus. Radiat Res 185:20–30
Rabin BM, Joseph JA, Shukitt-Hale B (2003) Long-term changes in amphetamine-induced reinforcement and aversion in rats following exposure to 56Fe particle. Adv Space Res 31(1):127–133
Rabin BM, Carrihill-Knoll KL, Shukitt-Hale B (2015) Comparison of the effectiveness of exposure to low-LET helium particles (4He) and gamma rays (137Cs) on the disruption of cognitive performance. Radiat Res 184:266–272
Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR (2004) Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 188(2):316–330
Roman DD, Sperduto PW (1995) Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys 31:983–998
Shukitt-Hale B, Casadesus G, McEwen JJ, Rabin BM, Joseph JA (2000) Spatial learning and memory deficits induced by exposure to iron-56-particle radiation. Radiat Res 154:28–33
Sienkiewicz ZJ, Saunders RD, Butland BK (1992) Prenatal irradiation and spatial memory in mice: investigation of critical period. Int J Radiat Biol 62:211–219
Silvers JM, Harrod SB, Mactutus CF, Booze RM (2007) Automation of the novel object recognition task for use in adolescent rats. J Neurosci Methods 166:99–103
Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130:843–852
Sona Y, Yang M, Wang H, Moon C (2015) Hippocampal dysfunctions caused by cranial irradiation: A review of the experimental evidence. Brain Behav Immun 45:287–296
Sorokina S, Zaichkina S, Rozanova O, Shemyakov A, Smirnova H, Romanchenko S, Dyukina A, Vakhrusheva O, Pikalov V (2017) The study of biological effects induced by accelerated 12C ions with an energy of 450 MeV/n on mice in vivo. Proceedings of the RAD, Budva, Montenegro, 12-16.06.2017; Publisher: RAD Association, Nis, Serbia, 2017, 2:15–18
Sweet TB, Panda N, Hein AM, Das SL, Hurley SD, Olschowka JA, Williams JP, O’Banion MK (2014) Central nervous system effects of whole-body proton irradiation. Radiat Res 182:18–34
Tada E, Parent JM, Lowenstein DH, Fike JR (2000) X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience 99:33–41
Tofilon PJ, Fike JR (2000) The radioresponse of the central nervous system: a dynamic process. Radiat Res 153:357–370
Tseng BP, Giedzinski E, Izadi A, Suarez T, Lan ML, Tran KK, Acharya MM, Nelson GA, Raber J, Parihar VK (2014) Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid Redox Signal 20:1410–1422
Veeraraghavan J, Natarajan M, Herman TS, Aravindan N (2011) Low-dose γ-radiation-induced oxidative stress response in mouse brain and gut: regulation by NFκB-MnSOD crosssignaling. Mutat Res 718:44–55
Wong CS, Van der Kogel AJ (2000) Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv 4(5):273–284
Wyrobek AJ, Britten RA (2016) Individual variations in dose response for spatial memory learning among outbred wistar rats exposed from 5 to 20 cGy of 56Fe particles. Environ Mol Mutagen 57:331–340
Zanni G, Deutsch HM, Rivera PD, Shih HY, LeBlanc JA, Amaral WZ, Lucero MJ, Redfield RL, DeSalle MJ, Chen BPC, Whoolery CW, Reynolds RP, Yun S, Eisch AJ (2018) Whole-body 12C irradiation transiently decreases mouse hippocampal dentate gyrus proliferation and immature neuron number, but does not change new neuron survival rate. Int J Mol Sci 19(10):3078
Acknowledgements
We thank our colleagues, Smirnova H.N. and Rozanova O.M., from Cellular Engineering Laboratory of ITEB RAS (Pushchino, Russia) for their comments on an earlier version of the manuscript. We would also like to show our gratitude to the Laryushkin D.P. for assistance with data processing that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sorokina, S.S., Malkov, A.E., Shubina, L.V. et al. Low dose of carbon ion irradiation induces early delayed cognitive impairments in mice. Radiat Environ Biophys 60, 61–71 (2021). https://doi.org/10.1007/s00411-020-00889-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-020-00889-0