Abstract
This article addresses the question of the role of morphallactic and epimorphic events in regeneration and asexual reproduction in annelids. It was shown that tissue reorganization and changes at the molecular level occur already at the very early stages of regenerative processes. These data indicate that morphallaxis is required for all progressive stages of full epimorphic regeneration, including initiation and completion, and de novo formation of missing parts in new zooids during asexual reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The ability of different organisms to recover lost tissues, organs, and even entire parts of the body (regeneration) has been known for a long time. However, the regeneration of missing parts of the body or their de novo formation in animals is also observed in different ways of asexual reproduction (Ivanova-Kazas, 1977; Korotkova, 1997; Bely and Wray, 2001; Kharin et al., 2006; Bely and Sikes, 2010; Stocum, 2012; Kostyuchenko et al., 2016). These two phenomena, which are forms of postembryonic development, have much in common. In particular, both regeneration and asexual reproduction proceed without the involvement of specialized germ cells but require active multiplication of somatic cells, resulting in the formation of temporary clusters of undifferentiated cells (typical for the epimorphosis) and/or reorganization of old tissues (reffered to as the morphallaxis). Asexually reproducing animals often exhibit excellent regenerative abilities. However, even in closely related species, the ability for such postembryonic morphogeneses may vary dramatically (Ivanova-Kazas, 1977; Korotkova, 1997; Bely, 1999, 2006; Bely and Nyberg, 2010; Zattara et al., 2019).
It is known that the types of regeneration and forms of asexual reproduction correlate with the organization of animals and, similarly to the animals themselves, may be very diverse. For example, along with typical regeneration, which leads to complete regeneration of the shape and function of an organ or part of the body but proceeds differently in the case of amputation or autotomy, variants of atypical regeneration (additions, hypertrophy, heteromorphoses, and incomplete regeneration) often occur (Vorontsova and Liosner, 1960; Korotkova, 1997). Features of the anatomical organization (fusion of segments, formation of a rigid external skeleton, etc.), for example, in insects, dramatically limit the ability for de novo formation. In many animals, the pattern of regeneration may be correlated with the specificity of the structure of the nervous system (Korotkova, 1997).
More fundamentally different basic types of asexual reproduction (fission, budding, cell aggregation, and polyembryony) correlate with the anatomical features, yet to a lesser extent than regeneration. For example, among the representatives of the same group of animals of the same species and even at the level of one individual, different variants of asexual reproduction can be observed depending on the environmental conditions, physiological state, and stage of the life cycle. However, disturbances of morphogenesis during asexual reproduction in animals are extremely rare and random (Ivanova-Kazas, 1977; Kharin et al., 2006; Kostyuchenko et al., 2016).
Obviously, to understand the mechanisms of postembryonic development of animals, models that are similar yet demonstrate subtle or fundamental differences are required. It can be either closely related groups or species differing in their abilities (e.g., the ability to regenerate) or phenomena similar in their basic characteristics. This approach helps to clarify the universal mechanisms, which may provide an opportunity to stimulate the regeneration of lost parts of the body even in animals with a reduced regenerative capacity under experimental conditions. An excellent example is the species of planarians, which normally cannot fully regenerate the head part but can recover the head under the influence of canonical Wnt signaling in experiments (Liu et al., 2013; Sikes and Newmark, 2013). Obviously, this approach is also important for creating models to study the evolution of the mechanisms of postembryonic development and to identify the possibly existing universal components of these mechanisms (Kharin et al., 2006; Bely and Sikes, 2010; Stocum, 2012; Kostyuchenko et al., 2016).
In this paper, on the example of annelids, which are one of the key groups of bilaterians for evolutionary biology, we will consider the characteristic features of morphogeneses during regeneration and asexual reproduction in terms of the type of cellular behavior, morphallaxis, and epimorphosis. Similarly to other spiral animals, annelids show a conservative pattern of embryonic development, although with elements of regulation (Kostyuchenko and Dondua, 2006, 2017; Schneider and Bowerman, 2007; Nakamoto et al., 2010; Kozin et al., 2013; Wanninger, 2015; Kozin and Kostyuchenko, 2016; Kozin et al., 2016; Carrillo-Baltodano and Meyer, 2017; Lanza and Seaver, 2018). It should be noted that the adult forms, which are markedly different in structure, life cycle, and environmental habitation conditions, are generally characterized by a pronounced ability for regeneration of whole parts of the body and often for asexual reproduction. Despite the great diversity of reparative morphogeneses, annelids are characterized by largely similar nature of postembryonic events based on transverse fissions while retaining the original body axes. This, together with the long history of research and the availability of modern technical advances, makes annelids a very promising model for studying both the molecular and cellular mechanisms of regeneration and asexual reproduction and the evolution of developmental programs (Iwanoff, 1928; Herlant-Meewis, 1964; Ivanova-Kazas, 1977; Korotkova, 1997; Bely, 1999, 2006; Kharin et al., 2006; Bely and Sikes, 2010; Zattara and Bely, 2011; Novikova et al., 2013; Kozin and Kostyuchenko, 2015; Kostyuchenko et al., 2016; Kozin et al., 2017; de Jong and Seaver, 2018).
REGENERATION AND ASEXUAL REPRODUCTION IN ANNELIDS: DIVERSITY AND ANCESTRY OF FORMS
In representatives of the phylum Annelida, similarly to other animals, the ability for regeneration and asexual reproduction is correlated with the features of the anatomical structure and organization of the body of the animal. Increasing specialization and secondary simplification have similar effects; namely, they lead to restriction of regeneration processes or their confinement to only certain regions of the body. Specialized forms such as leeches and dinophyllids exhibit very limited regeneration, mainly wound healing (Korotkova, 1997; Bely, 2006; Kostyuchenko et al., 2016; Zattara and Bely, 2016). At the same time, polychaetes with reduced septa between segments, such as Arenicola marina, die after amputation of even one segment without starting wound healing (Iwanoff, 1928; Bely, 2006). However, the majority of annelids are capable of posterior regeneration (i.e., regeneration of the lost tail region). Obviously, posterior regeneration is an ancestral trait of the entire phylum Annelida. The head, or anterior, regeneration (regeneration of the head and anterior segments) is characteristic of a smaller number of species. Nevertheless, in some cases, all the lost parts of an animal can be recovered even from a very small fragment of the body (Berrill, 1952; Herlant-Meewis, 1964; Korotkova, 1997; Bely, 2006; Özpolat and Bely, 2016). Phylogenetic analysis of the prevalence of various types of regeneration among annelids also showed the ancestry of the anterior regeneration, which, for certain reasons (including those described above), was lost secondarily in a fairly large number of species (Bely and Sikes, 2010; Zattara and Bely, 2016). Of course, annelids are capable of de novo formation of not only missing parts of the body but also of individual organs, such as parapodia, various appendages, and even gonads (Berrill, 1952; Herlant-Meewis, 1964; Korotkova, 1997; Tadokoro et al., 2006; Özpolat et al., 2016; Boilly et al., 2017). However, in all cases, regeneration proceeds in concert with the integrity of the anterior and posterior ends, which should be recovered, if possible, first of all (Korotkova, 1997; Kostyuchenko et al., 2016).
All annelids, which reproduce asexually, usually exhibit excellent regenerative capacity; however, in some cases the regeneration reactions are, probably, secondarily limited only to certain parts of the body (Bely and Nyberg, 2010). In annelids, asexual reproduction occurs widely (but only among polychaetes and oligochaetes) and unevenly in different families. Usually, it is represented by transverse fission. If the maternal zooid first undergoes fission to produce daughter zooids and then the missing structures are formed, this is considered as evidence of architomy. However, the new head and tail regions may be formed before the physical separation of the initial individual into two or more zooids. This phenomenon is called paratomy. Paratomy is relatively more common in oligochaetes, whereas architomy is more characteristic of polychaetes (Ivanova-Kazas, 1977; Zattara and Bely, 2016). For some species of polychaetes and oligochaetes, sexual reproduction has not been described, and it is assumed that populations of such species reproduce solely asexually. In the majority of species, regression of sex products upon the transition to asexual reproduction is observed. However, in the polychaetes of the family Syllidae, asexual reproduction results in the formation of sexual individuals (Ivanova-Kazas, 1977; Ribeiro et al., 2018). It should also be noted that some groups or species of annelids may have complicated forms of paratomy and architomy, up to forms close to budding (Ivanova-Kazas, 1977; Kostyuchenko et al., 2016; Ribeiro et al., 2018).
SPECIFICITY OF PROCESSES ALONG THE BODY AXES
Despite the similarity in many aspects, regeneration and asexual reproduction processes are characterized by specific features within the body axes, primarily along the anteroposterior axis. This applies equally to the completeness of the process and the spatial restriction of the specificity of regeneration of missing structures. For example, in animals with tagmosis, the paratomy zone with a new head end is laid down in the last third of the body. In animals that do not have obvious tagmata, the area of formation of the transverse fission zone is observed either in a particular segment or within any of the segments of primarily the middle part of the body (Figs. 1b, 1c). For example, the paratomy zone in Dero digitata is laid down at the level of the 38th segment; in the species of the genus Ophidonais, it is at the level of the 35th segment. In the course of stolonization, a special proliferating segment is formed in polychaetes of the family Syllidae, which develops new stolons (Ivanova-Kazas, 1977; Kostyuchenko et al., 2016; Ribeiro et al., 2018). However, in Nais communis, which comprises 21–41 segments, the fission zone is laid down within segments 12–23. In Pristina longiseta, the first site of separation of zooids appears on segments 14–18 (the length of the animal is 21–29 segments). A subsequent paratomy zone is formed successively, each time on the segment ahead of the previous one. Only when the set of fission-competent segments is exhausted does the animal cease to reproduce and grow until the required length (Kharin et al., 2006). In the case of architomy, both in polychaetes and oligochaetes, the body disintegrates either into two parts approximately in the middle or into several fragments consisting of one or more segments, each of which, retaining its polarity, regenerates the missing parts (Ivanova-Kazas, 1977).
Schematic representation of the localization of regenerative territories in reparative regeneration and competence zones during asexual reproduction in annelids: (a) regeneration, (b) architomy, and (c) paratomy. Designations: 1, the head region and cephalogenic part of the fission zone; 2, somatogenic part of the fission zone and its derivatives 3, cephalic regenerative territory; 4, posterior (“tail”) regenerative territory; 5, segment competence zone, within which animals can be separated during asexual reproduction; GZ, growth zone; HS, head segments; PZ, paratomy zone; Pg, pygidium.
Regardless of the presence or absence of tagmata in both oligochaetes and polychaetes, their ability to regenerate, if any, is usually limited only to the anterior part of the body (Fig. 1a). The size of the head regenerate, as well as the pattern and completeness of head regeneration, significantly changes depending on the distance between the amputation site and the original head. Its increase may result in abnormalities, up to regeneration of the “tail” end instead of the head on the wound surface. For example, in Autolitys pictus, the largest regeneration bud, which produces the prostomium, perestomium, and eight head segments, is formed when the amputation is performed at the level of the eighth segment. When operation is performed between the 9th and 13th segments, the regenerated head end is shorter by four segments, and when segments 14–42 are removed, only the prostomium is recovered (Korotkova, 1997). The head regeneration area in each species is limited to a species-specific set of segments. However, similarly to asexual reproduction, complete regeneration often involves the old segments adjoining the wound. Exactly in these segments, the proper parts of the gut are transformed into the stomach. The posterior regeneration shows less specificity along the anteroposterior axis. It is not only characteristic of a much greater number of species but is also usually possible in the case of amputation within almost the entire length of the worm, except for the head segments (Korotkova, 1997; Kostyuchenko et al., 2016).
Note that heteromorphic regeneration after the amputation of the anterior or posterior end or under asexual reproduction by transverse fission is observed extremely rarely. Most often it consists just in the creation of a second “tail” end instead of the head one, and vice versa, when operation is performed outside of the respective regeneration area. According to our observations, paratomy may entail the formation of an animal with a forked posterior end of the body. This is a very rare event occurring as a result of disturbance in the fission zone. In this case, only a new “tail” end of the anterior zooid is formed, growing at an angle to the anteroposterior axis of the animal’s body (Kostyuchenko, unpublished data).
Regeneration of the lost parts along the dorsoventral axis has its own specifics and indicates the importance of a topologically correct combination of systems and tissues, including the nervous system, gut, and the body wall, which have ventral and dorsal identity. This is confirmed by experiments on the transplantation of parapodia and portions of the body wall from a donor in the ectopic position on the host body surface. As a result, additional parapodia are formed in the area where the dorsal and ventral tissues are superimposed on one another. Thus, if the graft and the transplantation site have opposite dorsoventral polarity (for example, when a dorsal portion of the body wall is transplanted to the ventral side, and vice versa), morphogenesis resulting in the formation of new parapodia along the entire length of the graft is induced. If transverse amputation through the body region comprising two nerve cords (normal ventral and transplanted “dorsal”) is performed after successful transplantation of a fragment of the ventral nerve cord together with the adjacent body wall to the dorsal region of the normal recipient animal, the regeneration of the posterior end is observed. The resulting regenerate has a pygidium with a doubled number of anal cirri and segments with a doubled set of parapodia. Therefore, the ventral nerve cord and/or a band of the respective ventral wall of the body are sufficient to induce a second dorsoventral axis in the regenerate (Boilly et al., 2017).
Worms with “tails” that have a double nerve cord are sometimes found in nature. According to Boilly et al. (2017), these animals appear as a result of regeneration, because in all cases their posterior ends of the body show the same morphological characteristics as in the animals with a double nerve cord obtained as a result of transplantation. Thus, complete regeneration requires correct connection of tissues with different identity. Obviously, the change in the normal pattern along the dorsoventral axis by its superposition or inversion leads to additions, heteromorphoses, and other disorders. However, there are several species among the annelids that are characterized by laying down new individuals during asexual reproduction precisely within the secondary axis. Such pathways of complicated stolonization are commonly called budding. In Trypanosyllus asterobia, ventral budding leads to the formation of a large number of multisegmented posterior ends (each based on a separate segment of the maternal organism) on the ventral side of the posterior part of the animal. In the case of ventroterminal budding in several Trypanosyllus species, numerous stolons are formed on the basis of one or two posterior segments. In Syllus ramose, lateral (i.e., located in the dorsoventral plane of parapodia) stolons are formed in the course of lateral budding (Ivanova-Kazas, 1977; Ribeiro et al., 2018). Thus, the regenerative morphogeneses under changed/disturbed dorsoventral polarity lead to different consequences: deformities in the case of regeneration and a successful reproductive strategy in the case of asexual reproduction.
The above data leave no doubt that, in the case of asexual reproduction, the molecular patterning along the body axes has its own peculiarities. Our knowledge suggests an ancestry of almost unlimited regenerative capacities, the possiblity of secondary restriction of regeneration processes to certain parts of the body, and multiple independent occurrences of asexual reproduction in different groups of annelids on the basis of reparative regeneration mechanisms (Vorontsova and Liosner, 1960; Ivanova-Kazas, 1977; Korotkova, 1997; Bely and Wray, 2001; Kharin et al., 2006; Bely and Sikes, 2010; Zattara and Bely, 2011, 2016; Kostyuchenko et al., 2016). Accordingly, the question arises as to which fundamental changes in the molecular organization of the body along the axes affect the success of the regeneration of missing structures? Similarities and differences between various forms of regeneration and asexual reproduction and their representation in different groups of polychaetes and oligochaetes allow annelids to be considered as an ideal model for understanding the fundamental mechanisms of regeneration initiation and for determination of the cellular potencies and evolution of postembryonic developmental programs (including the maintenance or restriction of regenerative capacities). Despite the long period of study of both regeneration and asexual reproduction phenomena, the data obtained often describe only the overall picture at a macroanatomical level, especially when it concerns problems of asexual reproduction (Randolph, 1892; Herlant-Meewis, 1964; Ivanova-Kazas, 1977; Korotkova, 1997; Bely, 2006). The appearance of new models and the current state of research techniques make it possible to proceed to a critical analysis of changes at the cellular and tissue levels in interpretation of the molecular data (Kharin et al., 2006; Tadokoro et al., 2006; Bely and Sikes, 2010; Zattara and Bely, 2011; Novikova et al., 2013; Kozin and Kostyuchenko, 2015; Kostyuchenko et al., 2016; de Jong and Seaver, 2016, 2018; Zattara et al., 2016; Kozin et al., 2017).
EARLY EVENTS OF REGENERATION AND ASEXUAL REPRODUCTION
From the very beginning of active research on regeneration and asexual reproduction in annelids, as well as in other animals, the attention of researchers was focused on the cellular and tissue aspects (Randolph, 1892; Herlant-Meewis, 1964; Korotkova, 1997; Bely, 2006; Kostyuchenko et al., 2016). Morphogeneses during these two forms of postembryonic development are ensured by epimorphosis and morphallaxis. The epimorphic processes are characterized by the regeneration of missing parts due to proliferation of the cells derived by dedifferentiation of existing tissues and/or special reserve cells (including migratory ones). In most cases, a regenerative blastema, a set of dividing undifferentiated cells that later give rise to the regenerated organ (part of the body), is formed. During morphallaxis, a regenerate is developed due to changes in old tissues without a pronounced proliferation phase and in the absence of a blastema (Korotkova, 1997; Stocum, 2012; Kozin et al., 2017).
For more than a century, considerable amount of data about the events at the cellular level regarding primarily regeneration and, to a much lesser extent, asexual reproduction in annelids have been accumulated (Bely, 2014; Kostyuchenko et al., 2016). Epimorphosis is usually observed during the regeneration of missing parts at the organismal level and is well expressed, although the size of the blastema may greatly vary. In most cases, the blastema is large during anterior regeneration and is poorly developed in the case of posterior regeneration (Korotkova, 1997; Kostyuchenko et al., 2016; Kozin et al., 2017). Regeneration of the anterior or posterior ends of the body of annelids solely by the mechanism of morphallaxis, apparently, is not typical for complete regeneration. However, morphallaxis is evident in the de novo formation of individual organs (for example, gonads in the newly formed segments or the stomach from the gut of old segments), as well as in the respecification of the previously existing segments adjacent to the regenerated end (Korotkova, 1997; Tadokoro et al., 2006; Özpolat et al., 2016; Boilly et al., 2017).
Both amputation and subsequent regeneration and asexual reproduction require preventing the loss of body fluid. This prevention involves different cell types and proceeds in different ways. Typically, the cells of the muscular system play an important role, providing rapid wound closure by sharp contraction immediately after the break (regeneration after amputation and architomy). In addition, muscles (especially circular ones) are involved in the process of physical separation (autotomy or paratomy). However, muscular contractions alone are insufficient. The gut, due to a certain prolapse after amputation or sometimes rapid fusion with the epidermis, also provides wound closure. Various types of cells, the homology of which can hardly be established on the basis of the results of histological studies on fixed material, migrate to the wound site to form a regeneration plug and perform a protective function. Finally, constant wound closure is ensured by wound epithelial cells (Herlant-Meewis, 1964; Korotkova, 1997; Lesiuk and Drewes, 1999; Kawamoto et al., 2005; Kharin et al., 2006; Bely, 2014; Kostyuchenko et al., 2016; Kozin et al., 2017).
Wound healing due to wound epithelium formation or fusion of severed edges of the gut epithelium with the outer epidermis is critical for further regeneration. The animals that are unable to close the wound die without starting the regenerate formation (Korotkova, 1997; Stocum, 2012; Kostyuchenko et al., 2016). Most often, wound healing is completed within the first day, usually before the appearance of the signs of active division of epidermal cells. Thus, already the first critical stage of regeneration is ensured mostly by changing the shape and position of cells of old tissues (Hill, 1970; Kharin et al., 2006; Paulus and Muller, 2006; Zattara and Bely, 2011; Bely, 2014; Kostyuchenko et al., 2016; Kozin et al., 2017). Interestingly, during transverse fission of annelids (even closest to regeneration (architomy), let alone paratomy), wound healing per se and formation of the wound epithelium are not observed (Martinez et al., 2005; Kostyuchenko et al., 2016). However, even in this case, the beginning of the development of the required border formations, preventing the appearance of a wound after physical separation of one zooid from another, occurs due to changes in the preexisting architecture of the muscular system and local modification of the epidermal cells in the fission zone (Ivanova-Kazas, 1977; Kharin et al., 2006; Babahanova et al., 2012; Kostyuchenko et al., 2016).
Tissue remodeling after amputation are accompanied by significant changes in the interaction of cells with the extracellular matrix and in the cell–cell interactions near the wound (Coulon et al., 1989; Dupin et al., 1991; Stocum, 2012). In addition, individual cells begin to migrate to the wound (site of the fission zone formation) or away from it (Kostyuchenko et al., 2016; Zattara et al., 2016). Thus, the earliest stages of regeneration and asexual reproduction are associated with local changes in old tissues by the morphallaxis.
It is important to note that, since the very beginning, regeneration and asexual reproduction processes are accompanied by the “molecular morphallaxis.” This primarily applies to the systems of positional information and changes in the molecular profile of cells. For example, in the paratomy zone or at the wound site, genes of germ and multipotent cells (vasa, pl10, and piwi) are upregulated before the appearance of the first signs of proliferation (Smirnova and Kostyuchenko 2007; Kozin and Kostyuchenko, 2015). Early activation of the expression of the genes encoding transcription factors, including pioneer ones (Fig. 2), is also observed (Kostyuchenko et al., 2019; Kozin and Kostyuchenko, unpublished data). All these events proceed against the background of reorganization of the molecular patterns of the nervous system and molecular identity of body parts, with involvement of Hox genes and other homeobox-containing genes (Steinmetz et al., 2010; Novikova et al., 2013; Kostyuchenko et al., 2016).
Activation of gene expression at the early stages of posterior regenerate development in the polychaete Alitta virens, detected by in situ hybridization. The arrows indicate the localization of the mRNA signal. The wound surface is shown on the right. (a) Avi-twist at the stage of 8 h after amputation, inner cells. (b) Avi-foxA is expressed in the area of fusion of severed edges of the gut epithelium with the outer epidermis prior to cell proliferation, 20 h after amputation. Scale, 50 μm.
PROLIFERATION, GROWTH, AND DIFFERENTIATION
The interaction of tissues at the wound site or within the fission zone anlage, rearrangement, and cell migration create conditions for both epimorphosis with the formation of a blastema and further changes by the morphallaxis (Fontes et al., 1983; Korotkova, 1997; Boilly et al., 2017). The development of epimorphic regeneration, most likely, requires signals from the central nervous system. It is known that, in many species, the blastema is not developed without innervation. The wound end of the ventral nerve cord can activate regeneration and proliferative activity of cells (Coulon and Thouveny, 1984; Muller et al., 2003). The part of the nervous system that is located directly at the wound site undergoes specific changes. In the polychaete Alitta virens, the terminal zone of the last ganglion, cut in its anterior part and coated with the wound epithelium, begins to be rearranged already on the first day after amputation. This is accompanied by a local remodeling of the ventral nerve cord. At this stage, the neurites extending from the severed end of the ventral nerve cord and from the subepithelial network of the peripheral nervous system of the last old segment penetrate the wound epithelium (Kozin et al., 2017). The development of the nerve fiber network encompassing the entire blastema before the manifestations of all other regenerative processes was also shown for the oligochaete Enchytraeus japonensis, which reproduces by architomy (Yochida-Noro et al., 2000). Most likely, the structural, biochemical, and functional changes in the nervous system during the formation of new zooids are evidence suggesting key role of the nervous system during asexual reproduction (Martinez et al., 2005; Kharin et al., 2006; Zattara and Bely, 2011; Kostyuchenko et al., 2016). Possibly, other systems also affect the local remodeling processes and blastema mass development (Korotkova, 1997; Kostyuchenko et al., 2016).
Blastema formation may also be affected by the descendants of specific (presumably stem) cells located at some distance from the wound. Thus, the blastema, at least in part, may be formed by migrating cells (Korotkova, 1997; Bely, 2014; Kostyuchenko et al., 2016; De Jong and Seaver, 2018). However, there are many models on which it was shown that blastema formation may be due to local cell proliferation and may be accompanied by dedifferentiation of cells of old tissues (Korotkova, 1997; Bely, 2014; Kozin and Kostyuchenko, 2015; Kostyuchenko et al., 2016; Nikanorova and Kostyuchenko, 2018; Shalaev et al., 2018a, 2018b; Planques et al., 2019). Our data support the significant migrations and active proliferation of cells in the fission zone during paratomy in oligochaetes. It is obvious that the tissues of all three germ layers may be involved in the development of blastema masses (Kharin et al., 2006; Smirnova and Kostyuchenko, 2007; Zattara and Bely, 2011, 2013; Babakhanova et al., 2012; Kostyuchenko et al., 2016).
Further development of the regenerate or the fission zone is associated with the growth and differentiation of the blastema. Regardless of the origin, undifferentiated blastema cells proliferate actively. Proliferation was also detected in the epithelial layers of the wound epithelium, epidermis, and gut. It should be noted that pronounced epimorphic processes take place against the background of not so pronounced rearrangements of old tissues. First of all, this concerns the muscular and nervous systems. The structure of the muscular framework changes: it becomes less dense. However, by the time of differentiation of the regenerate or the paratomy zone, additional fibers appear in it, both by inserting new cellular elements and due to intercalary growth. The segmental ganglia closest to the wound or the paratomy zone undergo changes and form new fibers or nerve cords that grow into the blastema masses and give rise to new terminal structures of the central and peripheral nervous systems (Kharin et al., 2006; Zattara and Bely, 2011; Kostyuchenko et al., 2016; Özpolat and Bely, 2016; Kozin et al., 2017).
The growth and differentiation of epimorphic regenerates are accompanied by intercalations and other forms of cell rearrangements, as well as by changes in the degree of compactness of both epithelial and mesenchymal structures. In fact, all this reflects the next stage of morphallactic events taking place against the background of formation of new segments and parts of the existing systems of the organism. Note that, after the completion of the processes of regeneration and asexual reproduction, significant morphallactic changes within the old segments are often observed. For example, the intestine in the old segments adjacent to the newly formed anterior end of the animal is transformed into the stomach (if this structure was lost due to amputation). In other cases, the morphological identity of some of the old segments changes, etc. (Kharin et al., 2006; Zattara and Bely, 2011; Kostyuchenko et al., 2016; Özpolat and Bely, 2016; Kozin et al., 2017).
Of course, progress of regenerate development or the fission zone formation is accompanied by significant changes in gene expression. At this time, a marked expansion of expression domains of the genes of germ and multipotent cells is observed, followed by restriction and substitution with the expression domains of the tissue-specific and cell differentiation genes. This is accompanied by noticeable systemic changes in the molecular profile, not only local but also within the entire organism (Steinmetz et al., 2010; Novikova et al., 2013; Kozin and Kostyuchenko, 2015; Kostyuchenko et al., 2016; De Jong and Seaver, 2016, 2018; Kostyuchenko et al., 2019).
MORPHALLAXIS versus EPIMORPHOSIS?
A lot of time has passed since T. Morgan (1901) divided the regeneration processes into two main types by the “behavior” of cells and the state of organ residue—epimorphosis and morphallaxis. As a rule, in the academic literature, these two types are contrasted against one another. Indeed, epimorphosis is accompanied by a burst of mitotic activity and the formation of a population of undifferentiated cells called blastema, whereas morphallaxis is accompanied by the rearrangement of cells in the residue of an organ or body of an animal after wound epithelialization without blastema formation. However, some researchers believe that the division of regenerative morphogeneses into epimorphosis and morphallaxis is conditional, because in some cases these processes are combined (Korotkova, 1997).
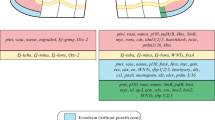
In this work, we have shown that the morphallactic rearrangements are observed during regeneration and asexual reproduction in annelids from the earliest stages (Fig. 3). The triggering mechanisms apparently differ, because in the case of asexual reproduction they reflect systemic regulation at the organismal level. The starting point of regeneration is wounding and displacement of tissues, leading to a local change in the positional information identity, etc. Obviously, morphallaxis is a necessary condition for further events by epimorphosis, which are well expressed in annelids. However, the morphallactic processes, though not so pronounced, accompany growth in the blastema mass, in this case at the level of local changes within certain systems of the body. At the final stage of regenerative morphogeneses in annelids, morphallaxis becomes apparent again, because it affects particular organs and metameric structures. Thus, morphallaxis is not only combined with the epimorphic processes but is also an essential component of morphogenesis in general, without which normal initiation of the formation of the blastema, its growth and differentiation, and the appearance of individual missing organs is impossible.
Scheme of the ratio of epimorphic and morphallactic events during reparative regeneration and asexual reproduction by the transverse fission in annelids. The color intensity in the gradient filling MORPHALLAXIS and EPIMORPHOSIS corresponds to the degree of expression of the process from the beginning until its completion. Vertical arrows show the relative time of separation of the animal into individual fragments in regeneration/architomy or zooids in paratomy.
REFERENCES
Babakhanova, R.A., Smirnova, N.P., and Kostyuchenko, R.P., Dedifferentiation of somatic cells as a probable source of regeneration of missing structures at asexual reproduction in annelids, Tsitologiya, 2012, vol. 54, no. 4, pp. 334–335.
Bely, A., Decoupling of fission and regenerative capabilities in an asexual oligochaete, Hydrobiologia, 1999, vol. 406, pp. 243–251.
Bely, A.E., Distribution of segment regeneration ability in the Annelida, Integr. Comp. Biol., 2006, vol. 46, pp. 508–518.
Bely, A.E., Early events in annelid regeneration: a cellular perspective, Integr. Comp. Biol., 2014, vol. 54, pp. 688–699.
Bely, A.E. and Nyberg, K.G., Evolution of animal regeneration: reemergence of a field, Trends Ecol. Evol., 2010, vol. 25, pp. 161–170.
Bely, A.E. and Sikes, J.M., Latent regeneration abilities persist following recent evolutionary loss in asexual annelids, Proc. Natl. Acad. Sci. U. S. A., 2010, vol. 107, pp. 1464–1469.
Bely, A. and Wray, G., Evolution of regeneration and fission in annelids: insights from engrailed and orthodenticle-class gene expression, Development, 2001, vol. 128, pp. 2781–2791.
Berrill, N.J., Regeneration and budding in worms, Biol. Rev., 1952, vol. 27, pp. 401–438.
Boilly, B., Boilly-Marer, Y., and Bely, A.E., Regulation of dorso-ventral polarity by the nerve cord during annelid regeneration: a review of experimental evidence, Regeneration, 2017, vol. 4, pp. 54–68.
Carrillo-Baltodano, A.M. and Meyer, N.P., Decoupling brain from nerve cord development in the annelid Capitella teleta: insights into the evolution of nervous systems, Dev. Biol., 2017, vol. 431, pp. 134–144.
Coulon, J. and Thouveny, Y., Relation entre l’innervation et l’activite proliferatrice des cellules blastematique au cours de la regeneration de l’annelide polychete Owenia fusiformis: etudes ultrastructurale et autoradiographique, Arch. Anat. Microsc., 1984, vol. 73, pp. 45–56.
Coulon, J., Diano, M., Arsanto, J.-P., and Thouveny, Y., Remodeling processes during anterior regeneration of Owenia fusiformis (Polychaeta, Annelidae): a morphological and immunocytochemical survey, Can. J. Zool., 1989, vol. 67, pp. 994–1005.
Dupin, F., Coulon, J., Le Parco, Y., Fontes, M., and Thouveny, Y., Formation of the extracellular matrix during the epimorphic anterior regeneration of Owenia fusiformis: autoradiographical and in situ hybridization studies, Int. J. Dev. Biol., 1991, vol. 35, pp. 109–119.
Evolutionary Developmental Biology of Invertebrates, vol. 2: Lophotrochozoa (Spiralia), Wanninger, A., Ed., Vienna: Springer Vienna, 2015.
Fontes, M., Coulon, J., Delgrossi, M.H., and Thouveny, Y., Muscle dedifferentiation and contractile protein synthesis during post-traumatic regeneration by Owenia fusiformis (polychaete annelid), Cell. Differ., 1983, vol. 13, pp. 267–282.
Herlant-Meewis, H., Regeneration in annelids, Adv. Morphogen., 1964, vol. 4, pp. 155–215.
Hill, S.D., Origin of the regeneration blastema in polychaete annelids, Am. Zool., 1970, vol. 10, pp. 101–112.
Ivanova-Kazas, O.M., Bespoloe razmnozhenie zhivotnykh (Asexual Reproduction of Animals), Leningrad: Leningr. Gos. Univ., 1977.
Iwanoff, P.P., Die Entwiklung der Larvalsegmente bei den Annelide, Z. Morph. Oekol. Tiere, 1928, vol. 10, pp. 62–161.
de Jong, D.M. and Seaver, E.C., Astable thoracic Hox code and epimorphosis characterize posterior regeneration in Capitella teleta,PLoS One, 2016, vol. 11, no. 2. e0149724–34.
de Jong, D.M. and Seaver, E.C., Investigation into the cellular origins of posterior regeneration in the annelid Capitella teleta,Regeneration, 2018, vol. 5, pp. 61–77.
Kawamoto, S., Yoshida-Noro, C., and Tochinai, S., Bipolar head regeneration induced by artificial amputation in Enchytraeus japonensis (Annelida, Oligochaeta), J. Exp. Zool. Pt. A Comp. Exp. Biol., 2005, vol. 303, pp. 615–627.
Kharin, A.V., Zagainova, I.V., and Kostyuchenko, R.P., Formation of the paratomic fission zone in freshwater oligochaetes, Russ. J. Dev. Biol., 2006, vol. 37, no. 6, pp. 354–365.
Korotkova, G.P., Regeneratsiya zhivotnykh (Regeneration of Animals), St. Petersburg: S.-Peterb. Gos. Univ., 1997.
Kostyuchenko, R.P. and Dondua, A.K., Development of the prototroch in embryogenesis of Nereis virens (Polychaeta), Russ. J. Dev. Biol., 2006, vol. 37, no. 2, pp. 69–76.
Kostyuchenko, R.P. and Dondua, A.K., Peculiarities of isolated blastomere development of the polychaete Alitta virens,Russ. J. Dev. Biol., 2017, vol. 48, no. 3, pp. 236–240.
Kostyuchenko, R.P., Kozin, V.V., and Kupryashova, E.E., Regeneration and asexual reproduction in annelids: cells, genes, and evolution, Biol. Bull. (Moscow), 2016, vol. 43, no. 3, pp. 185–194.
Kostyuchenko, R.P., Kozin, V.V., Filippova, N.A., and Sorokina, E.V., FoxA expression pattern in two polychaete species, Alitta virens and Platynereis dumerilii: examination of the conserved key regulator of the gut development from cleavage through larval life, post-larval growth and regeneration, Dev. Dyn., 2019, vol. 248, no. 8, pp. 728–743.
Kozin, V.V. and Kostyuchenko, R.P., Vasa, PL10, and Piwi gene expression during caudal regeneration of the polychaete annelid Alitta virens,Dev. Genes Evol., 2015, vol. 225, pp. 129–138.
Kozin, B.B. and Kostyuchenko, R.P., Evolutionary conservation and variability of the mesoderm development in Spiralia: a peculiar pattern of nereid polychaetes, Biol. Bull. (Moscow), 2016, vol. 43, no. 3, pp. 216–225.
Kozin, V.V., Babakhanova, R.A., and Kostyuchenko, R.P., Functional role for MAP kinase signaling in cell lineage and dorsoventral axis specification in the basal gastropod Testudinalia testudinalis (Patellogastropoda, Mollusca), Russ. J. Dev. Biol., 2013, vol. 44, no. 1, pp. 35–47.
Kozin, V.V., Filimonova, D.A., Kupriashova, E.E., and Kostyuchenko, R.P., Mesoderm patterning and morphogenesis in the polychaete Alitta virens (Spiralia, Annelida): Expression of mesodermal markers Twist, Mox, Evx and functional role for MAP kinase signaling, Mech. Dev., 2016, vol. 140, pp. 1–11.
Kozin, V.V., Filippova, N.A., and Kostyuchenko, R.P., Regeneration of the nervous and muscular system after caudal amputation in the polychaete Alitta virens (Annelida: Nereididae), Russ. J. Dev. Biol., 2017, vol. 48, no. 3, pp. 198–210.
Lanza, A.R. and Seaver, E.C., An organizing role for the TGF-β signaling pathway in axes formation of the annelid Capitella teleta,Dev. Biol., 2018, vol. 435, pp. 26–40.
Lesiuk, N. and Drewes, C., Autotomy reflex in a freshwater oligochaete, Lumbriculus variegates,Hydrobiologia, 1999, vol. 406, pp. 253–261.
Liu, S.-Y., Selck, C., Friedrich, B., Lutz, R., Vila-Farreґ, M., Dahl, A., Brandl, H., Lakshmanaperumal, N., Henry, I., and Rink, J.C., Reactivating head regrowth in a regeneration-deficient planarian species, Nature, 2013, vol. 500, pp. 81–84.
Martinez, V.G., Menger, G.J., 3rd, and Zoran, M.J., Regeneration and asexual reproduction share common molecular changes: upregulation of a neural glycoepitope during morphallaxis in Lumbriculus,Mech. Dev., 2005, vol. 122, no. 5, pp. 721–732.
Morgan, T.H., Regeneration, Columbia Univ. Biol. Ser., Norwood, MA: Macmillan, 1901.
Muller, M.C.M., Berenzen, A., and Westheide, W., Experiments on anterior regeneration in Eurythoe complanata (“Polychaeta,” Amphinomidae): reconfiguration of the nervous system and is function for regeneration, Zoomorphology, 2003, vol. 122, pp. 95–103.
Nakamoto, A., Nagy, L.M., and Shimizu, T., Secondary embryonic axis formation by transplantation of d quadrant micromeres in an oligochaete annelid, Development, 2010, vol. 138, pp. 283–290.
Nikanorova, D.D. and Kostyuchenko, R.P., Regeneration processes and gonad recovery features in Enchytraeus coronatus (Enchytraeidae, Oligochaeta), in Zoologiya bespozvonochnykh (Invertebrate Zoology), Novyi vek, 2018, p. 93.
Novikova, E.L., Bakalenko, N.I., Nesterenko, A.Y., and Kulakova, M.A., Expression of Hox genes during regeneration of nereid polychaete Alitta (Nereis) virens (Annelida, Lophotrochozoa), EvoDevo, 2013, vol. 4, no. 14.
Özpolat, B.D. and Bely, A.E., Developmental and molecular biology of annelid regeneration: a comparative review of recent studies, Curr. Opin. Genet. Dev., 2016, vol. 40, pp. 144–153.
Özpolat, B.D., Sloane, E.S., Zattara, E.E., and Bely, A.E., Plasticity and regeneration of gonads in the annelid Pristina leidyi,EvoDevo, 2016, vol. 7 (1), no. 22.
Paulus, T. and Muller, M.C.M., Cell proliferation dynamics and morphological differentiation during regeneration in Dorvillea bermudensis (Polychaeta, Dorvilleidae), J. Morphol., 2006, vol. 267, pp. 393–403.
Planques, A., Malem, J., Parapar, J., Vervoort, M., and Gazave, E., Morphological, cellular and molecular characterization of posterior regeneration in the marine annelid Platynereis dumerilii,Dev. Biol., 2019, vol. 445, pp. 189–210.
Randolph, H., The regeneration of the tail in Lumbriculus,J. Morphol., 1892, vol. 7, pp. 317–344.
Ribeiro, R.P., Bleidorn, C., and Aguado, M.T., Regeneration mechanisms in Syllidae (Annelida), Regeneration, 2018, vol. 5, pp. 26–42.
Schneider, S.Q. and Bowerman, B., β-Catenin asymmetries after all animal/vegetal-oriented cell divisions in Platynereis dumerilii embryos mediate binary cell-fate specification, Dev. Cell, 2007, vol. 13, no. 1, pp. 73–86.
Shalaeva, A.Yu., Kostyuchenko, R.P., and Kozin, V.V., The study of mitotic activity at the posterior regeneration of the White Sea polychaete Alitta virens, in Zoologiya bespozvonochnykh (Invertebrate Zoology), Novyi vek, 2018a, p. 128.
Shalaeva, A.Yu., Borisenko, I.E., Kostyuchenko, R.P., and Kozin, V.V., The kinetics of cell populations during regeneration and larval development in the White Sea polychaete Alitta virens: methodological aspects and preliminary results, in Morskie issledovaniya i obrazovanie: MaresEdu (Marine Research and Education: MaresEdu-2018), 2018b, pp. 93–97.
Sikes, J.M. and Newmark, P.A., Restoration of anterior regeneration in a planarian with limited regenerative ability, Nature, 2013, vol. 500, pp. 77–80.
Smirnova, N.P. and Kostyuchenko, R.P., Cellular sources of the paratomy zone development in the oligochaete Pristina longiseta (Naididae): cloning and analysis of gene expression markers of stem and poorly differentiated cells, Tsitologiya, 2007, vol. 49, no. 9, p. 794.
Steinmetz, P.R.H., Urbach, R., Posnien, N., Eriksson, J., Kostyuchenko, R.P., Brena, C., Guy, K., Akam, M., Bucher, G., and Arendt, D., Six3 demarcates the anterior-most developing brain region in bilaterian animals, EvoDevo, 2010, vol. 1, no. 14, pp. 1–9.
Stocum, D.L., Regenerative Biology and Medicine, 2nd ed., Amsterdam: Elsevier, 2012.
Tadokoro, R., Sugio, M., Kutsuna, J., Tochinai, S., and Takahashi, Y., Early segregation of germ and somatic lineages during gonadal regeneration in the annelid Enchytraeus japonensis,Curr. Biol., 2006, vol. 16, pp. 1012–1017.
Vorontsova, M.A. and Liosner, L.D., Asexual Propagation and Regeneration, London: Pergamon Press, 1960.
Yochida-Noro, C., Myohara, M., Kobari, F., and Tochinai, S., Nervous system dynamics during fragmentation and regeneration in Enchytraeus japonensis (Oligochaeta, Annelida), Dev. Genes Evol., 2000, vol. 210, pp. 311–319.
Zattara, E.E. and Bely, A.E., Evolution of a novel developmental trajectory: fission is distinct from regeneration in the annelid Pristina leidyi,Evol. Dev., 2011, vol. 13, pp. 80–95.
Zattara, E.E. and Bely, A.E., Investment choices in post-embryonic development: quantifying interactions among growth, regeneration, and asexual reproduction in the annelid Pristina leidyi, J. Exp. Zool. P B Mol.Dev. Evol., 2013, vol. 320, pp. 471–488.
Zattara, E.E. and Bely, A.E., Phylogenetic distribution of regeneration and asexual reproduction in Annelida: regeneration is ancestral and fission evolves in regenerative clades, Invert. Biol., 2016, vol. 135, no. 4, pp. 400–414.
Zattara, E.E., Turlington, K.W., and Bely, A.E., Long-term time-lapse live imaging reveals extensive cell migration during annelid regeneration, BMC Dev. Biol., 2016, vol. 16 (1), no. 6.
Zattara, E.E., Fernandez-Alvarez, F.A., Hiebert, T.C., Bely, A.E., and Norenburg, J.L., A phylum-wide survey reveals multiple independent gains of head regeneration ability in Nemertea, Proc. Biol. Sci., 2019, vol. 286, art. ID 20182524.
ACKNOWLEDGMENTS
This study was conducted using the equipment of the RMiKT Resource Center of St. Petersburg State University and the infrastructure of the Belomorskaya Educational and Scientific Base, St. Petersburg State University.
Funding
This study was supported by the Russian Foundation for Basic Research, project no. 16-04-00991-a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Kostyuchenko, R.P., Kozin, V.V. Morphallaxis versus Epimorphosis? Cellular and Molecular Aspects of Regeneration and Asexual Reproduction in Annelids. Biol Bull Russ Acad Sci 47, 237–246 (2020). https://doi.org/10.1134/S1062359020030048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359020030048