Abstract
Annelida are metameric, eucoelomate bilaterian worms belonging to Lophotrochozoa, which is a major group of protostomes. This phylum includes Polychaeta, Oligochaeta, Hirudinea (leeches), and Archiannelida, and it is an important link in the evolution of body plan, regeneration, and reproduction. Although annelids generally reproduce sexually, many species can switch to asexual reproduction, proliferating exponentially, as seen in planarians, hydras, and some other lower invertebrates. Asexual reproduction is achieved through dedifferentiation and through stem cells regeneration, instead of being based on germ cells as in the case of sexual reproduction. Thus, studies on regeneration mechanisms and germ cells are essential for understanding annelid reproduction. In this chapter, Annelida’s reproduction and germ cell formation and regeneration are reviewed. Based on our research on the oligochaete Enchytraeus japonensis, a unique process of germ cell regeneration during asexual reproduction is proposed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Since the Cambrian age, annelids have survived, selecting the most favorable mode of reproduction depending on circumstances and the environment. Almost all oligochaetes, leeches, and polychaetes can proliferate through sexual reproduction, although all groups also have hermaphroditic species. Oligochaetes and leeches are generally hermaphroditic, laying a few eggs wrapped in a cocoon (Brusca and Brusca 1990), which increases fertility, protecting eggs from environmental changes and bacteria. Hermaphroditic and dioecious species are typically found within polychaetes; many species lay a small number of eggs, whereas others lay a larger number of eggs as a strategy to increase their survival rate (Izuka 1903; Okada 1941; Hauenschild 1960; Bentley et al. 2001). The sexual reproduction and germ cell formation in annelids are described in this chapter.
Unlike leeches, oligochaetes and polychaetes generally possess a high regeneration ability (Bely 2006), allowing some species to reproduce asexually and proliferate exponentially. Some of these species divide their body into several fragments, each of which regenerates a new individual, whereas other species bud new generations from growth zones (Berrill 1952; Bell 1959; Christensen 1959; Bouguenec and Giani 1989; Nakamura 1993; Schmelz et al. 2000; Bely and Wray 2001). In asexual reproduction, stem cells, instead of germ cells, and dedifferentiation systems play a central role in reproduction. To date, regeneration processes have been well described in both oligochaetes and polychaetes based on histological analyses. Here, the regeneration process of oligochaetes is briefly described, focusing on Enchytraeus japonensis as an example (Nakamura 1993; Myohara et al. 1999). Interestingly, these worms can regenerate germ cells that are often lost by fission or accidental cutting (Tadokoro et al. 2006). Thus, in addition to the commonly known animal germ cell system, E. japonensis, uses the regeneration of somatic tissues and germ cells as a reproductive strategy. Although the origin of regenerating germ cells has been investigated in other oligochaetes and in polychaetes (Iwanoff 1928; Gates 1943; Herlant-Meewis 1964b; Vannini 1947; Dorsett 1961), it is still largely unknown. Previous and recent studies on germ cell regeneration will also be reviewed here.
2 Sexual Reproduction in Annelids
Oligochaetes and leeches are generally hermaphroditic, and their male and female gonads and germ cells are located around the coelomic cavity in special segments of the head region termed genital segments. These segments are characterized by a thickened epidermis called the clitellum (Fig. 10.1a) (Stephenson 1930; Brusca and Brusca 1990), and oligochaetes and leeches are therefore collectively classified as Clitellata (Stephenson 1930; Jamieson 1992). Most clitellate species have several pairs of ovaries and testes, and their location and number are used as taxonomic characters (Michaelsen 1929; Jamieson 2006).
Sexual reproduction in Clitellates. (a) Male and female reproductive organs and germline cells of oligochaete, Enchytraeus japonensis, colored in blue and pink, respectively. (b) Mating behavior of oligochaetes; Blue and pink circles indicate spermathecae and female genitalia, respectively. (c) Process of egg deposition and fertilization in an oligochaete. (d) Schematic illustration of embryogenesis in the leech Helobdella robusta; Darker gray areas indicate the teloplasm. N, O, P, and Q are ectodermal teloblasts and M is a mesodermal teloblast (redrawn from Weisblat et al. (1980))
Male reproductive organs comprise the testes, seminal vesicles, male funnel, seminiferous tube, and penis (Fig. 10.1a). During spermatogenesis, sperm stem cells (spermatogonia) within the testes are released into seminal vesicles, where they undergo repeated incomplete cell divisions with maintaining cytoplasmic connections. As a result, the spermatogonia develop a morula-like morphology (Fig. 10.1a) (Stephenson 1930; Jamieson 1992) that later differentiates into spermatids and sperm. Thereafter, mature sperm enter the male funnel and are transported to the penis via the seminiferous tube (Fig. 10.1a) (Brusca and Brusca 1990; Stephenson 1930; Jamieson 1992). The histological characterization of Spermatogonia and reproductive organs is a straightforward process. Oocyte stem cells (oogonia) are harbored in the ovary and mature within the ovisac, which is a vesicle-like structure derived from the septum (Fig. 10.1a) (Stephenson 1930; Jamieson 1992; Brusca and Brusca 1990). Oocytes are transported along the oviduct and deposited in the clitellum through the female pore (Stephenson 1930; Jamieson 1992).
At the onset of breeding, two worms join their head regions aligned in opposite directions and each releases sperm from the penis and deposits it into the partner’s spermatheca (Fig. 10.1b) (Stephenson 1930; Michaelsen 1929; Avel 1959; Brusca and Brusca 1990). After mating, the worms secrete cocoon material from the clitellum, surround the eggs with it, and displace the cocoon toward the anterior region by vermicular movements (Fig. 10.1c) (Edwards and Lofty 1972; Brusca and Brusca 1990). Eggs are fertilized by the partner’s sperm within the cocoon when it passes on the spermatheca’s pore (Fig. 10.1c) (Edwards and Lofty 1972; Brusca and Brusca 1990) and the cocoon is finally shed from the head of worm (Fig. 10.1c) (Edwards and Lofty 1972; Brusca and Brusca 1990).
Reproduction in polychaetes is more diverse than that in oligochaetes and leeches. Although most polychaetes are dioecious, some species are hermaphroditic; some individuals are born either as male or female and then change sex (Bacci and Bortesi 1961). Stem cells of both male and female germ cells are harbored in the gonads or in the coelomic epithelium and mature in the coelomic fluid (Olive and Clark 1978; Fischer 1974, 1975; Sawada 1975). Although in most species germ cells are located in specific segments, in primitive species these cells float in the coelomic cavity of all segments (Olive and Clark 1978; Fischer 1974, 1975; Sawada 1975). Mature oocytes are released through the nephridium or by rupturing the body wall (Goodrich 1945), as male and female ducts develop poorly compared with those of oligochaetes. In many cases, eggs are deposited in masses enclosed by a gel, and fertilization occurs after egg deposition (only a few species mate before egg laying) (Brusca and Brusca 1990). In some species of Nereididae, Syllidae, and Eunicidae, sexually mature worms, or parts of the body filled with germ cells (epitokes), simultaneously swim toward the sea surface, releasing numerous eggs and sperm on specific full moon nights (Izuka 1903; Okada 1941; Hauenschild 1960; Bentley et al. 2001). This swarming behavior has attracted the attention of biologists, particularly ecologists and ethologists. Although it has been shown that swarming behavior is activated by brain-controlled hormones (Hauenschild 1960), the molecular mechanisms regulating this periodic behavior remain unknown.
3 Germ Cell Formation During Embryogenesis
The origin of annelid germ cells has long been debated based on the traditional histological observations performed over the last 50 years. As a result of recent gene expression analyses and cell tracing experiments more details on the origin of annelid germ cells have emerged. Here, previous and current studies on the embryonic origin of germ cells are reviewed, mainly performed on the clitellates Helobdella robusta (leech) and Tubifex tubifex (oligochaete).
As background information for the observations and experiments described below, I present a brief review of H. robusta development (for details, see Weisblat et al. 1980). After fertilization,the H. robusta zygote divides into four blastomeres via two cleavage divisions (A, B, C, and D quadrants in Stage 3, Fig. 10.1d) (Weisblat et al. 1980). Almost synchronously, each blastomere produces micromeres in alternating directions (clockwise/counter-clockwise), which are spirally accumulated around the animal pole (spiral cleavage) (Fig. 10.1d, stages 4a and 4b). These micromeres contribute to the head’s ectoderm and mesoderm (Shankland and Savage 1997). The D quadrant divides into four pairs of ectodermal stem cells (N, O, P, and Q teloblasts) and a single pair of mesodermal stem cells (M teloblasts) on both sides of the blastomere (Fig. 10.1d, stages 4b–8) (Weisblat et al. 1980). The teloblasts then undergo asymmetric cell division, continuously generating primary blast cells in bandlets, which fasciculate together on each side, producing germinal bands (Fig. 10.1d, early stage 8) (Weisblat et al. 1980). The germinal bands on each side are displaced ventrally as they elongate and finally merge on the ventral midline, forming the tubular body of the worm (Fig. 10.1d, early stage 8 to late stage 8) (Weisblat et al. 1980). Embryogenesis of the oligochaete, Tubifex, is similar to that of H. robusta, despite the different timing of cleavage (Shimizu 1980).
Meyer (1929) reported that in Tubifex primordial germ cells (PGCs) emerge in situ around the presumptive genital segments during embryogenesis. Penners and Stäblein (1930) postulated that Tubifex PGCs derive from M teloblasts and migrate to the genital segments through amoeboidal movements. On the other hand, it has been suggested that in the oligochaete Eisenia fetida the first primary blast cells produced by M teloblasts do not contribute to PGCs (Devries 1971). Thus, it is difficult to determine the origin of PGCs based only on histological observation. Studies on the origin of germ cells have recently made substantial progress, as germ cell gene markers (for example, nanos, vasa, piwi) allow the unambiguous identification of germline cells.
In the twenty-first century, the research team of David Weisblat reported the expression pattern of the nanos gene in H. robusta (Kang et al. 2002). This gene encodes a translational repressor protein that is specifically expressed in the germline cells of almost all organisms (Kobayashi et al. 1996; Tsuda et al. 2003; Koprunner et al. 2001; Sato et al. 2006). During H. robusta early development, maternal transcripts of nanos are first accumulated in the yolk-free cytoplasm (teloplasm) of zygotes and then inherited by the D quadrant and its descendants DM and DNOPQ (Fig. 10.1d, stages 1–4b) (Kang et al. 2002). Until this stage, nanos mRNA behavior is identical to that displayed in the teloplasm (Fig. 10.1d, dark gray areas). Maternal expression of nanos decreases by stage 7 and zygotic transcripts are expressed in the ectodermal and mesodermal teloblasts, in their descendants (i.e., primary blast cells), and in germinal bands (Fig. 10.1d, early stage 8) (Kang et al. 2002). At this stage, nanos expression in the germinal bands almost disappears, except in the presumptive gonoblasts located in the 11 mid-body segments (Fig. 10.1d, stage 10, spots), which will develop into germ cells (Kang et al. 2002). Cell tracing experiments confirmed that these nanos-expressing gonoblasts derive from M primary blast cells (Kang et al. 2002) and might correspond to cells previously proposed to be PGCs.
Recently, the expression patterns of the conserved germline makers DEAD-box RNA helicase vasa and the ncRNA binding protein piwi (Shibata et al. 1999; Yoon et al. 1997; Fujiwara et al. 1994; Mochizuki et al. 2001; Cox et al. 1998, 2000; Lau et al. 2006; Kuramochi-Miyagawa et al. 2001; Deng and Lin 2002), were shown to be similarly distributed (Cho et al. 2014). Interestingly, during Helobdella embryogenesis, nanos and vasa/piwi mRNAs are preferentially expressed in male and female PGCs, respectively (Cho et al. 2014). Oyama and Shimizu (2007) showed that vasa is broadly expressed in Tubifex embryonic cells, including mesodermal cells, in patterns similar to those of nanos and vasa in Helobdella. Furthermore, based on cell ablation experiments, these authors determined that PGCs are generated from primary m cells (m10 and m11), corresponding to the presumptive genital segments, but not from cells deposited by M teloblasts before blast cell generation (Kato et al. 2013). As PGCs do not disappear when blast cells adjacent to m10 and m11 are ablated (Kato et al. 2013), the destiny of these blast cells might be determined at birth. However, it is still largely unknown how PGCs are determined in specific primary blast cells.
Previous studies on polychaetes have suggested that germ cells might also derive from the mesodermal linage corresponding to M teloblasts and/or bandlets (Malaquin 1925, 1934; Iwanoff 1928). Rebscher and co-authors (Rebscher et al. 2007, 2012) reported the expression pattern of vasa mRNA and its encoded protein in Platynereis dumerilii, showing that mesoblasts (4 d) deposit four vasa-positive cells that become PGCs after migration. These four vasa-positive cells appear to be identical to the cells that Schneider and Bowerman (2007) designate as “prospective PGCs.” In Capitella teleta, germline cells appear to emerge near mesodermal bands, although the details are still unclear (Giani et al. 2011; Dill and Seaver 2008). Thus, almost all studies suggest that annelids’ PGCs derive from mesodermal lineages generated in early embryogenesis, with the timing of segregation differing among species. It has often been debated whether PGCs are specified by preformation or by epigenesis: whereas in preformation the germline is specified by maternal cytoplasmic determinants, as is the case in fruit flies and nematodes, in epigenesis germ cell segregation is induced from the somatic lineage (Eddy 1975; Extavour and Akam 2003). In Helobdella, PGCs are specified around the 20th round of cell division, when most nanos maternal transcripts have disappeared, although PGCs’ specification by preformation generally occurs at earlier stages of embryogenesis. In Tubifex species, inheritable determinants explaining germline specification by preformation were not identified, despite the thorough assessment of vasa-expression from embryogenesis to juveniles. Thus, germline specification seems to be regulated by epigenesis in annelids, although it has not been experimentally verified at the molecular level.
4 Asexual Reproduction in Annelids
Agamic asexual reproduction has the advantage of producing a large number of offspring at a low cost; however, it rarely produces individuals able to adapt to a wide variety of environments, as is the case of sexual reproduction. Asexual reproduction is typically found among oligochaetes, but not among leeches. In the asexual phase, oligochaetes divide into several fragments, producing entire worms by regeneration and/or by growing head and tail structures, i.e., by autotomy or paratomy (Fig. 10.2a) (Brusca and Brusca 1990). In paratomy, which is typically observed in Naididae and Aeolosomatidae, fission occurs after the formation of head and tail structures in prospective cutting planes (budding zones) (Fig. 10.2a, right) (Bely and Wray 2001; Herlant-Meewis 1954). In contrast, species dividing by autotomy (e.g., those in Enchytraeidae) split without preforming a budding zone (Fig. 10.2a, left) (Bell 1959; Christensen 1959, 1964; Bouguenec and Giani 1989; Nakamura 1993; Schmelz et al. 2000). Asexually reproducing worms are able to completely regenerate head and tail structures, whereas sexually reproducing species can only regenerate their anterior region (Bely 2006).
Asexual reproduction in oligochaetes: Fission and regeneration. (a) Types of asexual reproduction in oligochaetes – autotomy and paratomy; Blue regions indicate newly formed body portions. (b) Anterior and posterior regeneration of the oligochaete Enchytraeus japonensis body parts, after amputation; Images were captured using a bright field stereomicroscope (Leica MZ10F with a coupled CCD Nikon DS-Ri1 camera); Dashed lines indicate amputation sites and arrowheads point out segmental borders; White arrows indicate cell aggregates in the blastema. Br brain, dpa days post amputation, Int intestinal tube, Pgz posterior growth zone, Ph pharynx, Pro prostomium, Pyg pygidium, VNC ventral nerve cord (Tadokoro, original figures)
In annelids, regeneration proceeds through a series of phases (Fig. 10.2b). Here I will describe the course of Enchytraeus japonensis regeneration, reviewing some previous studies (Nakamura 1993; Myohara et al. 1999; Yoshida-Noro and Tochinai 2010). After amputation, circular muscles adjacent to the wound immediately contract to prevent the discharge of fluid (Herlant-Meewis 1964a, b; Bilej 1994). Epidermal cells cover anterior stumps, whereas the intestinal wall and epidermis interconnect to cover posterior stumps (Herlant-Meewis 1964a, b; Myohara et al. 1999). This process is conserved amongst almost all annelids that are able to regenerate. Five hours post amputation, epidermal cells and stem-like cells begin to proliferate, resulting in the accumulation of a large number of undifferentiated cells around the stump. A regeneration blastema is formed during the first 24 h following amputation, i.e., 1 day post amputation (dpa) (Fig. 10.2, 1 dpa). During the next 24 h, the anterior blastema elongates and tiny cell aggregates form on the dorsal and ventral sides of the blastema (Fig. 10.2, 2 dpa). These aggregates are thought to correspond to primordial brain and ventral nerve cord. As the blastema grows further, major tissues and organs regenerate and become morphologically recognizable. Morphological segmentation is also visible in the epidermis on the third day after amputation (Fig. 10.2, 3 dpa). Subsequently, each organ and tissue grows, enlarges, and regeneration is complete at the fourth dpa (Fig. 10.2, 4 dpa). In the posterior blastema, the growth zone and pygidium are regenerated by the second dpa, and segments are newly formed via the growth zone (Fig. 10.2). The process of regeneration following amputation is more or less similar in asexual reproducing worms, regardless of species.
The regeneration processes that take place after blastema formation are mediated by genes involved in body patterning, morphogenesis, and organogenesis during embryogenesis (Akimenko et al. 1995; Bely and Wray 2001; Gardiner et al. 1995; Khan et al. 2002). In contrast, blastema formation is unique to the regeneration process, i.e., it is not found in embryogenesis, and the origin of blastema cells has long been debated. Histological analyses of annelid regeneration have been performed since the nineteenth century in parallel with those of hydras, planarians, and salamanders. Hydras and planarians regenerate all body parts from pluripotent stem cells scattered throughout their body (Baguna 2012), whereas blastema formation in salamanders depends on cell dedifferentiation (Lentz 1969; Lo et al. 1993).
Dedifferentiation and stem cells referred to as neoblasts are characteristic of oligochaete asexual reproduction (Randolph 1892). Neoblasts typically have a large nucleus, ovoid shape, and basophilic cytoplasm, and are located on both sides of each septum of the trunk segments, across the ventral nerve cord (Randolph 1892). Although it has long been debated if neoblasts are pluripotent stem cells similar to planarian neoblasts, several experiments, including 5′-bromo-2-deoxyuridine (BrdU) pulse-chase, have suggested that neoblasts migrate to the stump and mainly contribute to regeneration of mesodermal tissues (Tadokoro et al. 2006; Sugio et al. 2012). Nerve axons extend from the pre-existing ventral nerve cord into the blastema during the early phase of regeneration in E. japonensis (Yoshida-Noro et al. 2000; Muller 2004), and neurons of the ventral nerve cord and brain appear to be newly formed from the epidermis in Limnodrilus (Cornec et al. 1987). Pre-existing nerve cords must play an important role in regeneration as ablation of the nerve cord inhibits head regeneration (Avel 1959, 1961). Endodermal tissues, such as the esophagus, pharynx, and digestive tract, are derived from preexisting endodermal tissues (Tweeten and Reiner 2012). In E. japonensis regeneration of the digestive tract is also achieved by dedifferentiation and re-differentiation (Takeo et al. 2008). However, studies on annelid regeneration lag behind that of planarians and other model animals, and experimental demonstrations of the mechanisms underlying annelid regeneration have rarely been performed.
5 Early Studies on Annelid Germ Cell Regeneration
The abovementioned fission and accidental cutting frequently yield worm fragments that lack the original genital segments containing germ cells. However, these fragments might propagate sexually after anterior regeneration, suggesting that germ cells regenerate along with somatic cells. Based on histological observations, some researchers have postulated germ cell regeneration. For example, Iwanoff (1928) observed germ cells during the regeneration of spionid polychaetes, proposing that germ cells were regenerated from the peritoneum. Gates (1943) and Herlant-Meewis (1964b) suggested that Perionyx and Lumbriculus germ cells arose in the inter-segmental septum. During the regeneration of the polychaete Spirirbis, monogenital segments appear to be able to generate germ cells from stem-like cells located around the muscle and blood vessels (Vannini 1947). In other polychaete species, it has been hypothesized that germ cells formed from the ventral epithelium migrate to the genital segments (Dorsett 1961). Overall, these classic histological observations suggest that PGCs can regenerate from somatic cells or from stem cells although the origin of such PGCs might differ between species. However, these observations have not provided conclusive evidence, as the origin of regenerated PGCs is even more difficult to trace in histological studies than in embryogenesis when molecular markers are not used.
6 Germ Cell Regeneration in Enchytraeus japonensis
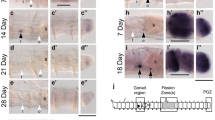
We previously addressed the question of germ cells origin using the oligochaete E japonensis found at the Agricultural Research Center for Tohoku Region (Fukushima Prefecture, Japan) (Nakamura 1993). As described in Sect. 10.5, this worm has one or two pairs of neoblasts in each segment and a strong regeneration capability, both anteriorly and posteriorly (Tadokoro et al. 2006; Yoshida-Noro and Tochinai 2010; Sugio et al. 2012). This species fragments into ~10 pieces by autotomy and within 5 days these fragments regenerate head and tail structures, producing individuals that propagate asexually under normal culture conditions (i.e., in high density) (Nakamura 1993). However, in worms cultured at extremely low densities after starvation and subsequent feeding, male and female germ cells, as well as other reproductive organs, develop in the seventh (sometimes in the sixth and seventh) and eighth segments, respectively, by the tenth day after fragmentation (Myohara et al. 1999). Thus, E. japonensis sexualization is controlled under experimental conditions, never occurring in high-density conditions. After autotomy and amputation, germ cell regeneration occurs as in other species (Tadokoro et al. 2006). The course of germ cell regeneration in E. japonensis was identified through expression patterns of piwi mRNA (Tadokoro et al. 2006). piwi mRNA, encodes a small RNA-associated protein (piwi interacting RNA; piRNA) (Lau et al. 2006), well known due to its involvement in Drosophila germline stem cell maintenance (Cox et al. 1998, 2000). Piwi expression pattern and function have recently been investigated in a wide variety of animals. For example, murine piwi homologs expressed in male germ cells regulate their differentiation to spermatocytes (Kuramochi-Miyagawa et al. 2001, 2004; Deng and Lin 2002). In planarians and jellyfish, piwi is expressed in germ cells and stem cells that differentiate to somatic and germ cells (Seipel et al. 2004; Reddien et al. 2005; Rossi et al. 2006). We isolated piwi from E. japonensis (Ej-piwi) and assessed its expression patterns in germ and stem cells (Tadokoro et al. 2006). Similar to what has been found in other animals, Ej-piwi mRNA is expressed in male germ cells (spermatogonia and differentiated spermatids) and female germ cells (oogonia and oocytes) but not in the somatic gonads of sexually mature worms (Tadokoro et al. 2006). In asexually reproducing worms, Ej-piwi-positive cells are found in tiny masses in the seventh and eighth segments, and dispersed single cells along the trunk region, dorsally to the ventral nerve cord (Fig. 10.3a); these Ej-piwi-positive cells are morphologically and positionally distinct from neoblasts (piwi-negative cells) (Tadokoro et al. 2006). These piwi-positive cells also express the vasa-related genes (vgl1 and vgl2) whereas neoblasts only express vgl2 (Sugio et al. 2008). The cell masses in the seventh and eighth segments are male and female germ cells or their precursors and remain silent during asexual reproduction. During the sexualization process, these cells expand markedly and differentiate to mature germ cells, thereby increasing piwi expression in the seventh and eighth segments (Fig. 10.3b) (Tadokoro et al. 2006). In addition, piwi-expressing cells in the trunk region change during head regeneration, but not during sexualization (Tadokoro et al. 2006). These cells start to proliferate near the anterior stump only 2 days after amputation, when the blastema has already derived from neoblasts and other cells (Fig. 10.3b) (Tadokoro et al. 2006). From the third to the fifth day after amputation, the piwi-expressing cells found in the blastema progressively change their distribution toward the seventh and eighth genital segments (Fig. 10.3b) (Tadokoro et al. 2006). Based on these data and on other evidence, E. japonensis germ cell regeneration appears to proceed as follows (Tadokoro et al. 2006): In preparation for incipient self-fragmentation, piwi-positive germline stem cells are stored in the ventral nerve cord of the trunk region; after the blastema is formed from somatic stem cells and others, germline stem cells migrate to the genital segments, regenerating male and female germ cells or their germ cell precursors. This mechanism ensures the maintenance of germ cells during repeated asexual reproduction and supports this reproductive strategy. Ozpolat and Bely (2015) have recently investigated piwi expression patterns during Pristina leidyi paratomy, and found that. piwi was expressed in newly formed tissues in the fission zone, although piwi signals were not found in E. japonensis regeneration blastema. However, piwi-expressing cells were distributed dorsally to the ventral nerve cord in an irregular pattern, which was very similar to the pattern found for piwi-expressing cells in E. japonensis, and seem to contribute to the germ cell regeneration. Statistical analyses and live imaging analyses revealed that piwi-expressing cells migrate toward the newly formed tissues in the fission zone, but not to the regeneration zones (Ozpolat and Bely 2015). Thus, piwi-expressing cells on the ventral nerve cord might be a conserved origin of germ cells, at least in asexual reproducing oligochaetes.
Germ cell regeneration in Enchytraeus japonensis. (a) piwi mRNA expression in an intact worm; masses of piwi-expressing cells are located on the ventral side of the 7th and 8th segments, which correspond to genital segments, and single piwi-expressing cells are sparsely distributed on the dorsal side of the ventral nerve cord; (a) pair of neoblasts is attached to the ventral side of each septum in the trunk region. (b) The process of germ cell regeneration during asexual reproduction; The dashed red line and red arrowheads indicate amputation sites; pictures on the right show the expression patterns of piwi mRNA during regeneration, and illustrations on the left summarize the model of germ cell regeneration on the 5 days following amputation (redrawn from Tadokoro et al. (2006, 2009)
7 Embryonic Origin of E. japonensis Germline Stem Cells
During E. japonensis anterior regeneration, germ cells in the seventh and eighth segments are regenerated from germline stem cells, but not from neoblasts. This raises the question of whether germline stem cells generate de novo from neoblasts when needed or are segregated during embryogenesis. To address this issue, our collaborators investigated the expression of piwi and vasa -related genes (Ej-vlg1 and Ej-vlg2), from embryogenesis to adult worm (Sugio et al. 2008). In adult and 20-segment worms, piwi-positive germline stem cells and germ cell precursors in the seventh and eighth segments also express Ej-vlg1 and Ej-vlg2, whereas neoblasts and cells in the posterior growth zone express Ej-vlg2 and Ej-vlg1/Ej-vlg2, respectively (Fig. 10.4e) (Sugio et al. 2008). These cell populations, which are characterized by different combinations of gene expression patterns, emerge in a step-wise fashion during embryogenesis (Sugio et al. 2008): Ej-vlg1 and Ej-vlg2 transcripts, which are initially detected in the zygote (Fig. 10.4a), are maternally supplied as they are also expressed in unfertilized oocytes; Ej-vlg1 and Ej-vlg2 are still expressed in M teloblasts and other blastomeres, but after nerve cord formation in late embryogenesis, these transcripts mainly accumulate in the posterior region, which might correspond to the presumptive posterior growth zone, although single Ej-vlg2-positive cells can be found scattered throughout the body (Fig. 10.4b, c); Ej-piwi-/Ej-vlg1-/Ej-vlg2-positive cells corresponding to germline cells emerge by the end of embryogenesis (Fig. 10.4b, c). These expression patterns suggest that neoblasts (Ej-vlg2-positive) and Ej-vlg1-/Ej-vlg2-positive cells are segregated during embryogenesis (Fig. 10.4b), and that germline stem cells (Ej-piwi-/Ej-vlg1-/Ej-vlg2-positive) are generated from the Ej-vlg1-/Ej-vlg2-positive cells in the posterior region by the end of embryogenesis (Fig. 10.4d) (Sugio et al. 2008). Recently, it has been reported that germline cells regenerate de novo around the posterior growth zone in the polychaete C. teleta, although PGCs are found in the anterior region (Giani et al. 2011). Although these results suggest that somatic cells are able to generate germline cells, the possible supply of germline stem cells by the posterior growth zone of E. japonensis adults remains to be explored. In addition, piwi-positive cells often proliferate in the trunk region of intact worms, implying that they might maintain themselves during posterior growth, without any supply from other cells (Tadokoro unpublished). Comparative analysis of germline and somatic stem cells lineages between asexually reproducing E. japonensis and sexual reproducing species might allow an improved understanding of how germline cells and stem cells arose during evolution.
Embryonic origin of Enchytraeus japonensis germline stem cells. Schematic illustrations showing the expression pattern of vasa-related gene 1 (vlg1), vasa-related gene 2 (vlg2), and piwi transcripts from the zygote to the 20-segment stage worm. (a) vlg1 and vlg2 are expressed in the perinuclear cytoplasm. (b–c) When the ventral nerve cord (yellow) is formed, vlg1 + /vgl2 + (purple) and vgl2 + (red) cells emerge, and then vlg1 + /vgl2 + increase. (d) Just after embryogenesis, vlg1 + /vgl2 + /piwi + (blue) cells corresponding to germline cells appear on the ventral nerve cord. E. At the 20-segment stage, vgl2 +-positive cells corresponding to neoblasts are located in each segment, behind the ventral side of the septum (redrawn from Sugio et al. (2008))
8 Conclusions
In this chapter, annelids’ reproduction has been described with special reference to the germ cell formation and somatic and germline cell regeneration. Asexually reproducing annelids, particularly oligochaetes in which germ cells are limited to the head region, continuously face the high risk of losing germ cells during repeated fission. To avoid this risk, all tissues containing germ cells are completely regenerated in E. japonensis using a dedifferentiation system and two types of stem cells: somatic and germline. In contrast, most sexually reproducing species do not have these stem cells and/or have less ability to dedifferentiate. As suggested from several studies, an organism’s stem cell and dedifferentiation ability, as well as their regeneration capacity, is highly correlated with their mode of reproduction. Oligochaetes and polychaetes, which are the ancestors of oligochaetes, are thus good models for understanding how stem cells and germ cells are established and the factors contributing to different regeneration abilities among species. Despite the histological, cell trace, and gene expression pattern analyses performed for annelids, these worms still lag behind other model animals in terms of gene manipulation techniques. Recently, the research group of Dr. Florian Raible and Dr. Kristin Tessmar-Raible established sophisticated technologies in Platynereis dumerilii, including transgenic lines, cell ablation, and gene targeting by genome editing (Backfisch et al. 2013, 2014; Bannister et al. 2014; Zantke et al. 2014). These innovations represent a significant advance in the study of annelid biology. By adapting these technologies to other annelid species, it might be possible to comprehensively elucidate on the reproduction, regeneration, reproductive behavior, and many biological issues of annelids within larger contexts such as evolution and environment.
References
Akimenko MA, Johnson SL, Westerfield M, Ekker M (1995) Differential induction of four msx homeobox genes during fin development and regeneration in zebrafish. Development 121(2):347–357
Avel M (1959) Classe des Anne’lides Oligoche’tes. Rev. Suisse Zool. Traite´ de Zoologie. Masson et Cie, Paris
Avel M (1961) L’influence du system nerveux sur la regeneration chez les urodeles et les oligochaetes. Bull Soc Zool Fr86
Bacci G, Bortesi O (1961) Pure males and females from hermaphroditic strains of Ophryotrocha puerilis. Experientia 17:229–230
Backfisch B, Veedin Rajan VB, Fischer RM, Lohs C, Arboleda E, Tessmar-Raible K, Raible F (2013) Stable transgenesis in the marine annelid Platynereis dumerilii sheds new light on photoreceptor evolution. Proc Natl Acad Sci U S A 110(1):193–198. https://doi.org/10.1073/pnas.1209657109
Backfisch B, Kozin VV, Kirchmaier S, Tessmar-Raible K, Raible F (2014) Tools for gene-regulatory analyses in the marine annelid Platynereis dumerilii. PLoS One 9(4):e93076. https://doi.org/10.1371/journal.pone.0093076
Baguna J (2012) The planarian neoblast: the rambling history of its origin and some current black boxes. Int J Dev Biol 56(1–3):19–37. https://doi.org/10.1387/ijdb.113463jb
Bannister S, Antonova O, Polo A, Lohs C, Hallay N, Valinciute A, Raible F, Tessmar-Raible K (2014) TALENs mediate efficient and heritable mutation of endogenous genes in the marine annelid Platynereis dumerilii. Genetics 197(1):77–89. https://doi.org/10.1534/genetics.113.161091
Bell AW (1959) Enchytraeus fragmentosus, a new species of naturally fragmenting oligochaete worm. Science (New York, NY) 129(3358):1278. https://doi.org/10.1126/science.129.3358.1278-a
Bely AE (2006) Distribution of segment regeneration ability in the Annelida. Integr Comp Biol 46(4):508–518. https://doi.org/10.1093/icb/icj051
Bely AE, Wray GA (2001) Evolution of regeneration and fission in annelids: insights from engrailed- and orthodenticle-class gene expression. Development (Cambridge, England) 128(14):2781–2791
Bentley MG, Olive PJW, Last K (2001) Sexual satellites, moonlight and the nuptial dances of worms: the influence of the moon on the reproduction of marine animals. Earth Moon Planet 85-86:67–84
Berrill NJ (1952) Regeneration and budding in worms. Biol Rev 27:401–438
Bilej M (1994) Cellular defense mechanisms. In: Vetvicka V, Sima P, Cooper EL, Bilej M, Roch P (eds) Immunology of annelids. CRC Press, Ann Arbor, pp 245–261
Bouguenec V, Giani N (1989) Biological studies upon Enchytraeus variatus in breeding cultures. Hydrobiologia 180:151–165
Brusca RC, Brusca GJ (1990) Invertebrates. SINAUER Associates, Sunderland, MA
Cho SJ, Valles Y, Weisblat DA (2014) Differential expression of conserved germ line markers and delayed segregation of male and female primordial germ cells in a hermaphrodite, the leech helobdella. Mol Biol Evol 31(2):341–354. https://doi.org/10.1093/molbev/mst201
Christensen B (1959) Asexual reproduction in the Enchytraeidae (Olig.) Nature 184:1159–1160
Christensen B (1964) Regeneration of a new anterior end in Enchytraeus bigeminus (Enchytraeidae, Oligochaeta). Vidensk Medd Dan Natrur Foren 127:259–273
Cornec JP, Cresp J, Delye P, Hoarau F, Reynaud G (1987) Tissue responses and organogenesis during regeneration in the oliogochaete Limnodrilus hoffmeisteri (Clap.) Can J Zool 65:403–414
Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H (1998) A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev 12(23):3715–3727
Cox DN, Chao A, Lin H (2000) piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127(3):503–514
Deng W, Lin H (2002) miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2(6):819–830
Devries J (1971) Origine de la lignee germinale chez le lombricien Eisenia foetida. Ann Embryol Morphogen 4:37–43
Dill KK, Seaver EC (2008) Vasa and nanos are coexpressed in somatic and germ line tissue from early embryonic cleavage stages through adulthood in the polychaete Capitella sp. I. Dev Genes Evol 218(9):453–463. https://doi.org/10.1007/s00427-008-0236-x
Dorsett DA (1961) The reproduction and meintenance of Polydora ciliata (Johnst.) at Whitstable. J Mar Biol Ass 41:383–396
Eddy EM (1975) Germ plasm and the differentiation of the germ cell line. Int Rev Cytol 43:229–280
Edwards CA, Lofty JR (1972) Biology of earthworms. Chapman and Hall, London
Extavour CG, Akam M (2003) Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130(24):5869–5884. https://doi.org/10.1242/dev.00804
Fischer A (1974) Stage and stage distribution in early oogenesis in the annelid, Platynereis dumerlii. Cell Tissue Res 156:35–45
Fischer A (1975) The structure of symplasmic early oocytes and their enveloping sheath cells in the polychaete, Platynereis dumerilii. Cell Tissue Res 160:327–343
Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T (1994) Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci U S A 91(25):12258–12262
Gardiner DM, Blumberg B, Komine Y, Bryant SV (1995) Regulation of HoxA expression in developing and regenerating axolotl limbs. Development 121(6):1731–1741
Gates GE (1943) Some further notes on regeneration in Perionyx excavatus. Proc Nat Acad Sci India 13:168–179
Giani VC Jr, Yamaguchi E, Boyle MJ, Seaver EC (2011) Somatic and germline expression of piwi during development and regeneration in the marine polychaete annelid Capitella teleta. EvoDevo 2:10. https://doi.org/10.1186/2041-9139-2-10
Goodrich C (1945) Tbe study of nephridia and genital ducts since 1895. Quart Jour micr Sci 86:113–392
Hauenschild C (1960) Lunar periodicity. Cold Spring Harb Symp Quant Biol 25:491–497
Herlant-Meewis H (1954) Etude histologique des Aeolosomatida au cours de la reproduction asexuee. Arch Biol 65:73–134
Herlant-Meewis H (1964a) Reconstitution du germen chez Lumbricillus lineatus (Enchytraeides). Arch Biol Paris 57:197–306
Herlant-Meewis H (1964b) Contribution a l’etude de la regeneration chez les Oligochetes. Reconstitution du germen chez Lumbricillus lineatus (Enchytraeides). Premiere partie: elements regenerateurs. Ann Soc Zool Belg 77:5–47
Iwanoff PP (1928) Die entwicklung der Larvalsegmente bei den Anneliden. Z Morphol Okol 10:62–161
Izuka A (1903) Observations on the Japanese palolo, Ceratocephale osawai. J coll Sci Tokyo 17:1–37
Jamieson BGM (1992) Annelida, Chapter 3 oligochaeta. In: Harrison FW, Gardiner SL (eds) Microscopics anatomy of invertebrates, vol 7. Wiley-Liss, New York
Jamieson BGM (2006) Non leech Clitellata. Reproductive biology and phylogeny of annelida. SP Science Publishers, Enfield
Kang D, Pilon M, Weisblat DA (2002) Maternal and zygotic expression of a nanos-class gene in the leech Helobdella robusta: primordial germ cells arise from segmental mesoderm. Dev Biol 245(1):28–41. https://doi.org/10.1006/dbio.2002.0615
Kato Y, Nakamoto A, Shiomi I, Nakao H, Shimizu T (2013) Primordial germ cells in an oligochaete annelid are specified according to the birth rank order in the mesodermal teloblast lineage. Dev Biol 379(2):246–257. https://doi.org/10.1016/j.ydbio.2013.04.028
Khan P, Linkhart B, Simon HG (2002) Different regulation of T-box genes Tbx4 and Tbx5 during limb development and limb regeneration. Dev Biol 250(2):383–392
Kobayashi S, Yamada M, Asaoka M, Kitamura T (1996) Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature 380(6576):708–711. https://doi.org/10.1038/380708a0
Koprunner M, Thisse C, Thisse B, Raz E (2001) A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev 15(21):2877–2885. https://doi.org/10.1101/gad.212401
Kuramochi-Miyagawa S, Kimura T, Yomogida K, Kuroiwa A, Tadokoro Y, Fujita Y, Sato M, Matsuda Y, Nakano T (2001) Two mouse piwi-related genes: miwi and mili. Mech Dev 108(1–2):121–133
Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, Nakano T (2004) Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131(4):839–849. https://doi.org/10.1242/dev.00973
Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE (2006) Characterization of the piRNA complex from rat testes. Science (New York, NY) 313(5785):363–367. https://doi.org/10.1126/science.1130164
Lentz TL (1969) Cytological studies of muscle dedifferentiation and differentiation during limb regeneration of the newt Triturus. Am J Anat 124(4):447–479. https://doi.org/10.1002/aja.1001240404
Lo DC, Allen F, Brockes JP (1993) Reversal of muscle differentiation during urodele limb regeneration. Proc Natl Acad Sci U S A 90(15):7230–7234
Malaquin A (1925) La ségrégation, au cours de l’ontogenèse, de deux cellules sexuelles primordiales, souches de la lignée germinale, chez Salmacina dysteri (Huxley). C R Acad Sci Paris 180:324–327
Malaquin A (1934) Nouvelles observations sur la lignée germinale de l’Annélide Salmacina dysteri, Huxley. C R Acad Sci Paris 198:1804–1805
Meyer A (1929) Die Entwicklung der Nephridien und Gonoblasten bei Tubifex rivulorum Lam. nebst Bemerkungen zum natürlichen System der Oligochâten. Z Wiss Zool 133:517–562
Michaelsen W (1929) Zur Stammesgeschichte der Oligochaten. Z Wiss Zool 134:693–716
Mochizuki K, Nishimiya-Fujisawa C, Fujisawa T (2001) Universal occurrence of the vasa-related genes among metazoans and their germline expression in Hydra. Dev Genes Evol 211(6):299–308
Muller MC (2004) Nerve development, growth and differentiation during regeneration in Enchytraeus fragmentosus and Stylaria lacustris (Oligochaeta). Develop Growth Differ 46(5):471–478. https://doi.org/10.1111/j.1440-169x.2004.00763.x
Myohara M, Yoshida-Noro C, Kobari F, Tochinai S (1999) Fragmenting oligochaete Enchytraeus japonensis: a new material for regeneration study. Develop Growth Differ 41(5):549–555
Nakamura Y (1993) A new fragmenting Enchytaeid species, Enchytraeus japonensis from a cropped Kuroboku soil in Fukushima, Northern Japan (Enchytraeids in japan 5). Edaphologia 50:37–39
Okada K (1941) The gametogenesis, the breeding habits, and the early development of Arenicola cristata Stimpson, a tubicolous polychaete. Sci Rep Tohoku Imp Univ Biol 16:99–145
Olive PJW, Clark RB (1978) Physiology of annelids. Physiology of reproduction. Academic, New York
Oyama A, Shimizu T (2007) Transient occurrence of vasa-expressing cells in nongenital segments during embryonic development in the oligochaete annelid Tubifex tubifex. Dev Genes Evol 217(10):675–690. https://doi.org/10.1007/s00427-007-0180-1
Ozpolat BD, Bely AE (2015) Gonad establishment during asexual reproduction in the annelid Pristina leidyi. Dev Biol. https://doi.org/10.1016/j.ydbio.2015.06.001
Penners A, Stäblein A (1930) Über die Urkeimzellen bei Tubificiden (Tubifex rivulorum Lam. und Limnodrilus udekemianus Claparede). Z Wiss Zool 137:606–626
Randolph H (1892) The regeneration of the tail in Lumbriculus. J Morphol 7:17–344
Rebscher N, Zelada-Gonzalez F, Banisch TU, Raible F, Arendt D (2007) Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii. Dev Biol 306(2):599–611. https://doi.org/10.1016/j.ydbio.2007.03.521
Rebscher N, Lidke AK, Ackermann CF (2012) Hidden in the crowd: primordial germ cells and somatic stem cells in the mesodermal posterior growth zone of the polychaete Platynereis dumerillii are two distinct cell populations. EvoDevo 3:9. https://doi.org/10.1186/2041-9139-3-9
Reddien PW, Oviedo NJ, Jennings JR, Jenkin JC, Sanchez Alvarado A (2005) SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science (New York, NY) 310(5752):1327–1330. https://doi.org/10.1126/science.1116110
Rossi L, Salvetti A, Lena A, Batistoni R, Deri P, Pugliesi C, Loreti E, Gremigni V (2006) DjPiwi-1, a member of the PAZ-Piwi gene family, defines a subpopulation of planarian stem cells. Dev Genes Evol 216(6):335–346. https://doi.org/10.1007/s00427-006-0060-0
Sato K, Shibata N, Orii H, Amikura R, Sakurai T, Agata K, Kobayashi S, Watanabe K (2006) Identification and origin of the germline stem cells as revealed by the expression of nanos-related gene in planarians. Develop Growth Differ 48(9):615–628. https://doi.org/10.1111/j.1440-169X.2006.00897.x
Sawada N (1975) Electron microscope study on sperm differentiation in marine annelid worms. II. Sperm differentiation in Arenicola brasiliensis. Develop Growth Differ 17:89–99
Schmelz RM, Collado R, Myohara M (2000) A taxonomic study of Enchytraeus japonensis (Enchytraeidae, Oligochaeta): morphological and biochemical comparisons with E. bigeminus. Zool Sci 17:505–516
Schneider SQ, Bowerman B (2007) beta-Catenin asymmetries after all animal/vegetal- oriented cell divisions in Platynereis dumerilii embryos mediate binary cell-fate specification. Dev Cell 13(1):73–86. https://doi.org/10.1016/j.devcel.2007.05.002
Seipel K, Yanze N, Schmid V (2004) The germ line and somatic stem cell gene Cniwi in the jellyfish Podocoryne carnea. Int J Dev Biol 48(1):1–7
Shankland M, Savage RM (1997) Annelids, the segmented worms. In: Gilbert SF, Raunio AM (eds) Embryology: constructing the organisms. Sinauer, Sunderland
Shibata N, Umesono Y, Orii H, Sakurai T, Watanabe K, Agata K (1999) Expression of vasa(vas)-related genes in germline cells and totipotent somatic stem cells of planarians. Dev Biol 206(1):73–87. https://doi.org/10.1006/dbio.1998.9130
Shimizu T (1980) Development in the freshwater oligochaete tubifex. Developmental biology of freshwater invertebrates. Alan R Liss, New York
Stephenson J (1930) The Oligochaeta. Clarendon Press, Oxford
Sugio M, Takeuchi K, Kutsuna J, Tadokoro R, Takahashi Y, Yoshida-Noro C, Tochinai S (2008) Exploration of embryonic origins of germline stem cells and neoblasts in Enchytraeus japonensis (Oligochaeta, Annelida). Gene Expr Patterns GEP 8(4):227–236. https://doi.org/10.1016/j.gep.2007.12.008
Sugio M, Yoshida-Noro C, Ozawa K, Tochinai S (2012) Stem cells in asexual reproduction of Enchytraeus japonensis (Oligochaeta, Annelid): proliferation and migration of neoblasts. Develop Growth Differ 54(4):439–450. https://doi.org/10.1111/j.1440-169X.2012.01328.x
Tadokoro R (2009) Germ cell regeneration of Enchytraeus japonensis. Saishinigaku 93:81–93
Tadokoro R, Sugio M, Kutsuna J, Tochinai S, Takahashi Y (2006) Early segregation of germ and somatic lineages during gonadal regeneration in the annelid Enchytraeus japonensis. Curr Biol 16(10):1012–1017. https://doi.org/10.1016/j.cub.2006.04.036
Takeo M, Yoshida-Noro C, Tochinai S (2008) Morphallactic regeneration as revealed by region-specific gene expression in the digestive tract of Enchytraeus japonensis (Oligochaeta, Annelida). Dev Dyn Off Publ Am Assoc Anatomists 237(5):1284–1294. https://doi.org/10.1002/dvdy.21518
Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y (2003) Conserved role of nanos proteins in germ cell development. Science (New York, NY) 301(5637):1239–1241. https://doi.org/10.1126/science.1085222
Tweeten KA, Reiner A (2012) Characterization of serine proteases of Lumbriculus variegatus and their role in regeneration. Invertebr Biol 131:322–332
Vannini E (1947) Neoblasti e rigenerazione dei segmenti genitali nel serpulide ermafrodita Salmacina incrustans., vol 1st
Weisblat DA, Harper G, Stent GS, Sawyer RT (1980) Embryonic cell lineages in the nervous system of the glossiphoniid leech Helobdella triserialis. Dev Biol 76(1):58–78
Yoon C, Kawakami K, Hopkins N (1997) Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 124(16):3157–3165
Yoshida-Noro C, Tochinai S (2010) Stem cell system in asexual and sexual reproduction of Enchytraeus japonensis (Oligochaeta, Annelida). Develop Growth Differ 52(1):43–55. https://doi.org/10.1111/j.1440-169X.2009.01149.x
Yoshida-Noro C, Myohara M, Kobari F, Tochinai S (2000) Nervous system dynamics during fragmentation and regeneration in Enchytraeus japonensis (Oligochaeta, Annelida). Dev Genes Evol 210(6):311–319. https://doi.org/10.1007/s004270050318
Zantke J, Bannister S, Rajan VB, Raible F, Tessmar-Raible K (2014) Genetic and genomic tools for the marine annelid Platynereis dumerilii. Genetics 197(1):19–31. https://doi.org/10.1534/genetics.112.148254
Acknowledgement
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK, part of Springer Nature
About this chapter
Cite this chapter
Tadokoro, R. (2018). Reproductive Strategies in Annelida: Germ Cell Formation and Regeneration. In: Kobayashi, K., Kitano, T., Iwao, Y., Kondo, M. (eds) Reproductive and Developmental Strategies. Diversity and Commonality in Animals. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56609-0_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-56609-0_10
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56607-6
Online ISBN: 978-4-431-56609-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)