Abstract
The first part of the review provides general information about the solvent extraction of organic compounds from solid samples and discusses various methods for its implementation: extraction in a Soxhlet apparatus, ultrasonic extraction, microwave-assisted extraction. Based on an analysis of review papers, information on the features of sample preparation using these methods is systematized, experimental parameters affecting extraction efficiency are considered, and examples of using these methods for isolating organic compounds in the analysis of solid environmental samples, food products, and plants are given.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Organic compounds, especially in low concentrations and in the analysis of complex samples, are most often determined by chromatography–mass spectrometry. The current level of the development of these methods makes it possible to achieve sensitivity and selectivity that would have been difficult to imagine just a few decades ago [1–3]. However, despite this, determination in most cases is still impossible without sample preparation [4–6]. During sample preparation, the target analytes are extracted from the samples being analyzed; the components interfering with the determination are removed; matrix effects are eliminated; analytes are preconcentrated, sometimes derivatized, and converted into a matrix compatible with the subsequent determination method [6]. This stage of analysis is one of the most complex and time-consuming. In particular, sample preparation, according to some estimates, takes up to approximately 60% of the time spent for laboratory analysis and is the source of about 30% of experimental errors [7].
Over the past twenty-five years, various miniaturized and sometimes simplified sample preparation procedures, consistent with the principles of green analytical chemistry, have been developed and successfully applied to the extraction of organic compounds from liquid samples [7, 8]. During this time, many new methods for the microextraction isolation and preconcentration of organic compounds from aqueous solutions have appeared, such as single-drop microextraction [9, 10], hollow fiber liquid-phase microextraction [10, 11], dispersive liquid–liquid microextraction [12, 13], homogeneous liquid–liquid microextraction [14, 15], pipette-tip solid-phase microextraction [16, 17], solid-phase microextraction [18, 19], stir-bar sorptive extraction [20, 21], microextraction by packed sorbent [22, 23], magnetic solid-phase extraction [24, 25], and dispersive solid-phase extraction [25, 26].
An analysis of a number of recent reviews devoted to the sample preparation of soils and other solid environmental samples [27–40], food products [41–47], plants [48–54], and cosmetic and personal care products [55–58], indicates that shaking extraction, Soxhlet extraction, ultrasound-assisted extraction, microwave-assisted extraction, pressurized liquid extraction, subcritical water extraction, supercritical fluid extraction, matrix solid-phase dispersion, and QuEChERS are used to separate organic compounds from solid samples. The non-selective nature of this primary processing necessitates the subsequent purification of the resulting extract, first by removing insoluble portions of the sample and then, if necessary, by purifying and/or preconcentrating the analytes in the resulting extracts using the previously mentioned various liquid–liquid or solid-phase extraction/microextraction versions.
The first part of this review summarizes review articles describing traditional methods for the extraction of organic compounds from solid samples, such as shaking extraction, Soxhlet extraction, ultrasound-assisted extraction, and microwave-assisted extraction. The general description of the methods is given, methods of implementation are considered, experimental parameters affecting the efficiency of the extraction of organic compounds are listed, and examples of the application of methods to the process of the sample preparation of various species are given.
SOLID–LIQUID EXTRACTION
Solvent extraction from solid matrices (solid–liquid extraction, SLE) is based on the distribution of a substance in a solid–liquid system (usually an organic solvent, less often water). The classic option for performing liquid–liquid extraction from solid matrices is to mechanically shake an analyzed solid sample with a selected solvent for a certain time [59, 60]. To do this, a weighed portion of a carefully ground solid sample is placed in a shaking vessel (the optimal particle size depends on the sample being analyzed and varies from 0.5 to 8 mm), the selected solvent is added, and the contents are stirred for a certain time (usually from 15–30 min to several hours). The phases are separated by filtration. The extraction process occurs in several stages. First, the extractant wets the solid substance and penetrates into the internal voids—micro- and macrocracks of solid phase particles. Next, the extracted substance is dissolved and released into the extractant located inside the solid phase, then into the near-surface layer of the extractant—the diffusion boundary layer, and then into the bulk of the extractant. The boundary diffusion layer formed on the surface of solid particles possesses high resistance to the further transfer of the extracted substances into the extractant. The thickness of this layer depends on the rate of stirring the extractant. The higher the stirring rate, the smaller the thickness of the boundary layer [59].
Extraction is a complex physicochemical process affected by a number of factors, the main of which is the nature of the solvent, the correct selection of which determines not only the completeness of the extraction of the desired component, but also the selectivity of extraction [48, 59]. It is desirable that the selected solvent is selective and dissolves the desired analytes to a maximum extent and other substances present in the solid sample to a minimum extent. In addition, in choosing a solvent, parameters such as volatility, purity, toxicity, availability, and cost are taken into account. It is important to select a proper solvent-to-solid sample ratio; recovery of compounds increases with increasing solvent volume. To reduce the volume of the extract, it is better to carry out several successive extractions in small portions of the extractant than one extraction in a large portion. In addition, the amount of the extracted substance depends on the degree of sample grinding, the intensity of stirring, and the time of phase contact [59].
Acetonitrile, methanol, ethanol, acetone, ethyl acetate, and their mixtures with water are most often used as solvents for extracting hydrophilic organic compounds from soils, plants, food, and other solid materials [38, 45, 48, 49, 59]. To extract hydrophobic organic compounds, diethyl ether, pentane, hexane, mixtures of hexane with acetone, toluene, methylene chloride, and a number of other solvents are used [31, 46, 59]. In recent years, supramolecular solvents, ionic liquids, and deep eutectic solvents have begun to be used as alternative solvents that have found application to the extraction of organic compounds from solid matrices [48, 49, 61–64].
In addition to the reviews cited above, separate sections in other reviews [27, 32, 41, 45, 46, 50, 51, 53–55, 58] were devoted to the use of solvent extraction for the isolation of organic compounds from solid samples. In these reviews, informative tables provide information on the conditions for the extraction of 4-alkylphenols and bisphenol A from river and marine sediments [32]; pesticides from soils [40]; pesticides [41] and neonicotinoids [45] from various foods; phthalates, benzothiazoles, and benzotriazoles from marine products [46]; pesticides from spices and plants [50]; quercetin and its glycosides [51], polyphenols, and other biologically active substances from plants [49, 51, 53, 54] and agricultural residues [49]; and various organic compounds from sewage sludge [27] and cosmetic and personal care products [55, 58].
The main disadvantage of this oldest and simplest method of sample preparation for solid samples is the slow establishment of an equilibrium and, as a consequence, significant time spent for sample preparation. In addition, the disadvantages of the method include the incomplete extraction of the target analytes. To increase the efficiency of the extraction, heat and ultrasonic and microwave radiation are used. A distinction is made between extraction in a Soxhlet apparatus, ultrasound-assisted extraction, and microwave-assisted extraction. Below we present a more detailed description of these options for the solvent extraction from solid matrices.
OPTIONS FOR SOLVENT EXTRACTION FROM SOLID MATRICES
Soxhlet extraction. Detailed information about the features of Soxhlet extraction can be found in the reviews [65–69]. Soxhlet extraction is one of the oldest methods of solid–liquid extraction, which was proposed in 1879 by the German chemist Franz von Soxhlet for separating fat from milk. Classical Soxhlet extraction is carried out in a Soxhlet apparatus, which consists of a solvent flask, an extractor, and a ball cooler. The operating principle of the extractor is as follows: 30–100 mL of a selected solvent is poured into a 0.5–1 L round-bottom flask, and a crushed solid material (1–10 g), packed in extraction sleeves made of high-purity cellulose (filter paper) or in a gauze bag, is placed in the extractor. When the flask is heated, solvent vapors rise and condense in the refrigerator. The resulting condensate enters the extractor. As the level of the solvent rises, increasing amounts of extracted components pass into it. Once the solvent level reaches the top level of the siphon, the solvent is drained into the flask and the process continues. In the end of the extraction, the solvent with the separated components is transferred from the flask to a suitable container and evaporated to the required volume. Thus, the device allows for multiple extractions in a continuous mode because of the reuse of a relatively small volume of the solvent, while the extracted substance accumulates in the main flask. Another advantage of the method is that, after extraction, there is no need in separating the remaining solid sample by filtration [65, 67, 69].
Soxhlet extraction has typically two major problems to be solved: within almost the entire extraction period, the extract is at the boiling point of the solvent, which can lead to the decomposition of thermally unstable extracts, and the resulting extract is usually highly diluted with the solvent. The disadvantages of the method also include the duration of the process: usually extraction in a Soxhlet apparatus is carried out for 12–24 h, and sometimes extraction time can be increased to several days [65, 69].
Over time, the disadvantages inherent in the classical version of Soxhlet extraction were partially eliminated due to the advent of commercially available automated systems, which were commercially named Soxtec (1975) [65, 68, 69]. Manufacturers of fully automated and semi-automated extraction systems have focused on the simplicity and safety of the traditional Soxhlet procedure, as well as on speeding up the extraction process. Modern automated Soxhlet units are mainly 2-, 4-, 6-place systems that provide increased sample throughput. Automated extraction systems operate 5–6 times faster than conventional Soxhlet systems [69]. In addition, modified versions of the Soxhlet extractor have been developed, such as focused microwave-assisted extractors [66, 68, 69], high-pressure extractors [68, 69], ultrasonic-assisted extractors [68, 69], and a number of others [69].
Currently, Soxhlet extraction is used to isolate polycyclic aromatic hydrocarbons (PAHs) [33, 35–37], polybrominated biphenyls [31], alkylphenols and bisphenol A [30, 32], and many other moderately and poorly volatile organic compounds [27–30, 34] from soils and bottom sediments; carotenoids [42] and fats [43] from food products. In the reviews listed above, the tables present the conditions for the Soxhlet extraction of organic compounds: the solvents, sample weights and volumes of solvents are listed; and extraction time and the recovery of the analytes are indicated.
Methods based on Soxhlet extraction are still used as reference and standard methods in many laboratories to compare the performance of other methods for the separation of organic compounds from solid samples [68]. For example, Soxhlet extraction is recommended by the US Environmental Protection Agency (EPA) and the National Oceanic and Atmospheric Administration (NOAA) for the extraction of PAHs from sediment samples [35]. In 1994, automated Soxhlet extraction was approved by the EPA as a standard method [27]. Many official procedures use Soxhlet extraction as the main method for isolating fats from various foods [44].
Ultrasound-assisted extraction, UAE. A list of reviews on the use of ultrasound for intensifying solvent extraction of organic compounds from various solid matrices is given in the chronological order in Table 1 [70–86]. Historical information about the development of the method is given in the review [79]. All reviews dealing with this method note that ultrasound-assisted extraction is an effective and an environmentally friendly method for extracting organic analytes from various types of solid samples because of the reduced solvent volume and extraction time compared to the classical solid–liquid extraction procedures.
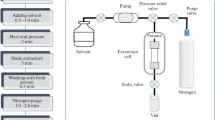
To carry out UAE, one must have an ultrasonic bath or an ultrasonic probe. Unlike an ultrasonic bath, the irradiation power of which is low and ranges from 1–5 W/cm2, the direct immersion of ultrasonic probes provides 100 times higher ultrasonic power. Extraction using ultrasonic probes was named focused UAE [80, 85]. The choice of the type of an ultrasonic device depends on the solution of a specific analytical problem. Ultrasonic probes generally provide higher recoveries of analytes within significantly less time (5–10 min) compared to ultrasonic water baths (10–60 min), but they are not very convenient in working with large numbers of samples, because only one sample is sonicated in one run. In addition, in working with probes, it is necessary to take into account the high probability of the loss and destruction of organic compounds as a result of the enhanced effect of the degassing and heating of the medium [80]. In contrast, an ultrasonic bath is more economical and easier to use, but is characterized by low reproducibility [82]. Examples of various commercial ultrasonic devices that have found application to UAE can be found in the reviews [70, 71, 75, 76, 78, 79]. A scheme of ultrasound-assisted extraction from solid samples using an ultrasonic probe or an ultrasonic bath is shown in Fig. 1 [80].
Scheme of ultrasonic extraction from solid samples using (a) an ultrasonic probe or (b) an ultrasonic bath [80].
Ultrasonic vibrations have a variety of effects on a solid–liquid system; they can be reduced to the following effects: thermal effects as a result of the absorption of ultrasonic energy; increased mass transfer in the pores of the solid phase due to abnormally deep penetration of the liquid into the capillaries and narrow cracks of the solid matrix; acceleration of diffusion processes. The main mechanisms of the effect of ultrasound on solids include acoustic flows and cavitation. Acoustic flows are vortex in nature and always arise when ultrasonic energy is absorbed by a liquid, causing its regular movement and, as a consequence, the intensification of the mass transfer processes. Ultrasonic cavitation consists of the formation of a large number of pulsating bubbles filled with steam, gas, or a mixture of them, in the liquid under ultrasonic treatment (Fig. 2). Cavitation bubbles in a certain region of the liquid arise whenever the rarefaction phase of an ultrasonic wave reaches this region. As a rule, cavitation bubbles do not live long: the compression phase, which follows the rarefaction, leads to the collapse of most of them, the so-called cavitation collapse. There are many thousands of such bubbles in a liquid, at the moment of the collapse of which the pressure and temperature increase (according to some data, up to 1000 atm (100 MPa) and 1000°C). The changes in temperature and pressure resulting from the collapse generate shock waves (at a speed of 100 m/s), which in turn lead to the enhanced mass transfer of the target compounds to the solvent. High local temperatures within the collapsing cavitation bubbles can cause an increase in the analyte solubility and solvent diffusion within the solid particles. The high pressure generated during the microbubble explosion improves permeability and solvent transfer. The surface renewal caused by particle fragmentation allows more analyte to come into contact with the solvent. In biological samples, cell destruction occurs, followed by the release of encapsulated analytes. The oxidation of organic matrices should also be facilitated by the formation of oxidative radicals in the bulk of the liquid. More details on the mechanism of the effect of ultrasound on a solid–liquid extraction system can be found in the reviews [73, 74, 76, 78, 79].
The cavitation phenomenon. (a) Development and collapse of cavitation bubbles. (b) Cavitation collapse at the solid–liquid interface. The sequence (1)−(3) shows the scheme of the fragmentation or destruction of solid particles, which leads to a decrease in their size (increase in surface area) [73].
The experimental parameters affecting the UAE are systematized in the reviews [76, 78, 80, 82, 86]. The completeness of the extraction of organic compounds primarily depends on the frequency and power of the ultrasound, the nature of the solvent, and temperature. The frequency and power of ultrasonic radiation are regulated by the type of the equipment used, and these parameters cannot always be varied, because most ultrasonic systems used in analytical laboratories operate at a certain frequency. Most often, UAE is carried out at frequencies from 20 to 100 kHz and powers from 20 to 700 W, with higher extraction efficiency observed in the low-frequency range (20–40 kHz) [78, 82]. The choice of a solvent is determined by the solubility of the target analytes, as well as solvent properties, such as viscosity, surface tension, and vapor pressure, which influence cavitation and, in particular, the cavitation threshold [76]. The sample temperature has an ambiguous effect on the completeness of the extraction of organic compounds. As the temperature increases, the solubility of the analytes in the selected solvent increases and the rate of diffusion of the compounds increases, which contributes to their more complete extraction. On the other hand, an increase in temperature leads to a decrease in viscosity and surface tension, and also causes an increase in vapor pressure, which can reduce extraction efficiency by weakening the cavitation effect [78, 80, 82]. Other factors that influence extraction efficiency are sonication time, sample particle size, and solid-to-solvent ratio [76, 78, 82].
Information on the applications of UAE to the extraction of organic compounds before their chromatographic determination can be found not only in the reviews listed in Table 1 [72, 74, 77, 81–86], but also in reviews devoted to the extraction of certain classes of organic compounds from solid matrices [27, 34–37, 39, 42, 46–51, 53–58]. This method of sample preparation is often used in the analysis of solid environmental samples [27, 35–37, 39, 40, 74, 75, 77, 80, 85], food products [42, 46, 47, 72, 74, 75, 78, 80, 83–85], plants and fruits [48–51, 53, 54, 79, 81, 82, 86], and cosmetics and personal care products [34, 55–58]. In these reviews, the tables show the conditions for ultrasonic sample preparation, which were used for the extraction of pesticides [40, 47, 72, 83, 85], PAHs [35–37, 72, 77, 83, 85], medicinal substances [34, 72, 77, 83, 85], biologically active compounds [49–51, 53, 54, 81, 82, 86], dyes [83, 84], phthalates [39, 46], carotenoids [42], and many other organic compounds. A review published in 2023 [85] provides information on the conditions for carrying out UAE for the multicomponent (from 11 to 180 compounds) extraction of organic compounds of different classes from sediments, soils, and food products. The methods that were used for the additional purification of the obtained extracts are also indicated. Currently, UAE is undoubtedly one of the most widely used methods for the extraction of organic compounds from solid samples due to the simplicity of the method, the availability and low cost of ultrasonic baths and probes, the ability of using a wide range of solvents with different polarities and applicability in the analysis of a wide variety of organic analytes and samples.
Microwave-assisted extraction, MAE. In sample preparation processes, microwave energy has been used since the early 1970s: first for the mineralization of various solid matrices before determining elements in them [87], and after 1986, for the extraction of organic compounds from solid samples [88]. The remarkable property of microwave radiation to accelerate the extraction of organic compounds from solid matrices and make it more efficient contributed to the rapid development of the method, as indirectly evidenced by the list of reviews in the chronological order presented in Table 1 [89–109]. From the viewpoint of “green” analytical chemistry, MAE has a number of advantages, the main of which are the use of a small amount of solvents, a significant reduction of extraction time, the partial or complete automation of the analytical process, and a possibility of its online combination with subsequent determination methods [94, 97, 103]. Historical information about the development of the MAE method is given in the reviews [89, 91, 96]. The development of the MAE method was largely facilitated by the emergence of commercially available analytical equipment – closed or open type MW systems [92]. In the last 10 years, MAE has been increasingly used in technological processes for the extraction of biologically active substances from plant materials [104, 105, 107, 108].
The theoretical foundations of microwave heating and the basic principles of using microwave energy for extraction are briefly outlined in the reviews [79, 90–93, 98, 99, 102]. In MAE, microwave energy is used to heat solvents in contact with a solid sample. Microwave heating is based on the direct effect of microwaves on solvent molecules through ionic conduction and dipole rotation. Ionic conductivity refers to the induced electrophoretic migration of ions under the influence of an electromagnetic field. The solution’s resistance to this flow of ions results in the friction and thus heating of the solution. Dipole rotation occurs when bipolar molecules try to navigate the electric field created by microwaves. At the 2450 MHz frequency used in commercial microwave systems, dipoles reorient 4.9 × 109 times per second, and this forced molecular motion also results in heating. Unlike traditional thermal heating, microwave heating is carried out uniformly throughout the entire volume, as a result of which the temperature of the solution is higher than that of the surrounding objects (vessel walls, gas phase above the solution, etc.), and the solution can be heated to a temperature exceeding the boiling point under atmospheric pressure, which significantly reduces extraction time.
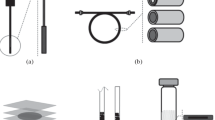
Microwave-assisted extraction is carried out using special equipment produced by a number of companies (Fig. 3). There are open- and closed-type microwave extractors. In open-type extractors, the extraction process takes place in a flask, which is connected to the atmosphere through a reflux condenser. Microwave radiation is focused on the bottom of the flask where the sample is located. Only one sample can be extracted using this method in one run. Most often, closed-type microwave systems are used in analytical laboratories, in which samples are exposed to microwave energy at controlled pressure and temperature [90, 91, 94]. A closed microwave system consists of a magnetron (magnetron tube) and temperature and pressure control devices. Using a waveguide, the magnetron generates microwave radiation into the working volume of the furnace, in which sealed extraction vessels capable of withstanding significant pressure (about 100 atm and above) are located on a rotating rotor. Modern systems for MAE allow the simultaneous sample preparation of up to 40 samples in just 10–15 min [106]. Fast heating, stirring systems, and rapid cooling in the end of the reaction make it possible to control heating time, which leads to more reproducible results. The equipment for MAE is constantly being improved, and, in addition to closed-type microwave systems, systems have been developed that allow vacuum MAE, microwave extraction with nitrogen protection, as well as ultrasonic and dynamic MAE [94, 100]. The design features of different types of microwave systems can be found in the reviews [90, 91, 94, 97, 105, 106, 108, 109].
(a) Principle of microwave extraction in closed vessels. (b) Commercial microwave extraction device for 40 samples [106].
The experimental parameters influencing the MAE are discussed in detail in the reviews [89–91, 99, 100, 107–109]. The main parameters affecting the completeness of the extraction of organic compounds by the MAE method are the following: the nature of the extractant solvent and its volume, temperature, power, and time of exposure to microwave radiation, and the nature of the matrix.
In choosing a solvent, it is necessary to take into account not only the solubility of the target analytes in it, but also its dielectric characteristics, which determine the ability of the solvent to absorb microwave energy and convert it into heat. Solvents such as methanol, ethanol, acetonitrile, ethyl acetate, acetone, as well as their mixtures with water (additives to the extractant at a level of 10–15%) are heated quickly and strongly. Hydrocarbon solvents with low dielectric constants (hexane, toluene, etc.) are used in a mixture with more polar solvents, e.g., acetone. Interest in the use of surfactants and ionic liquids as an alternative to classical solvents in MAE has increased in recent years [100, 103]. The choice of the volume of a solvent depends on the type and size of the sample: the volume of the solvent should be such that the entire sample is immersed in it. On average, the volume of solvent in MAE varies from 5 to 20 mL, which is significantly less than in other methods of solid–liquid extraction.
An important parameter to consider in choosing MAE conditions is the water content of the sample, which must be controlled to obtain reproducible results. The choice of power, temperature, and appropriate irradiation time depends on the type of the sample and solvent used, as well as the number of samples processed per extraction cycle in closed microwave systems. In most cases, with increasing temperature (up to a certain value), the recovery of analytes increases due to the increased diffusion of the solvent into the internal parts of the matrix and the increased desorption of the components from the active centers of the matrix. However, too high temperature may cause the loss of volatile compounds and the decomposition of some compounds. As mentioned above, microwave-assisted extraction allows the achievement of high extraction rates within short time (15–30 min), while the consumption of solvents is significantly reduced. The gain in time is achieved by increasing the boiling point of the solvent, high pressure, and the specific effect of microwave radiation on the solution, as well as constant stirring. More precise control over reaction parameters (temperature, time) allows for more reproducible results.
Microwave-assisted extraction has established itself as one of environmentally friendly methods of the sample preparation of solid samples, including the online version of a combination of sample preparation methods and the subsequent determination (Fig. 4). Reviews [89–91, 96] provide references to early works on the use of MAE for the extraction of PAHs, polychlorinated biphenyls, and phenols from soils and bottom sediments. In 1997, the US Environmental Protection Agency approved and validated Standard Method 3546, “Microwave Extraction,” for MAE from soils, clays, sediments, silts, and other particulate samples of moderately volatile organic compounds, such as organophosphorus and organochlorine pesticides, chlorinated and phenoxy acid herbicides, and substituted phenols [96]. Method 3546 is a relatively simple and a versatile method for the preparation of various solid samples, providing the simultaneous extraction of more than 100 target analytes belonging to different classes. Validation of the method confirmed that the recovery of the compounds coincided with the recovery in the Soxhlet apparatus, while less solvents were consumed (50–75 mL instead of 500–600 mL), and the sample preparation itself took much less time (minutes rather than hours or days) [96].
Installation for online microwave extraction followed by determination [103].
In the last 20 years, MAE is increasingly used for the extraction of organic compounds from environmental samples [27, 35, 36, 40, 99, 102, 103, 106], food products [41, 42, 45, 47, 101–103, 109], plants [100, 101, 104, 105, 108], and biological samples [98]. The range of separated compounds has also expanded. In the informative tables given in the reviews listed above, one can find information about the MAE conditions (solvent and its volume, temperature, power, extraction duration) for various organic compounds: antibiotics, veterinary drugs and other pharmaceuticals [98, 99, 101–103, 106, 109], hormones [101, 103, 106], carotenoids [42], pesticides [40, 41, 102, 103, 109], phthalates [39, 102], flame retardants [99, 102], surfactants [99, 102], personal care products [55, 99, 102, 106], and biologically active compounds [48, 49, 53, 54, 100, 101, 104, 105, 108].
REFERENCES
Picó, Y., Curr. Opin. Environ. Sci. Health, 2020, vol. 18, p. 47. https://doi.org/10.1016/j.coesh.2020.07.002
Lopez-Ruiz, R., Romero-Gonzalez, R., and Frenich, A.G., TrAC, Trends Anal. Chem., 2019, vol. 118, p. 170.
Rathod, R.H., Chaudhari, S.R., Patil, A.S., and Shirkhedkar, A.A., Future J. Pharm. Sci., 2019, vol. 5, p. 2. https://doi.org/10.1186/s43094-019-0007-8
Kanu, A.B., J. Chromatogr. A, 2021, vol. 1654, p. 462444.
Gu, Y., Peach, J.T., and Warth, B., TrAC, Trends Anal. Chem., 2023, vol. 166, p. 117151.
Chen, Y., Guo, Z., Wang, X., and Qiu, C., J. Chromatogr. A, 2008, vol. 1184, p. 191. https://doi.org/10.1016/j.chroma.2007.10.026
Câmara, J.S., Perestrelo, R., Berenguer, C.V., Andrade, C.F.P., Gomes, T.M., Olayanju, B., Kabir, A., Rocha, C.M.R., Teixeira, J.A., and Pereira, J.A.M., Molecules, 2022, vol. 27, p. 2953. https://doi.org/10.3390/molecules27092953
Picot-Allain, C., Mahomoodally, M.F., Ak, G., and Zengin, G., Curr. Opin. Food Sci., 2021, vol. 40, p. 144. https://doi.org/10.1016/j.cofs.2021.02.009
Kailasa, S.K., Koduru, J.R., Park, T.J., Singhal, R.K., and Wu, H.-F., Trends Environ. Anal. Chem., 2021, vol. 29, p. e00113.
Dmitrienko, S.G., Apyari, V.V., Tolmacheva, V.V., and Gorbunova, M.V., J. Anal. Chem., 2021, vol. 76, p. 907.
Gjelstad, A., TrAC, Trends Anal. Chem., 2019, vol. 113, p. 25.
Dmitrienko, S.G., Apyari, V.V., Tolmacheva, V.V., and Gorbunova, M.V., J. Anal. Chem., 2020, vol. 75, no. 10, p. 1237.
Sajid, M., TrAC, Trends Anal. Chem., 2022, vol. 152, p. 116636.
Dmitrienko, S.G., Apyari, V.V., Gorbunova, M.V., Tolmacheva, V.V., and Zolotov, Yu.A., J. Anal. Chem., 2020, vol. 75, no. 11, p. 1371.
Ramezani, A.M., Ahmadi, R., and Yamini, Y., TrAC, Trends Anal. Chem., 2022, vol. 149, p. 116566.
Turoňová, D., Kujovská Krčmová, L., and Švec, F., TrAC, Trends Anal. Chem., 2021, vol. 143, p. 116404.
Carasek, E., Mores, L., and Huelsmann, R.D., Anal. Chim. Acta, 2022, vol. 1192, p. 339383.
Jalili, V., Barkhordari, A., and Ghiasvand, A., Microchem. J., 2020, vol. 152, p. 104319.
Nolvachai, Y., Amaral, M.S.S., Herron, R., and Marriott, P.J., Green Anal. Chem., 2023, vol. 4, p. 104319.
David, F., Ochiai, N., and Sandra, P., TrAC, Trends Anal. Chem., 2019, vol. 112, p. 102.
Hasan, C.K., Ghiasvand, A., Lewis, T.W., Nesterenko, P.N., and Paull, B., Anal. Chim. Acta, 2020, vol. 1139, p. 222.
Yang, L., Said, R., and Abdel-Rehim, M., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, vol. 1043, p. 33.
Pereira, J.A.M., Goncalves, J., Porto-Figueira, P., Figueira, J.A., Alves, V., Perestrelo, R., Medina, S., and Câmara, J.S., Analyst, 2019, vol. 144, p. 5048.
Capriotti, A.L., Cavaliere, C., La Barbera, G., Montone, C.M., Piovesana, S., and Laganà, A., Chromatographia, 2019, vol. 82, p. 1251.
Ghorbani, M., Aghamohammadhassan, M., Chamsaz, M., Akhlaghi, H., and Pedramrad, T., TrAC, Trends Anal. Chem., 2019, vol. 118, p. 793.
Dmitrienko, S.G., Apyari, V.V., Tolmacheva, V.V., Gorbunova, M.V., and Furletov, A.A., J. Anal. Chem., 2024, vol. 79, no. 2, p. 105. https://doi.org/10.1134/S1061934824020060
Zuloaga, O., Navarro, P., Bizkarguenaga, E., Iparraguirre, A., Vallejo, A., Olivares, M., and Prieto, A., Anal. Chim. Acta, 2012, vol. 736, p. 7. https://doi.org/10.1016/j.aca.2012.05.016
Tadeo, J., Sánchez-Brunete, C., Albero, B., García-Valcárcel, A., and Pérez, R., Cent. Eur. J. Chem., 2012, vol. 10, p. 480.
Martin-Pozo, L., de Alarcón-Gómez, B., Rodríguez-Gómez, R., García-Córcoles, M.T., Çipa, M., and Zafra-Gómez, A., Talanta, 2019, vol. 192, p. 508. https://doi.org/10.1016/j.talanta.2018.09.056
Grześkowiak, T., Czarczyńska-Goślińska, B., and Zgoła-Grześkowiak, A., TrAC, Trends Anal. Chem., 2016, vol. 75, p. 209. https://doi.org/10.1016/j.trac.2015.07.005
Kim, M., Li, L.Y., Gorgy, T., and Grace, J.R., Environ. Pollut., 2017, vol. 220, p. 753. https://doi.org/10.1016/j.envpol.2016.10.053
Salgueiro-González, N., Castiglioni, S., Zuccato, E., Turnes-Carou, I., López-Mahía, P., and Muniategui-Lorenzo, S., Anal. Chim. Acta, 2018, vol. 1024, p. 39. https://doi.org/10.1016/j.aca.2018.02
Wang, C., Wang, Y., and Herath, H.M.S.K., Org. Geochem., 2017, vol. 114, p. 1. https://doi.org/10.1016/j.orggeochem.2017.09
Pérez-Lemus, N., López-Serna, R., Pérez-Elvira, S.I., and Barrado, E., Anal. Chim. Acta, 2019, vol. 1083, p. 19. https://doi.org/10.1016/j.aca.2019.06.044
Ling, W., Rui, S., Yongxin, L., and Sun, C., Trends Environ. Anal. Chem., 2019, p. e00074. https://doi.org/10.1016/j.teac.2019
Galmiche, M., Delhomme, O., François, Y.-N., and Millet, M., TrAC, Trends Anal. Chem., 2021, vol. 134, p. 116099. https://doi.org/10.1016/j.trac.2020.116099
Song, N., Tian, Y., Luo, Z., Dai, J., Liu, Y., and Duan, Y., RSC Adv., 2022, vol. 12, p. 6099. https://doi.org/10.1039/D1RA08633B
Chen, X., Wu, X., Luan, T., Jiang, R., and Ouyang, G., J. Chromatogr. A, 2021, vol. 1640, p. 461961. https://doi.org/10.1016/j.chroma.2021.461961
Hidalgo-Serrano, M., Borrull, F., Marcé, R.M., and Pocurull, E., TrAC, Trends Anal. Chem., 2022, vol. 151, p. 116598. https://doi.org/10.1016/j.trac.2022.116598
Brinco, J., Guedes, P., Gomes da Silva, M., Mateus, E.P., and Ribeiro, A.B., Microchem. J., 2023, vol. 195, p. 109465. https://doi.org/10.1016/j.microc.2023
Lambropoulou, D.A. and Albanis, T.A., Anal. Bioanal. Chem., 2007, vol. 389, p. 1663. https://doi.org/10.1007/s00216-007-1348-2
Saini, R.K. and Keum, Y.-S., Food Chem., 2018, vol. 240, p. 90. https://doi.org/10.1016/j.foodchem.2017.07.099
Madej, K., Kalenik, T.K., and Piekoszewski, W., Food Chem., 2018, vol. 269, p. 527. https://doi.org/10.1016/j.foodchem.2018.07.007
Hewavitharana, G.G., Perera, D.N., Navaratne, S.B., and Wickramasinghe, I., Arab. J. Chem., 2020, vol. 13, p. 6865. https://doi.org/10.1016/j.arabjc.2020.06.039
Watanabe, E., J. Chromatogr. A, 2021, vol. 1643, p. 462042. https://doi.org/10.1016/j.chroma.2021.462042
Castro, Ó., Borrull, F., and Pocurull, E., TrAC, Trends Anal. Chem., 2022, vol. 157, p. 116743. https://doi.org/10.1016/j.trac.2022.116743
Mandal, S., Poi, R., Hazra, D.K., Ansary, I., Bhattacharyyaa, S., and Karmakar, R., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2023, vol. 1215, p. 123587.
Lefebvre, T., Destandau, E., and Lesellier, E., J. Chromatogr. A, 2020, vol. 1635, p. 461770. https://doi.org/10.1016/j.chroma.2020.461770
Mir-Cerda, A., Núñez, O., Granados, M., Sentellas, S., and Saurina, J., TrAC, Trends Anal. Chem., 2023, vol. 161, p. 116994.
Parrilla Vázquez, P., Ferrer, C., Martinez Bueno, M.J., and Fernández-Alba, A.R., TrAC, Trends Anal. Chem., 2019, vol. 115, p. 13. https://doi.org/10.1016/j.trac.2019.03.022
Hamed, M., Abdallah, I.A., Bedair, A., and Mansour, F.R., Microchem. J., 2023, vol. 194, p. 109233.
Bessonova, E.A., Karpitskii, D.A., and Kartsova, L.A., J. Anal. Chem., 2023, vol. 78. S. 883.
Bitwell, C., Indra, S.S., Luke, C., and Kakoma, M.K., Sci. Afr., 2023, vol. 19, p. e01585.
Milevskaya, V.V., Prasad, S., and Temerdashev, Z.A., Microchem. J., 2019, vol. 145, p. 1036.
Cabaleiro, N., de la Calle, I., Bendicho, C., and Lavilla, I., Anal. Methods, 2013, vol. 5, p. 323. https://doi.org/10.1039/c2ay25830g
Piao, C., Chen, L., and Wang, Y., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, vol. 969, p. 139. https://doi.org/10.1016/j.jchromb.2014.08.015
Abedi, G., Talebpour, Z., and Jamechenarboo, F., TrAC, Trends Anal. Chem., 2018, vol. 102, p. 41. https://doi.org/10.1016/j.trac.2018.01.006
Celeiro, M., Garcia-Jares, C., Llompart, M., and Lores, M., Molecules, 2021, vol. 26, p. 4900. https://doi.org/10.3390/molecules26164900
Chanioti, S., Liadakis, G., and Tzia, C., in Food Engineering Handbook, Varzakas, T. and Tzia, C., Eds., Boca Raton: CRC, 2014, p. 253.
Naviglio, D., Scarano, P., Ciaravolo, M., and Gallo, M., Foods, 2019, vol. 8, p. 245. https://doi.org/10.3390/foods8070245
Chemat, F., Vian, M.A., Ravi, H.K., Khadhraoui, B., Hilali, S., Perino, S., and Tixier, A.S.F., Molecules, 2019, vol. 24, p. 3007. https://doi.org/10.3390/molecules24163007
Didion, Y.P., Tjalsma, T.G., Su, Z., Malankowska, M., and Pinelo, M., Sep. Purif. Technol., 2023, vol. 320, p. 124147. https://doi.org/10.1016/j.seppur.2023.124147
Kaoui, S., Basaid, K., and Chebli, B., Sustainable Chem. Pharm., 2023, vol. 31, p. 100937. https://doi.org/10.1016/j.scp.2022.100937
Vakh, C. and Koronkiewicz, S., TrAC, Trends Anal. Chem., 2023, vol. 165, p. 117143.
Luque de Castro, M.D., and García-Ayuso, L., Anal. Chim. Acta, 1998, vol. 369, p. 1. https://doi.org/10.1016/s0003-2670(98)00233-5
Luque-García, L.J. and Luque de Castro, M.D., Talanta, 2004, vol. 64, p. 571.
Jensen, W.B., J. Chem. Educ., 2007, vol. 84, p. 1913. https://doi.org/10.1021/ed084p1913
De Castro, M.L. and Priego-Capote, F., J. Chromatogr. A, 2010, vol. 1217, p. 2383. https://doi.org/10.1016/j.chroma.2009.11.027
Zygler, A., Słomińska, M., and Namieśnik, J., Compr. Sampling Sample Prep., 2012, vol. 2, p. 65. https://doi.org/10.1016/B978-0-12-381373-2.00037-5
Luque-García, L.J. and Luque de Castro, M.D., TrAC, Trends Anal. Chem., 2003, vol. 22, p. 41. https://doi.org/10.1016/s0165-9936(03)00102-x
Santos, H. and Capelo, J., Talanta, 2007, vol. 73, p. 795. https://doi.org/10.1016/j.talanta.2007.05.039
Tadeo, J.L., Sánchez-Brunete, C., Albero, B., García-Valcárcel, A.I., J. Chromatogr. A, 2010, vol. 1217, p. 2415. https://doi.org/10.1016/j.chroma.2009.11.066
Bendicho, C., De La Calle, I., Pena, F., Costas, M., Cabaleiro, N., and Lavilla, I., TrAC, Trends Anal. Chem., 2012, vol. 31, p. 50. https://doi.org/10.1016/j.trac.2011.06.018
Seidi, S. and Yamini, Y., Cent. Eur. J. Chem., 2012, vol. 10, p. 938. https://doi.org/10.2478/s11532-011-0160-1
Picó, Y., TrAC, Trends Anal. Chem., 2013, vol. 43, p. 84. https://doi.org/10.1016/j.trac.2012.12.005
Tiwari, B.K., TrAC, Trends Anal. Chem., 2015, vol. 71, p. 100.
Albero, B., Sánchez-Brunete, C., García-Valcárcel, A.I., Pérez, R.A., and Tadeo, J.L., TrAC, Trends Anal. Chem., 2015, vol. 71, p. 110. https://doi.org/10.1016/j.trac.2015.03.015
Chemat, F., Rombaut, N., Sicaire, A.G., Meullemiestre, A., Fabiano-Tixier, A.S., and Abert-Vian, M., Ultrason. Sonochem., 2017, vol. 34, p. 540.
Vinatoru, M., Mason, T.J., and Calinescu, I., TrAC, Trends Anal. Chem., 2017, vol. 97, p. 159. https://doi.org/10.1016/j.trac.2017.09.002
Albero, B., Tadeo, J.L., and Perez, R.A., TrAC, Trends Anal. Chem., 2019, vol. 118, p. 739. https://doi.org/10.1016/j.trac.2019.07.007
Ojha, S., Aznar, R., O’Donnell, C., and Tiwari, B.K., TrAC, Trends Anal. Chem., 2019, p. 115663. https://doi.org/10.1016/j.trac.2019.115663
Kumar, K., Srivastav, S., and Sharanagat, V.S., Ultrason. Sonochem., 2021, vol. 70, p. 105325. https://doi.org/10.1016/j.ultsonch.2020.105325
Jinadasa, B.K.K.K., Moreda-Pineiro, A., and Fowler, S.W., Food Rev. Int., 2021, vol. 39, p. 1. https://doi.org/10.1080/87559129.2021.1967378
Das, P., Nayak, P.K., and Kesavan, R., Food Chem. Adv., 2022, vol. 1, p. 100144.
Pérez, R.A. and Albero, B., TrAC, Trends Anal. Chem., 2023, vol. 166, p. 117204. https://doi.org/10.1016/j.trac.2023.117204
Shen, L., Pang, S., Zhong, M., Sun, Y., Qayum, A., Liu, Y., Rashid, A., Xu, B., Liang, Q., Ma, H., and Ren, X., Ultrason. Sonochem., 2023, vol. 101, p. 106646.
Smith, F.E. and Arsenault, E.A., Talanta, 1996, vol. 43, p. 1207. https://doi.org/10.1016/0039-9140(96)01882-6
Ganzler, K., Salgó, A., and Valkó, K., J. Chromatogr. A, 1986, vol. 371, p. 299. https://doi.org/10.1016/s0021-9673(01)94714-4
Letellier, M. and Budzinski, H., Analusis, 1999, vol. 27, p. 259. https://doi.org/10.1051/analusis:1999116
Camel, V., TrAC, Trends Anal. Chem., 2000, vol. 19, p. 229. https://doi.org/10.1016/S0165-9936(99)00185-5
Eskilsson, C.S. and Bjorklund, E., J. Chromatogr. A, 2000, vol. 902, p. 229. https://doi.org/10.1016/s0021-9673(00)00921-3
Camel, V., Analyst, 2001, vol. 126, p. 1182. https://doi.org/10.1039/b008243k
Kubrakova, I.V., Russ. Chem. Rev., 2002, vol. 71, no. 4, p. 283. https://doi.org/10.1070/RC2002v071n04ABEH000699
Luque-García, J.L. and Luque de Castro, M.D., TrAC, Trends Anal. Chem., 2003, vol. 22, p. 90. https://doi.org/10.1016/S0165-9936(03)00202-4
Srogi, K., Anal. Lett., 2006, vol. 39, p. 1261. https://doi.org/10.1080/00032710600666289
Belanger, J.M.R. and Pare, J.R.J., Anal. Bioanal. Chem., 2006, vol. 386, p. 1049.
Chen, L., Song, D., Tian, Y., Ding, L., Yu, A., and Zhang, H., TrAC, Trends Anal. Chem., 2008, vol. 27, p. 151. https://doi.org/10.1016/j.trac.2008.01.003
Madej, K., TrAC, Trends Anal. Chem., 2009, vol. 28, p. 436. https://doi.org/10.1016/j.trac.2009.02.002
Sanchez-Prado, L., Garcia-Jares, C., and Llompart, M., J. Chromatogr. A, 2010, vol. 1217, p. 2390. https://doi.org/10.1016/j.chroma.2009.11.080
Chan, C.H., Yusoff, R., Ngoh, G.C., and Kung, F.W.L., J. Chromatogr. A, 2011, vol. 1218, p. 6213. https://doi.org/10.1016/j.chroma.2011.07.040
Tatke, P. and Jaiswal, Y., Res. J. Med. Plant, 2011, vol. 5, p. 21.
Sanchez-Prado, L., Garcia-Jares, C., Dagnac, T., and Llompart, M., TrAC, Trends Anal. Chem., 2015, vol. 71, p. 119. https://doi.org/10.1016/j.trac.2015.03.014
Wang, H., Ding, J., and Ren, N., TrAC, Trends Anal. Chem., 2016, vol. 75, p. 197. https://doi.org/10.1016/j.trac.2015.05.005
Mandal, V. and Tandey, R., TrAC, Trends Anal. Chem., 2016, vol. 82, p. 100. https://doi.org/10.1016/j.trac.2016.05.020
Kala, H.K., Mehta, R., Sen, K.K., Tandey, R., and Mandal, V., TrAC, Trends Anal. Chem., 2016, vol. 85, p. 140. https://doi.org/10.1016/j.trac.2016.09.007
Llompart, M., Celeiro, M., and Dagnac, T., TrAC, Trends Anal. Chem., 2019, vol. 116, p. 136. https://doi.org/10.1016/j.trac.2019.04.029
Bagade, S.B. and Patil, M., Crit. Rev. Anal. Chem., 2021, vol. 51, p. 138. https://doi.org/10.1080/10408347.2019.1686966
López-Salazar, H., Camacho-Díaz, B.H., Ocampo, M.L.A., and Jiménez-Aparicio, A.R., BioResources, 2023, vol. 18, p. 6614. https://doi.org/10.15376/biores.18.3
Ferrara, D., Beccaria, M., Cordero, C.E., and Purcaro, G., J. Sep. Sci., 2023, vol. 46, p. e2300390. https://doi.org/10.1002/jssc.202300390
ACKNOWLEDGMENTS
The authors are grateful to the Interdisciplinary Scientific and Educational School of Moscow University “The Future of the Planet and Global Environmental Changes.”
Funding
The work was carried out within the framework of a state assignment, topic no. AAAA-A21-121011990021-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by V. Kudrinskaya
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dmitrienko, S.G., Apyari, V.V., Tolmacheva, V.V. et al. Methods for the Extraction of Organic Compounds from Solid Samples: 1. Solvent Extraction. Review of Reviews. J Anal Chem 79, 999–1010 (2024). https://doi.org/10.1134/S1061934824700382
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934824700382