Abstract
We created glassy carbon electrodes (GCEs) modified with CeO2 nanoparticles and various surfactants and determined the voltammetric characteristics of tartrazine oxidation on them. The electrode modified with the cationic surfactant cetyltriphenylphosphonium bromide (CTPPB) ensures a 72.5-fold increase in the oxidation currents of tartrazine compared to a GCE. The oxidation of tartrazine on the CeO2-CTPPB/GCE proceeds irreversibly, involves one electron, and is controlled by surface processes. A procedure was developed for the voltammetric determination of tartrazine on a CeO2-CTPPB/GCE. The analytical range is 1.00–250 µM of tartrazine with a limit of detection of 0.4 µM. The procedure was applied to quantify tartrazine in the Tarkhun beverage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Dyes are widely used to restore color lost during processing or to improve the appearance of food products. Among the wide variety of food colorants, tartrazine (trisodium salt of 5-hydroxy-1-(p-sulfophenyl)-4-[(p-sulfophenyl)-azo]-pyrazole-3-carboxylic acid) can be noted. It is labeled as food additive E102 [1]. Tartrazine is found in soft drinks, juices, jellies, sweets, cakes, cereals, soups, and other products [2]. However, like other synthetic dyes, tartrazine exhibits toxic properties depending on its concentration. A high concentration of tartrazine combined with sodium benzoate, a food preservative, can cause hyperactivity in children, allergies, and asthma [1]. The decomposition of the dye in a body under the action of azoreductase yields toxic aromatic amines [2]. In this regard, it is necessary to control tartrazine concentration in food products, which requires rapid, sensitive, and selective methods for its determination.
The azo and hydroxyl groups in the structure of tartrazine are electrochemically active; therefore, many electrochemical methods have been proposed to determine tartrazine. For example, Lipskikh et al. [3] studied the electroreduction of tartrazine on a glassy carbon electrode (GCE) in an acidic medium. Under the conditions of adsorptive stripping voltammetry, the range of determined concentrations is 0.05–0.50 mg/L with a limit of detection of 0.011 mg/L. To increase the sensitivity of the determination of tartrazine, several chemically modified electrodes based on surfactants [4], carbon nanomaterials [5, 6], metal nanoparticles [7, 8], polymer coatings [9], and their combinations [10–12] were developed. To improve the selectivity of the determination of tartrazine, electrodes based on molecularly imprinted polymers have been proposed [13–15]. The determination was carried out by recording a decrease in the reduction currents of K3Fe(CN)6 in the presence of tartrazine [13, 15]. The performance characteristics of the voltammetric determination of tartrazine on modified electrodes are presented in Table 1.
CeO2 nanoparticles in combination with surfactants are promising as electrode surface modifiers. This approach significantly increases sensitivity and lowers the limits of detection for the target analytes, which was shown on an example of natural phenolic antioxidants [16–19]. The modified electrodes do not exhibit electrochemical activity. This enables the recording of the own analytical signal of the analyte, increasing its selectivity. Such a combination of modifiers was not described in publications regarding food colorants.
In this work, we propose a GCE modified with CeO2 nanoparticles and surfactants for determining tartrazine.
EXPERIMENTAL
Reagents and solutions. We used tartrazine (85%, Sigma, United States), cetylpyridinium bromide (CPB) (98%, Aldrich, Germany), cetyltrimethylammonium bromide (CTAB) (99%, Acros Organics, Belgium), cetyltriphenylphosphonium bromide (CTPPB) (synthesized at the Department of Macromolecular and Organoelement Compounds, Butlerov Institute of Chemistry, Kazan Federal University), sodium dodecyl sulfate (SDS) (pharmacopoeial grade, Panreac, Spain), Brij® 35 (Acros Organics, Belgium), and Triton X-100 (Aldrich, Germany). Other reagents were of cp grade.
A standard 10 mM tartrazine solution was prepared by dissolving its accurately weighed portion in 5.0 mL of distilled water. Diluted solutions were prepared immediately before measurements in 5.0-mL volumetric flasks, bringing them to the mark with distilled water.
Surfactant solutions (5.0 mM solutions of CPB and CTAB, 3.0 mM CTPPB, 10 mM SDS, Brij® 35, and Triton X-100) were prepared by dissolving their accurately weighed portions in distilled water. Working solutions were prepared by diluting the stock solutions.
Modification of the electrode. An aqueous dispersion containing 10 wt % of CeO2 nanoparticles (particle size <25 nm, Sigma-Aldrich, United States) was used as an electrode surface modifier. Working dispersions in solutions of surfactants of various nature and concentrations were prepared by sequential dilutions followed by ultrasonic treatment for 10 min. The concentration of nanoparticles was varied from 0.5 to 1.5 mg/mL.
A 3-μL portion of the suspension was applied to a GCE surface by drop evaporation. Before modification, the GCE working surface was renewed mechanically by polishing it with alumina with a particle size of 0.05 µm. Then, the electrode was rinsed with acetone and distilled water.
Voltammetric and chronoamperometric measurements were carried out in a 10.0-mL three-electrode electrochemical cell using an Autolab PGSTAT 320N potentiostat/galvanostat (Eco Chemie, Netherlands) with the NOVA 1.10.1.9 software. We used a working GCE (surface area 7.07 mm2, CH Instruments, United States) and a GCE modified with CeO2 nanoparticles, a saturated silver–silver chloride reference electrode, and an auxiliary platinum electrode. For calculations in voltammetry, baseline correction was applied using the NOVA 1.10.1.9 software, which ensured a more accurate measurement of the peak parameters. A supporting electrolyte and an aliquot portion of a tartrazine solution (the total volume of the solution in the cell was 5.0 mL) were added to the electrochemical cell, and differential pulse voltammograms were obtained at a pulse amplitude of 50 mV and a pulse time of 50 ms or cyclic voltammograms were recorded from 0.4 to 1.5 V at various potential scan rates. For the quantitative determination of tartrazine, voltammetry with linear potential sweep from 0.4 to 1.6 V at a rate of 250 mV/s was used.
An Ekspert-001 pH meter (Ekoniks-Ekspert, Russia) was used to determine the pH of the supporting electrolyte.
Real samples were analyzed after preliminary degassing in an ultrasonic bath for 10 min. Five hundred microliters of a Tarkhun beverage was added to the electrochemical cell, and voltammograms were recorded with linear potential sweep in the range from 0.4 to 1.6 V at a potential scan rate of 250 mV/s.
The statistical processing of the results was carried out for five measurements at a confidence level of 0.95. The results were presented as X ± ΔX, where X was the mean value and ΔX was the confidence interval. The random error of the determination was estimated by the relative standard deviation (RSD). Regression analysis was performed using the OriginPro 8.0 software (OriginLab, United States).
RESULTS AND DISCUSSION
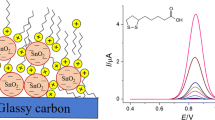
Tartrazine is oxidized on the GCE at 0.890 V in a phosphate buffer solution of pH 7.0 (Fig. 1). Despite the high concentration of tartrazine in the cell (100 µM), the oxidation currents are 12 ± 4 nA even in the differential pulse mode, which impedes using the GCE for analytical purposes. Therefore, we considered GCEs modified with CeO2 nanoparticles dispersed in surfactants. CeO2 nanoparticles have a partial negative surface charge at pH 7.0 [20], which causes their electrostatic interaction with positively charged “heads” of cationic surfactants. Electrostatic repulsion is observed for anionic SDS under these conditions, while in the case of nonionic surfactants, hydrophobic interactions probably play the key role. Thus, cationic surfactants are more strongly held on the electrode surface.
Variation of the nature of surfactants showed that, for all the surfactants under consideration, the oxidation currents of tartrazine increased compared to the GCE (Table 2). Higher oxidation currents were observed for electrodes modified with cationic surfactants, which is explained by electrostatic interactions of the cationic surfactant with tartrazine present at pH 7.0 as an anion (pKa1 = 2.0, pKa2 = 5.0, pKa3 = 10.89) [21]. A comparison of the oxidation currents for CTAB, CPB, and CTPPB illustrates the contribution of hydrophobic interactions, in particular, interactions of aromatic rings in the structure of surfactants and tartrazine. The maximum currents were observed for CTPPB, which has the highest hydrophobicity among the studied cationic surfactants. The tartrazine oxidation peak split into two for cationic CTAB and CPB (Table 2). A similar effect was described for eugenol oxidized on the CeO2-SDS/GCE [16]. This phenomenon is characteristic of the electrooxidation of organic compounds in surfactant-containing media and is explained by a change in the electron transfer rate [22]. The shift in tartrazine oxidation potentials also supports this.
We estimated the effect of the concentrations of CeO2 nanoparticles and CTPPB in the dispersion on tartrazine’s voltammetric characteristics. The oxidation potentials did not change in this case, and the oxidation currents varied significantly (Fig. 2). With an increase in the concentration of CeO2 nanoparticles to 1.0 mg/mL, oxidation currents increased, regardless of the concentration of CTPPB. A change in the concentration of CeO2 nanoparticles to 1.5 mg/mL decreased the currents, while for the CTPPB concentration of 0.10 mM, this decrease was statistically unreliable. For the further studies, we selected an electrode based on 1.0 mg/mL of CeO2 nanoparticles in a 0.10 mM CTPPB solution.
The electrooxidation of tartrazine on the CeO2-CTPPB/GCE was studied (Fig. 3). Varying the pH of the supporting electrolyte from 4.8 to 8.0 showed that the oxidation potential of tartrazine shifted to lower values as the pH of the supporting electrolyte was increased (Fig. 3a), which indicates the involvement of protons in the electrode reaction. Tartrazine oxidation currents gradually increased up to pH 7.5 and then sharply decreased (Fig. 3b). The further studies were carried out at pH 7.5.
To determine the nature of the electrochemical process, we assessed the effect of the rate of potential change on the oxidation currents of tartrazine (Fig. 4). Oxidation was controlled by the adsorption of the analyte, which was confirmed by the linear dependence of oxidation currents on V (Eq. (1)) and the slope of the dependence of ln Ip on ln v (Eq. (2)), that is,
The absence of a cathodic step in the cyclic voltammograms and the shift of the oxidation potential of tartrazine to more positive values with increasing v indicate irreversible oxidation. The oxidation potential increased linearly as a function of ln v according to the equation
According to the Laviron equation [23] (Eq. (4)), αan = 0.51.
For an irreversible process, αa = 0.5 [24]. Then, the number of electrons participating in the reaction is 1, which agrees well with the data for other modified electrodes [9–12]. Thus, considering the data [9–12], tartrazine is oxidized on the CeO2-CTPPB/GCE with the involvement of one electron and one proton according to Scheme 1.

Scheme 1 . Oxidation of tartrazine on a glassy carbon electrode modified with CeO2 nanoparticles and cetyltriphenylphosphonium bromide.
To quantify tartrazine, we used voltammetry with a linear potential sweep. The voltammograms showed a pronounced oxidation peak at 1.17 V (Fig. 5), the height of which was linearly related to the tartrazine concentration in the ranges of 1.0–15 and 15–250 µM (Eqs. (5) and (6)).
Voltammograms with a linear potential sweep for tartrazine at different concentrations on a CeO2-CTPPB/GCE in phosphate buffer solution at pH 7.5: (a) (1) 1.0, (2) 2.5, (3) 5.0, (4) 7.5, (5) 10, and (6) 15 µM of tartrazine and (b) (1) 15, (2) 25, (3) 50, (4) 75, (5) 100, and (6) 250 µM of tartrazine; potential sweep rate, 250 mV/s.
The limit of detection for tartrazine is 0.40 µM. The characteristics obtained are superior to those described for other chemically modified electrodes (Table 1). The proposed electrode is also characterized by a simpler and faster manufacturing procedure.
We performed the determination of tartrazine in model solutions. The accuracy of the procedure was evaluated by the standard addition method (Table 3). The relative added-found did not exceed 2%. The values of the recovery point to the high reliability of the results obtained using the developed approach.
The selectivity of the response of tartrazine in the presence of 1000-fold amounts of inorganic ions (K+, Mg2+, Ca2+, \({\text{NO}}_{3}^{ - },\) Cl–, and \({\text{SO}}_{4}^{{2 - }}\)), 100-fold amount of glucose, rhamnose, sucrose, arabinogalactan, ascorbic acid, and Sunset Yellow FCF food colorant often used together with tartrazine was demonstrated.
The developed approach was applied to the determination of tartrazine in a Tarkhun beverage. The voltammograms showed a clearly pronounced peak of tartrazine oxidation at 1.17 V (Fig. 6), which was confirmed by the standard addition method. The percentage recovery was 100–101%, indicating high determination accuracy and an absence of matrix effects.
The results of the quantification of tartrazine are presented in Table 4. The results are consistent with the data of the spectrophotometric determination [25] and do not exceed the regulated upper limit for the tartrazine concentration in drinks [26]. The values calculated by the t-test and F-test were smaller than the tabulated values at P = 0.95, which indicates the absence of a systematic error and the equivalence of the methods of voltammetry and spectrophotometry. The proposed method is characterized by high sensitivity and accuracy, simplicity, and availability, which ensure its use in laboratories for the quality control of beverages.
REFERENCES
Aguilar, F., Charrondiere, U.R., Dusemund, B., Galtier, P., Gilbert, J., Gott, D.M., Grilli, S., Guertler, R., Koenig, J., Lambre, C., Larsen, J.-C., Leblanc, J.-C., Mortensen, A., Parent-Massin, D., Pratt, I., Rietjens, I.M.C.M., Stankovic, I., Tobback, P., Verguieva, T., and Woutersen, R.A., EFSA J., 2009, vol. 7, no. 11, 1331.
Demirkol, O., Zhang, X., and Ercal, N., J. Verbraucherschutz Lebensmittelsicherh., 2012, vol. 7, no. 3, p. 229.
Lipskikh, O.I., Nikolaeva, A.A., and Korotkova, E.I., J. Anal. Chem., 2017, vol. 72, no. 4, p. 396.
Raril, C. and Manjunatha, J.G., Portugal Electrochim. Acta, 2021, vol. 39, no. 1, p. 59.
Song, Y.Z., Xu, J.M., Lv, J.S., Zhong, H., Ye, Y., and Xie, J.M., Russ. J. Phys. Chem. A, 2012, vol. 86, no. 2, p. 303.
Song, X., Shi, Z., Tan, X., Zhang, S., Liu, G., and Wu, K., Sens. Actuators, B, 2014, vol. 197, p. 104.
Asadpour-Zeynali, K. and Aleshi, M., Portugal Electrochim. Acta, 2014, vol. 32, no. 6, p. 369.
Ghoreishi, S.M., Behpour, M., and Golestaneh, M., J. Chin. Chem. Soc., 2013, vol. 60, no. 1, p. 120.
Manjunatha, J.G., Heliyon, 2018, vol. 4, no. 11, e00986.
He, Q., Liu, J., Liu, X., Li, G., Deng, P., Liang, J., and Chen, D., Sensors, 2018, vol. 18, no. 6.
Tahtaisleyen, S., Gorduk, O., and Sahin, Y., Anal. Lett., 2020, vol. 53, no. 11, p. 1683.
Karim-Nezhad, G., Khorablou, Z., Zamani, M., Dorraji, P.S., and Alamgholiloo, M., J. Food Drug Anal., 2017, vol. 25, no. 2, p. 293.
Jiang, S., Xu, J., Xu, P., Liu, L., Chen, Y., Qiao, C., Yang, S., Sha, Z., and Zhang, J., Anal. Lett., 2014, vol. 47, no. 2, p. 323.
Zhao, L.J., Zeng, B.Z., and Zhao, F.Q., Electrochim. Acta, 2014, vol. 146, p. 611.
Zhao, X., Liu, Y., Zuo, J., Zhang, J., Zhu, L., and Zhang, J., J. Electroanal. Chem., 2017, vol. 785, p. 90.
Ziyatdinova, G., Ziganshina, E., Romashkina, S., and Budnikov, H., Electroanalysis, 2017, vol. 29, no. 4, p. 1197.
Ziyatdinova, G., Ziganshina, E., Nguyen Cong, Ph., and Budnikov, H., Food Anal. Methods, 2017, vol. 10, no. 1, p. 129.
Ziyatdinova, G., Ziganshina, E., Shamsevalieva, A., and Budnikov, H., Arab. J. Chem., 2020, vol. 13, no. 1, p. 1524.
Ziyatdinova, G.K., Zakharova, S.P., Ziganshina, E.R., and Budnikov, H.C., J. Anal. Chem., 2019, vol. 74, no. 8, p. 816.
Berg, J.M., Romoser, A., Banerjee, N., Zebda, R., and Sayes, C.M., Nanotoxicology, 2009, vol. 3, no. 4, p. 276.
Klett, C., Barry, A., Balti, I., Lelli, P., Schoenstein, F., and Jouini, N., J. Environ. Chem. Eng., 2014, vol. 2, no. 2, p. 914.
Ziyatdinova, G.K., Ziganshina, E.R., and Budnikov, H.C., J. Anal. Chem., 2012, vol. 67, no. 11, p. 869.
Laviron, E., J. Electroanal. Chem., 1974, vol. 52, no. 3, p. 355.
Bard, A.J. and Faulkner, L.R., Electrochemical Methods: Fundamentals and Applications, New York: Wiley, 2001, 2nd ed.
Güler, Z., J. Food Qual., 2005, vol. 28, no. 1, p. 98.
SanPiN (Sanitary Rules and Norms) 2.3.2.1293-03: Hygienic Requirements for the Use of Food Additives, Moscow, 2003.
ACKNOWLEDGMENTS
The authors are grateful to Prof. I.V. Galkina (Department of Macromolecular and Organoelement Compounds, Butlerov Institute of Chemistry, Kazan Federal University) for providing cetyltriphenylphosphonium bromide.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Ziyatdinova, G.K., Budnikov, H.C. Voltammetric Determination of Tartrazine on an Electrode Modified with Cerium Dioxide Nanoparticles and Cetyltriphenylphosphonium Bromide. J Anal Chem 77, 664–670 (2022). https://doi.org/10.1134/S106193482206017X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106193482206017X