Abstract
Sensors for the potentiometric determination of mefenamic and phenylanthranylic acids are prepared on the basis of single- and the double-layer plasticized polyvinylchloride membranes. Ion pairs of perchlorate and mefenamic and phenylanthranylic acid with basic fuchsin are synthesized for the fabrication of membranes. The composition of compounds is confirmed by spectrophotometry and IR spectrometry. Effects of different factors on the electrode characteristics are studied, the composition of the membranes is optimized. The proposed procedure for the fabrication of double-layer membranes ensures the improvement of the properties of sensors for mefenamic and phenylanthranylic acids. The developed procedures are applied to the analysis of pharmaceutical preparations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mefenamic and phenylanthranylic acids (Scheme 1) are similar in structures, but differ in the fields of application. Phenylanthranylic acid (Phen, C13H11NO2, diphenylamine-2-carboxylic acid) is used as a reactant for the synthesis of biologically active agents and also in analytical chemistry as a reagent for the determination of metal ions and as a widespread redox indicator [1]. Some of Phen derivatives are physiologically active (anti-inflammatory and stress-protective properties, stimulation of plant growth) and are antioxidants. Mefenamic acid (Mef, C15H15NO2 2-[(2,3-dimethylphenyl)amino]benzoic acid) is a derivative of phenylanthranylic acid. It is used in pharmacy as an anesthetic and an anti-inflammatory agent. Except for the typical properties of nonsteroid anti-inflammatory drugs, mefenamic acid stimulates the formation of interferon and has pronounced febrifugal effect. In the ingestion of mefenamic acid by an organism, protein ultrastructures and cell membranes are stabilized, the permeability of vessels is reduced, oxidative phosphorylation processes are interrupted, the synthesis of mucopolysaccharides is suppressed, cell resistance is increased, and wound healing is stimulated. Because of the above features of the physiological effect on an organism, mefenamic acid is often used in medical practice [2].

Scheme. 1. Structural formulae of (a) phenylanthranylic and (b) mefenamic acids.
Mefenamic acid is in most cases determined by chromatographic methods of analysis [3–12]. The drawbacks of chromatographic procedures are the high cost of equipment and also the use of toxic acetonitrile as the main component of the mobile phase. The majority of present-day chromatographic procedures include the preconcentration of samples containing mefenamic acid by solid-phase [13–15] or dispersive microextraction [16]. Spectrophotometric procedures based on the formation of colored complexes [17–20], ion pairs (IPs) [21, 22], or products of redox transformations [23, 24] are also known. However, the majority of these procedures are not selective, require careful control of the acidity of the medium, and sometimes of temperature conditions, which complicates the performance of analysis. The described mercury–mefenamate electrode for potentiometric determinations [25] and the carbon paste electrode for voltammetric measurements [26–28] are insufficiently selective and work in a narrow pH range of the medium (Table 1).
Using modern technologies and materials, one can manufacture sensors with certain properties (potentiometric, conductometric, optical), selective for different ions. Sensors including ionophores can be effective for analytical control [29, 30]. They are simple in use and service and require rather simple and inexpensive facilities for recording an analytical signal. Sensors for the determination of mefenamic acid manufactured on the basis of nanomaterials were described in a number of publications [31–35]. However, the above methods have not found wide application to analysis. The European Pharmacopeia has still recommended the alkalimetric determination of mefenamic acid in ethanol using phenolic red as an indicator [36]. Much less methods are known for the determination of phenylanthranylic acid. Thus, titrimetric methods with visual or potentiometric detection of the equivalence point are known [37].
The aim of this work was the creation of sensors for the determination Mef and Phen with modified membranes based on ion pairs of basic fuchsin (BF) and a study of effects of various factors on the main characteristics of sensors. To solve this problem, we prepared double–layer membranes in which the first layer contained ion pairs of basic fuchsin with Mef (Phen), and second (inner) layer, ion pairs of basic fuchsin with the perchlorate ion. Sensors were used for the determination of phenylanthranylic acid in model solutions and mefenamic acid in pharmaceutical preparations.
EXPERIMENTAL
Standard 0.01 M solutions of Mef and Phen were prepared by dissolving precisely weighed portions of preparations in 10 mL of a 0.5 M NaOH solution followed by the addition of distilled water and a universal buffer mixture to pH 9. A stock 0.01 M solution of the BF basic dye was prepared by dissolving a precisely weighed portion of the dye salt in a small amount of methanol followed by dilution with distilled water. A NaClO4 solution (0.01 M) was prepared by dissolving a precisely weighed portion of the salt in distilled water.
To obtain ion pairs, 0.01 M solutions of BF and Mef (Phen, \({\text{ClO}}_{4}^{ - }\)) were mixed in the ratio 1 : 1. The obtained mixture was allowed to stand at room temperature for 8–10 h. The precipitate formed was filtered off, several times washed with cold distilled water, and dried at room temperature.
Plasticized polyvinylchloride (PVC) membranes were prepared according to recommendations [38]. A precise amount of an extracted ion pair (0.05–0.25 g) was weighed, 0.075 g of PVC was added, and the mixture was carefully stirred. Then 0.15 mL of a plasticizer (dibutyl phthalate (DBF), dinonyl phthalate (DNF), dioctyl phthalate (DOF), dibutyl sebacate (DBC) or tricresyl phosphate (TCP)) and 0.5 mL of a solvent (tetrahydrofuran) were added, and the contents were carefully stirred to obtain a homogeneous mixture. The obtained mixture was transferred to a glass template (ring 1.7–2.0 cm in diameter, densely glued to a glass substrate) and dried in air for 12–15 h. A disk 0.5–0.6 cm in diameter was cut from the obtained films and glued to a face of a polyvinyl chloride tube. After the complete drying of the glue, the fabricated electrode was filled with a standard Mef solution and a copper wire was immersed in it.
Double-layer membranes were prepared similarly. We obtained two mixtures for membranes on the basis of ion pairs of BF with ClO4– and mefenamic or phenylanthranylic acids. To obtain the first layer, a mixture on the basis of ion pairs of BF with \(\text{CO}_{4}^{-}\) was placed in a glass template. After 50–60 min, the second mixture with ion pairs (BF+) (Mef–) or (BF+) (Phen–) was poured over the first mixture. After the complete drying of the membrane, a disk was cut from it and glued to a polyvinyl chloride tube so that the first layer was directed inside the tube. The inner solution was a 0.01 M NaClO4 solution.
The absorption spectra of solutions were studied on an SF-2000 spectrophotometer (LOMO, Russia) in 1-cm quartz cells and IR spectra were recorded on a Nicolet iS10 spectrometer with a Continuum microscope in the wavelength region 4000–650 cm–1. Potentiometric measurements were performed using an AI-123 potentiometer with an ion-selective electrode (MLsoft Instruments, Ukraine); the measurement error did not exceed ±0.02 mV/pc. The reference electrode was silver–silver chloride electrode. The pH of solutions was controlled using an AI-123 or a I-160 M potentiometer with a glass electrode. All measurements were performed at room temperature. The acidity of the medium was maintained with a buffer mixture (0.04 M CH3COOH, H3BO3, H3PO4 and a 0.2 M NaOH solution). Measurements were performed according to the classical scheme of construction of an electrochemical cell:
Method for determining mefenamic acid in pharmaceutical preparations. Twenty tablets or the contents of 20 capsules were homogenized in an agate mortar to a homogeneous powder. A weighed portion of the obtained powder, equivalent to the mass of one tablet or one capsule, was dissolved in 10 mL of a 0.5 M NaOH solution. The solution was placed in a 100-mL volumetric flask, diluted with distilled water to the tag with adjusting acidity to pH 9.5 ± 0.5 using a universal buffer solution. The solution obtained was transferred to a 150-mL beaker, electrodes are put in it, and the electrode potential was measured. The concentrations of mefenamic and phenylanthranylic acids were determined by a calibration graph constructed under similar conditions.
RESULTS AND DISCUSSION
The precipitation of ion pairs is followed by a change in the color of solutions followed by the formation of a finely crystalline precipitate. The acidity of the solutions is highly important, because mefenamic and phenylanthranylic acids can occur in solution as singly charged ions only in alkaline media (pH 8–12). The scheme of ion pair formation can be presented as follows:
where An– is the organic Mef or the Phen anion, Ct+ us the cation of the BF basic dye.
The formation of ion pairs of basic fuchsin with mefenamic and phenanthranilic acids and also with the perchlorate anion was studied by IR spectrometry (Fig. 1). The spectra of Mef exhibited characteristic peaks at 1255 cm–1 (stretching vibrations of the –OH group and vibrations of –COOH groups), 1647 cm–1 (stretching vibrations of the –NH group), 1572 cm–1 (C=O stretchings), 1504 cm–1 (aromatic –CH in-plane vibrations), 1163 cm–1 (aromatic –O–CH3) [39].
Phenylanthranylic acid exhibits characteristic peaks at 1657 cm–1, corresponding to the C=O stretching vibration of the carboxylic group; peaks at 1261 cm–1 can be assigned to stretching vibrations of the CN group in the Phen molecule [40].
The basic fuchsin molecule contains nitrogen atoms, whose stretching vibrations ν(NH) appear at 3361 and 3212 cm–1, bending vibrations δ(NH) appear at 1632, 1595, 1550, 1514 cm–1 and ν(СN), at 1375, 1284, 1174 cm–1.
During the formation of ion pairs (Fig. 1, curves 2–4), the intensity and positions of the main absorption band changed. There appeared a wide band at 3650–3190 cm–1, corresponding to the stretching vibration of NH bonds. The absorption band of bending vibrations of the NH–group was observed at 1595 cm–1, and the band of ν(СH) vibrations, at 1285–1014 cm–1.
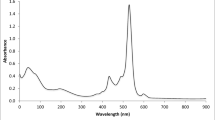
As was found by spectrophotometry, an insignificant increase in the amount of Mef or Phen in solution (at a constant concentration of the dye) caused a bathochromic shift of the absorption band (Fig. 2).
The appearance of an isobestic point indicated the formation of compounds of a constant composition. Using absorption spectra of ion pair solutions, one can calculate their association constants Kas, the values of which for Mef and Phen were 5.82 × 103 and 2.58 × 103, respectively.
The ion pairs obtained by precipitation were used as ionophores in the fabrication of sensors. The dependence of the chemico-analytical properties of the sensors on the composition of the membranes was studied by fabricating identical membranes with constant concentrations of all components except for the studied one.
It is known from [41] that the nature of the plasticizer used in the membrane significantly affects the electrode response. As can be seen in Fig. 3, the best characteristics were observed for sensor membrane plasticized by TCP (the slope of the electrode function was 54.2 ± 0.3 and 69.0 ± 0.3 mV/pc for Mef and Phen, respectively).
Effect of the nature of plasticizer on the response of sensors to (a) phenylanthranylic and (b) mefenamic acids: 6% of the (BF+) (Phen–)ion pair; 65% of plasticizer: (1) TCP; (2) DBF; (3) DNF; (4) the DBC. (b) 6% of the (BF+) (Mef–) ion pair; 65% of plasticizer: (1) TCP; (2) DBF; (3) DNF; (4) DOF; (5) DBC.
The electrochemical characteristics of membranes based on (BF+) (Mef–) and (BF+) (Phen –) ion pairs plasticized by TCP are presented in Table and 2. It can be seen that all membranes ensure the slope of the electrode function characteristic for singly charged ions. Membranes with the high concentration of TCP (65–75%) are more elastic; this property determines the life time of the sensor. The optimized composition of electrode membranes for the determination of mefenamic and phenylanthranylic acids is 4% of ion pairs, 65% of TCP, 31% of PVC and 6% of ion pairs and 70% of TCP, 24% of PVC, respectively.
In certain cases, the addition of a lipophilic component to the membrane can change the parameters of sensor response to the potential-determining ion [42–45]. We studied the effect of an additive of tetramethylethylene diamine (TMED) on the electrochemical parameters of sensors fabricated on the basis of membranes with the concentration of ion pairs (BF+) (Mef–) 2, 4, 6, 8, and 10%. It was found that the addition of 0.02–0.08 mL of TMED to the membrane leads to minor changes in the sensor characteristics (Fig. 4).
Effect of the concentration of the (BF+) (Mef–) ion pair and of the lipophilic additive to the membrane on the slope of the electrode function and the limit of detection (LOD) for mefenamic acid. (1) Ion pairs (BF+) (Mef–); (2) ion pairs (BF+) (Mef–) and TMED; (3) ion pairs (BF+) (Mef–); (4) ion pairs (BF+) (Mef–) and TMED.
To improve the characteristics of sensors, we prepared double-layer membranes on the basis of ion pairs ClO–4, Mef, and Phen with BF. It was found that use of double-layer membranes improved the properties of sensors (Fig. 5). In addition, this technology allowed us to replace inner Mef or Phen solutions unstable in time with a solution of an inorganic salt, a 0.01 M NaClO4 solution.
The obtained sensor samples work in the pH range of solutions 8.5–12. A stable value of electrode potentials was attained already in 10–15 s. The synthesized membranes are suitable for work within not less than 4 months.
To determine the values of the selectivity coefficients of sensors, we used the method of separate solutions. For this purpose, we constructed a dependence of potential on the concentration of an interfering ion in the solution and derived the ratio of activities of the studied aA and foreign aB ions at the achievement of equal potentials:
The obtained selectivity coefficients (pKA/B) for single-layer and double-layer membranes are summarized in Table 3. It can be seen that the selectivities of double-layer membranes to certain ions (bromides, iodides, thiocyanates, perchlorates, borates, phosphates, salicylates) were enhanced. An important advantage of the created sensors in comparison with the known mercury–mefenamate graphite sensor [25] is a possibility of performing measurements in the solutions containing chloride ions.
The prepared sensors were used for the determination of phenylanthranylic acid in model solutions and mefenamic acid in pharmaceutical preparations. The results are summarized in Tables 4 and 5.
REFERENCES
Zapała, L. and Kalembkiewicz, J., Talanta, 2006, vol. 69, no. 3, p. 601.
Voitenko, N.G., Ukr. Med. Zh., 2011, vol. 81, no. 1, p. 75.
Maron, N. and Wright, G., J. Pharm. Biomed. Anal., 1990, vol. 8, no. 1, p. 101.
United States Pharmacopeia National Formulary, USP 26, NF21, Rockville, 2003.
Niopas, I. and Mamzoridi, K., J. Chromatogr. B: Biomed. Sci. Appl., 1994, vol. 656, no. 2, p. 447.
Rouini, M.R., Asadipour, A., Hoseinzadeh Ardakani, Y., and Aghdasi, F., J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, vol. 800, p. 189.
Sun, Y., Takaba, K., Kido, K., Nakashima, M.N., and Nakashima, K., J. Pharm. Biomed. Anal., 2003, vol. 30, no. 5, p. 1611.
Hoshina, K., Horiyama, S., Matsunaga, H., and Haginaka, J., J. Pharm. Biomed. Anal., 2011, vol. 55, no. 1, p. 916.
Ibrahim, F., Sharaf El-Din, M.K., El-Din, A.K., and Shimizu, K., J. Chromatogr. Sci., 2017, vol. 55, no. 1, p. 23.
Morcoss, M.M., Abdelwahab, N.S., Ali, N.W., and Elsaady, M.T., J. Chromatogr. Sci., 2017, vol. 55, no. 7, p. 766.
Twinkle, P.K. and Dhananjay, M.B., Int. J. Pharm. Sci. Drug Res., 2015, vol. 7, no. 4, p. 361.
Rivai, H., Putri, W.K., and Armin, F., J. Chem. Pharm. Res., 2016, vol. 8, no. 1, p. 565.
Wan Ibrahim, W.N., Sanagi, M.M., Mohamad Hanapi, N.S., Kamaruzaman, S., Yahaya, N., and Wan Ibrahim, W.A., J. Sep. Sci., 2018, vol. 41, p. 2942.
Asiabi, H., Yamini, Y., and Shamsayei, M., Talanta, 2018, vol. 185, p. 80.
Al-Khazrajy, O.S.A. and Boxall, A.B.A., Anal. Methods, 2017, vol. 9, p. 4190.
Arghavani-Beydokhti, S., Rajabi, M., and Asghari, A., Anal. Chim. Acta, 2018, vol. 997, p. 67.
Rashid, M.H., Sarsam, S.W., and Al-Sabea, N., Al-MustansiriyahJ. Pharm. Sci., 2017, vol. 7, no. 2, p. 7.
Alarfaj, N.A., Altamini, S.A., and Almarshady, L.Z., Asian J. Chem., 2009, vol. 21, no. 1, p. 217.
Das, S., Sharma, S.C., Talwar, S.K., and Sethi, P.D., Analyst, 1989, vol. 114, no. 1, p. 101.
Zseltvay, I., Zheltvay, O., and Antonovich, V., Acta Pol. Pharm., 2011, vol. 68, no. 5, p. 629.
Sastry, C.S., Prasad Tipirneni, A.S., and Suryanarayana, M.V., Analyst, 1989, vol. 114, no. 4, p. 513.
Kormosh, Zh.A., Matviichuk, O.Yu., and Bazel’, Ya.R., J. Anal. Chem., 2014, vol. 69, no. 10, p. 960.
Othman, N.S. and Awadis, L.S., RafidainJ. Sci., 2009, vol. 20, no. 1, p. 8.
Hashemi, M., Zohrabi, P., and Torkejokar, M., Sep. Purif. Technol., 2017, vol. 176, p. 126.
Santini, A.O., Pezza, H., and Pezza, L., Sens. Actuators, B, 2007, vol. 128, no. 1, p. 117.
Hasanzadeh, M., Shadjou, N., Saghatforoush, L., and Dolatabadi, J.E., Colloids Surf., B, 2012, vol. 92, p. 91.
Babaei, A., Khalilzadeh, B., and Afrasiabi, M., J. Appl. Electrochem., 2010, vol. 40, p. 1537.
Liu, L. and Song, J., Anal. Biochem., 2006, vol. 354, no. 1, p. 22.
Pechenkina, I.A. and Mikhelson, K.N., Russ. J. Electrochem., 2015, vol. 51, no. 2, p. 93.
Mikhel’son, K.N. and Peshkova, M.A., Russ. Chem. Rev., 2015, vol. 84, no. 6, p. 555.
Shetti, N.P., Nayak, D.S., Malode, S.J., Kakarla, R.R., and Shukla, S.S., Anal. Chim. Acta, 2019, vol. 1051, p. 58.
Tezerjani, M.D., Benvidi, A., Rezaeinasab, M., Jahanbani, S., and Ardakani, M.M., J. Serb. Chem. Soc., 2017, vol. 82, p. 1273.
Akbarian, Y., Shabani-Nooshabadi, M., and Karimi-Maleh, H., Sens. Actuators, B, 2018, vol. 273, p. 228.
Naeemy, A., Gholam-Shahbazi, R., and Mohammadi, A., Electrochem. Sci. Technol., 2017, vol. 8, p. 282.
Tarlekar, P. and Chatterjee, S., J. Electroanal. Chem., 2017, vol. 803, p. 51.
European Pharmacopeia 5.0, Strasbourg: Council of Europe, 2001, p. 1534.
Kobzar, N.P., Isaev, S.G., Svechnikova, O.M., and Pavlii, O.O., Zh. Org. Farm. Khim., 2006, vol. 4, no. 16, p. 67.
Cammann, K., Das Arbeiten mit Ionenselektiven Elektroden (Working with Ion-Selective Electrodes), Heidelberg: Springer, 1977.
Romero, S., Escalera, B., and Bustamante, P., Int. J. Pharm., 1999, vol. 178, no. 2, p. 193.
Mudit, D., Keshavarao, K.P., and Selvam, P., Int. Res. J. Pharm., 2011, vol. 2, no. 4, p. 207.
Morf, W., The Principles of Ion-Selective Electrodes and of Membrane Transport, Budapest: Akadémiai Kiadó, 1981.
Bakker, E. and Chumbimuni-Torres, K., J. Braz. Chem. Soc., 2008, vol. 19, no. 4, p. 621.
Mikhelson, K.N., Metody Ob”ekty Khim.Anal., 2006, vol. 1, no. 1, p. 73.
Ivanova, A.D., Koltashova, E.S., Solovyeva, E.V., Peshkova, M.A., and Mikhelson, K.N., Electrochim. Acta, 2016, vol. 213, p. 439.
Mikhelson, K.N., Ion-Selective Electrodes: Lecture Notes in Chemistry, Heidelberg: Springer, 2013.
Funding
This study was supported by the VEGA Agency on Scientific Grants of the Ministry of Education of Slovak Republic and the Slovak Academy of Sciences (grant no. 1/0033/20).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Rykova
Rights and permissions
About this article
Cite this article
Kormosh, Z.A., Matviichuk, O.Y., Antal, I.P. et al. Sensors Based on Single- and Double-Layer Plasticized Membranes for the Potentiometric Determination of Mefenamic and Phenylanthranylic Acids. J Anal Chem 75, 820–828 (2020). https://doi.org/10.1134/S1061934820060131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820060131