Abstract
The use of neonicotinoid insecticides has been constantly revised because of their impact on bees, causing their decrease and bee malady. Unfortunately, because of the worldwide differences in pesticide regulation, chlothianidin is still allowed in European Union for greenhouse use and worldwide in some cases without any restictions. Lately, it was detected on soil particles and in raw and drinking waters. The preparation of drinking waters implies different purification processes, including chlorination, ozonation, and UV irradiation and nowadays advanced oxidation processes, including TiO2. The TiO2 photocatalytic degradation of chlothianidin in the presence of oxygen, nitrate, and humic acids was followed by kinetic studies, whereas the photoproducts formed were identified by liquid chromatography/tandem mass spectrometry. The efficiency of different set-ups of the photocatalytic degradation of chlothianidin was evaluated by the identification of photoproducts and bioluminescence inhibition of bacteria Vibrio fischeri. The results indicate that less harmful photoproducts are generated in the samples with added humic acids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Neonicotinoids are a class of systemic insecticides used for foliar and soil tillage. Clotianidin ((E)-N-(2-chlorothiazol-5-ylmethyl)-N '-methyl-N "-nitroguanidine) is one of neonicotinoids, missing space, redundant space which can translocate in the xylem of plants from the soil to leaves, fruits, flowers, pollen, nectar, and plant fluid. One of undesirable consequences of the use of neonicotinoids, first reported in the case of using imidacloprid, include bee maladies [1, 2]. Currently, bees may be exposed to other neonicotinoids, such as chlothianidin, when they feed on the nectar, pollen, and guttation fluid of treated plants [3]. On a short term scale sublethal residue levels may affect foraging intensity, food consumption and orientation capability. However, honey bees can also be subjected to sublethal effects if they intensively forage on seed-dressed crop plants over longer periods [4]. Thus, in the long run, the egg-laying capacity of the queen of honey bearing bees may be seriously impaired. Experts believe that chlothianidin is one of many possible causes of a decrease in the number of bees, and the recent bee malady termed colony collapse disorder [5, 6].
The United States Environmental Protection Agency [7] (US EPA 2003) defined chlothianidin ti be from “mobile to highly mobile” compound. Clothianidin is expected to leach into groundwater, based on its persistence and ability to bind with soil particles [8]. It is usually used as a seed coating that remains stable throughout the year in soil pore water [9]. According to the published data, the half-lives of chlothianidin in soils are highly variable ranging from 17 days to several years [10]. The moisture content of soils has a positive effect on degradation rates; however, chlothianidin was observed to be more persistent than other neonicotinoids, i.e. imidacloprid and thiamethoxam, in the soil of tropical ecosystem [11]. A recent study showed that degradation of clothianidin in soils turned out to be a rather slow process with half-lives ranging from 90 (high organic carbon) to 280 (low organic carbon) days [12].
The registration and re-registration procedure for pesticides is being constantly revised. The EU Commission adopted the Rules for the complete ban of the use of imidacloprid, chlothianidin, and thiamethoxam in open air only on May 29th 2018. These data were published in the Official Journal of the European Union on May 30th 2018 [13]. According to the regulations, the use of chlothianidin in the future will be allowed only in greenhouses. However, the situation in other countries may be completely different, e.g., in China and India, which have implemented their own pesticide management systems, chlothianidin is still used because of legislation being inadequate or sometimes poorly enforced [14]. Thus, chlothianidin itself, or its transformation products, can be transferred with water streams or, e.g., birds [15] to neighboring territories, leading to negative consequences, primarily for beekeeping. Therefore, further work is required to introduce a consistent approach to regulating the use of pesticides in different jurisdictions.
Since our society produces more and more xenobiotics, especially pesticides, which are released into the environment, an efficient degradation employing heterogeneous (TiO2) photocatalysis is needed [16–18]. The photocatalytic degradation of chlothianidin was described by our group several years ago [19]; however, there is still no information regarding studies on the enhanced photocatalytic removal of chlothianidin from environmental samples in the presence of humic acids (HA) and nitrate ions \(\left( {{\text{NO}}_{3}^{ - }} \right)\). Many examples, summarized in the review [20], show that HA, nitrite ions, and nitrate ions can be sources of hydroxyl radicals and, therefore, may play a significant role in the removal of pollutants.
Within this research we have studied the photocatalytical degradation of chlothianidin using TiO2 films in order to determine the effect of the addition of \({\text{NO}}_{3}^{ - }\) and HA. Besides, chlorination as the final step of pesticides removal from ground water was studied. Degradation of chlothianidin was compared to the degradation of flubendiamide and imidacloprid. The efficiency of removal was linked to the toxicity evaluation, since it is known from our previous experience that removal does not always match the decreased toxicity [17]. The addition of \({\text{NO}}_{3}^{ - }\) and HA was made in order to assess the influence of the environment on the formation of the chlothianidin degradation products.
EXPERIMENTAL
Reagents and materials. Chlothianidin was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Analytical standards of sodium nitrate (Riedel-de Haen, Honeywell, Seelze, Germany), whereas HA, acetonitrile (HPLC grade), Pluronic F-127 copolymer, disodium hydrogen phosphate, and sodium hydrocarbonate were all purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Titanium (IV) isopropoxide was purchased from Acros Organics (Thermo Fisher Scientific, Pittsburgh, Pennsylvania, USA), potassium dihydrogen phosphate and sodium carbonate were obtained from Carlo Erba (Milano, Italy). Photocatalytic decomposition experiments using TiO2. The reactor cell consisted of a Durand glass tube (280 mm, inner diameter 80 mm) with an effective volume of 1.5 L. The reactor cell was placed in a photoreactor chamber equipped with four ultraviolet A (UVA) lamps (15 W, 265 × 16 mm, Philips Cleo; broad maximum at 355 nm) mounted on the reflecting surface of polished aluminum which was placed behind the lamps. Twelve glass sheets with an immobilized TiO2 catalyst [21] were fixed around the axis of a special Teflon holder immersed in the center of the reactor cell.
For photocatalytic experiments, 10 mg of chlothianidin in deionized water (10 mg/L) was diluted with distilled water up to 1000 mL. Then the solution was transferred to the reactor chamber. A Teflon holder with 12 glass sheets with immobilized TiO2 was immersed in the center of the cell. A gentle purging of oxygen (1–2 mL/min) allowed the mixing of the solution and provided the contact of the pesticide with the catalysts. The addition of \({\text{NO}}_{3}^{ - }\), HA, and active chlorine was performed to achieve the final concentration of the added substance 10 mg/L. The reaction time was 2 h. The irradiated samples for high-performance liquid chromatography (HPLC) analysis were collected by sampling a volume of 1 mL at different time intervals (0, 5, 15, 30, 45, 60, 90, and 120 min). After 2 h of photocatalytic irradiation, 75 mL of the final solution was extracted with solid-phase extraction (SPE) C18 cartridges (Supelco, Discovery DSC-18), washed with 1 mL of acetonitrile, and analyzed by liquid chromatography–mass spectrometry (LC–MS) and liquid chromatography–tandem mass spectrometry (LC–MS/MS).
Kinetic studies—measurements by HPLC−UV (UV-Vis). Aqueous solutions of chlothianidin were analyzed by an Agilent 1100 HPLC−UV-Vis chromatograph (Agilent Technologies, Palo Alto, CA, USA). Separation was performed using a 5 μm Supelco Ascentis® Express C18 column (5 μm, 150 × 4.6 mm) at 25°C. The injection volume was 20 μL with the flow rate of 1.0 mL/min, the wavelength was set at 260 nm. Analyses were carried out under isocratic conditions using a mobile phase consisting of acetoniltrile (40%) and 1.0% (vol.) of an acetic acid solution (60%). For quantification purposes, calibration curves in the range from 0.5 to 50 mg/L were prepared.
Identification of the transformation products of chlothianidin by LC–MS and LC–MS/MS. All LC–MS/MS analyses were performed using LC-30AD HPLC (Shimadzu, Japan) coupled to a hybrid Orbitrap QExactive Plus mass spectrometer (Thermo Fisher Scientific, USA) with a heated electrospray ionization source (HESI). The analytes were separated on a Kinetex C18 column (150 ± 2.1 mm, 1.7 μm) at 25°C using the following gradient program (mobile phase A – water with 0.1% formic acid, phase B – acetonitrile with 0.1% formic acid): 0.01 min, 10% B; 10.0 min, 70% B; 11 min, 70% B; 17 min, 10% B; followed by a 1-min washing step at 10% B. The flow rate was 450 μL min–1, and the injection volume 10 μL. The mass spectrometer was operated in the positive ionization mode and data were acquired in the product ion scan mode. The source temperature was set at 300°C, spray voltage was 3.8 kV, and the used gas flows (N2) were as follows: sheath gas 50, auxiliary gas 25, and sweep gas 10 arbitrary . units. Accurate mass measurements were carried out in an Orbitrap analyzer with 35000 FWHM resolving power. The elemental composition of each fragment ion was calculated within 5 ppm mass accuracy. MS/MS experiments were carried out using higher-energy collision-induced dissociation (HCD) at 27.5 V. The m/z range for separating precursor ions for MS/MS was 1 Da. The system was controlled by the Xcalibur software, which was also used for data collection and processing.
Bioluminescence inhibition assay with Vibrio fisc-heri. The luminescent bacteria test with the marine bacterium Vibrio fischeri has become one of the basic tests for the ecotoxicological testing of chemicals, wastewaters, and soil and sediment eluates over the last decades [22]. The luminescence produced by bacteria is proportional to their metabolic activity and measured using a photomultiplier (LUMIStox 300 luminometer, Hach Dr. Lange GmbH, Germany). The inhibition of natural light radiation in the presence of the sample was determined using a negative control (2% NaCl at the adjusted pH) after incubation at 30-min intervals at 15°C ± 1°C. Because of the natural fall of luminescence over time, the correction of the results with respect to the negative control was made. Fluorescence losses due to the absorption of colored solutions were compensated by the integrated color correction applied during the test. For positive control, a solution of potassium dichromate (K2Cr2O7) with a concentration of 100 mg/L was used, causing 50 ± 5% inhibition. The resulting measurements were processed using the LUMIS soft 4 software.
Statistical analysis. All experiments and measurements were performed in triplicate to assess the reproducibility of the measurements. Data are expressed as the mean ± standard deviation.
RESULTS AND DISCUSSION
Photocatalytic degradation of chlothianidin. Neonicotinoids are highly water soluble and, therefore, excellent systemic insecticides. Besides, they are resistant to hydrolysis and, for this reason, have rather large half-lives in soil and water, both at neutral or acidic pH and under anaerobic conditions [23]. The two major degradation products were chlothianidin-urea (m/z 205) and dechlorinated chlothianidin-urea (m/z 171). The same degradation product (chlothianidin-urea) was found in the case of the chlothianidin degradation in different soils (with the reaction rate constant k ranging from 0.0049 to 0.0128 d–1 in a sterilized soil and from 0.0074 to 0.0440 d–1 in unsterilized conditions), suggesting a significant contribution of biodegradation to the removal of chlothianidin via the nitrate reduction, cyano hydrolysis, and chloropyridinyl dechlorination reactions [24].
The chemical stability of neonicotinoids (chlorotanidine, imidacloprid, and thiamethoxam) has been recently confirmed by their presence in raw and drinking water of agricultural regions of Southern Ontario, Canada [25]. In this regard, the ways of their degradation and transformation should be carefully studied. In fact it is known, that the advanced oxidation processes, chlorination and ozonation may form some new pollutants [17, 19, and 26–28].
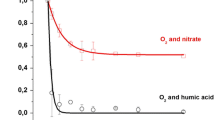
Figure 1 demonstrates the rate of photodegradation of chlothianidin with TiO2 in the presence of HA and \({\text{NO}}_{3}^{ - }\). It is important to note that the addition of HA and \({\text{NO}}_{3}^{ - }\), which are usually present in natural waters of agricultural use, reduce the rate of the photodegradation of chlothianidin.
First-order kinetic curves of chlothianidin degradation as a function of irradiation time during photocatalysis in the presence of TiO2, TiO2 and O2, TiO2 and \({\text{NO}}_{3}^{ - }\), and TiO2 and HA, as well as inhibition of bioluminescence of V. fischeri bacteria in chlothianidin solutions after 0 and 120 min of photocatalytic irradiation in (a) deionized water and water with additives of (b) HA and (c) \({\text{NO}}_{3}^{ - }\).
The first-order kinetic constants depend on the experimental setup and on the conditions used. In our case, it was found that the most efficient photocatalysis left at least half of the initial compound in the mixture. The results suggest that chlothianidin photocatalysis is enhanced by the precence of oxygen, but is stopped in the presence of HA and/or \({\text{NO}}_{3}^{ - }\).
LC−MS investigation of formed by-products. Photocatalytical degradation in the presence of TiO2 of neonicotinoids, mainly imidacloprid, has been well studied in terms of efficieny and kinetics [18, 29], as well as and in terms of identification of photoinduced products [19, 30–32].
Clothianidin has been shown to be resistant to hydrolysis at environmental pH values temperatures. Besides, its metabolic degradation takes place very slowly in aerobic soil [7]. Mammals metabolize chlothianidin into several products: N-methyl-N '-nitroguanidine (MNG), N-(2-chloro-1,3-thiazol-5-ylmethyl)-N'-methylurea (TZMU), N-(2-chloro-1,3-thiazol-5-ylmethyl)-N'-nitroguanidine (TZNG), 2‑chloro-1,3-thiazole-5-carboxylic acid (MTCA), ((2-chloro-1,3-thiazole-5-yl)methyl)urea (TZU), N‑nitroguanidine (NTG), and methylguanidine (MG) [33, 34]. On the other hand, chlothianidin metabolism in plants has a predominant amount of one metabolite, N-(2-chloro-1,3-thiazol-5-yl)methyl)-N'-methylguanidine (TMG) [35]. In photocatalytic experiments with pure chlothianidin solutions in deionized water, two main products were identified, one previously known as TZMU and one unknown degradation product from the group of amidodiazenes [19]. But to the best of our knowledge, there was a lack of information regarding the photocatalysis with added known sensitizes, like HA and \({\text{NO}}_{3}^{ - }\). Photocatalytic experiments without additives and with the addition of HA and \({\text{NO}}_{3}^{ - }\) resulted in the formation of two main products—TZNG and MNG, whereas the product TZMU, previously identified by Žabar et al. [19], was detected only in the case of added of HA. In this study, one completely new product was also found. Considering the elemental composition of all the observed fragment ions (Fig. 2, Scheme 1) and the fragmentation patterns of other chlothianidin degradation products, we assumed the structure of N-[(2-chloro-1,3-thiazol-5-yl)methyl]-N '-formyl-N "-nitroguanidine (TANG), which formed only in the experiment with the addition of HA. For more precise identification a corresponding standard should be analyzed to prove the assumed structure. All identified products are presented in Table 1.
Toxicity.Figure 1 shows the proportion of inhibited Vibrio fischeri marine bacteria by initial and final chlothianidin samples irradiated for 120 min. All of the above chlothianidin degradation products were found in samples prepared in a similar way. It can be seen, that after 120 min of irradiation, samples containing chlothianidin ((58 ± 8)% of the initial concentration of chlothianidin) and its degradation products (MNG and TZNG) do not change their toxicity (from 2.9 ± 8.0 to 22.0 ± 0.8%). A similar toxicity result was obtained by the photocatalytic degradation of chlothianidin in the presence of \({\text{NO}}_{3}^{ - }\), where the same two decomposition products MNG and TZNG were identified, but no stimulating effect of \({\text{NO}}_{3}^{ - }\) was observed when the initial compound degraded (concentrations of chlothianidin from 103.3 ± 0.1% at the beginning to 92.5 ± 16% at the end of the experiment). The most interesting result was observed in the photocatalytic degradation of chlothianidin in the presence of HA: a decrease in the concentration of the initial compound, shown by HPLC (Fig. 1), was accompanied by halved bioluminescence inhibition (from 19.8 ± 0.5% to 1.4 ± 2.1%). According to our results, the role of HA as a photosensitizer resulted in the formation of several degradation products, which exhibit lower toxicity compared to photocatalytic products of chlothianidin in deionised water and water with added \({\text{NO}}_{3}^{ - }\). Since the concentration of chlothianidin did not decrease in photocatalytic irradiation with the addition of HA, it is assumed that HA act as antagonists with chlothianidin in the case of bacteria inhibition. This is a promising fact for the development of purification procedures of raw agricultural waters containing chlothianidin.

Scheme 1. Fragmentation scheme of the protonated molecule of the new chlothianidin degradation product .
CONCLUSIONS
The photocatalytical degradation of chlothianidin using TiO2 films in the presence of nitrate ions and humic acids turned out to be less effective than the photocatalytic decomposition of pure chlothianidin in oxygenated solutions. The photocatalytical degradation of chlothianidin in oxygenated solutions with added nitrates and humic acids resulted in the formation of two main decomposition products: N-[(2-chloro-1,3-thiazol-5-yl)methyl]-N'-nitroguanidine and N-methyl-N'-nitroguanidine. However, humic acids stimulated the formation of two additional products, one of which is the previously known N-[(2-chloro-1,3-thiazol-5-yl)methyl]-N '-methylurea, and another completely new product, identified as N-[(2-chloro-1,3-thiazol-5-yl)methyl]-N '-formyl-N "-nitroguanidine. The toxicity measurements before and after photocatalytical irradiation showed that only the presence of humic acids reduces the inhibition of bacteria, indicating their antagonistic effect in the photocatalytical chlothianidine degradation.
REFERENCES
El Hassani, A.K., Dacher, M., Gary, V., et al., Arch. Environ. Contam. Toxicol., 2008, vol. 54, no. 4, p. 653.
Tapparo, A., Giorio, C., Marzaro, M., et al., J. Environ. Monit., 2011, vol. 13, no. 6, p. 1564.
Blacquière, T., Smagghe, G., Van Gestel, C.A.M., and Moammaerts, V., Ecotoxicology, 2012, vol. 21, no. 4, p. 973.
Pohorecka, K., Skubida, p., Miszczak, A., et al., J. Apic. Sci., 2012, vol. 56, no. 2, p. 115.
Van der Sluijs, J.P., Simon-Delso, N., Goulson, D., et al., Curr. Opin. Environ. Sustainability, 2013, vol. 5, nos. 3–4, p. 293.
Brandt, A., Gorenflo, A., Siede, R., et al., J. Insect Physiol., 2016, vol. 86, p. 40.
EPA, United States Environmental Protection Agency. (2003) Name of chemical: Clothianidin, Reason for issuance: Conditional Registration, Date Issued: May 30, 2003. http://www.epa. gov/opprd001/factsheets/clothiandin.pdf. Accessed May 20, 2018.
Miranda, G.R.B., Raetano, C.G., Silva, E., et al., Hum. Ecol. Risk Assess., 2011, vol. 17, no. 4, p. 981.
Whiting, S.A., Strain, K.E., Campbell, L.A., et al., Sci. Total Environ., 2014, vol. 497, p. 534.
Goulson, D., J. Appl. Ecol., 2013, vol. 50, no. 4, p. 977.
Ramasubramanian, T., Water, Air, Soil Pollut., 2013, vol. 224, p. 1468.
Li, Y., Su, P., Li, Y., et al., Chemosphere, 2018, vol. 207, p. 708.
Commission Implementing Regulation (EU) 2018/784 of 29 May 2018 Amending Implementing Regulation (EU) no. 540/2011 as Regards the Conditions of Approval of the Active Substance Clothianidin (Text with EEA relevance). http://data.europa.eu/eli/reg_impl/2018/784/oj. Accessed May 20, 2018.
Handford, C.E., Elliott, C.T., and Campbell, K., Integr. Environ. Assess. Manage., 2015, vol. 4, no. 4, p. 525.
Lebedev, A.T., Poliakova, O.V., Karakhanova, N.K., et al., Sci.Total Environ., 1998, vol. 221, nos. 2–3, p. 153.
Burrows, H.D., Canle, M.L., Santaballa, J.A., et al., J. Photochem. Photobiol., B, 2002, vol. 67, no. 2, p. 71.
Kralj, B.M., Černigoj, U., Franko, M., et al., Water. Res. 2007, vol. 41, no. 19, p. 4504.
Černigoj, U., Lavrenčič Štangar, U., and Trebše, P., Appl. Catal., B, 2007, vol. 75, nos. 3–4, p. 229.
Žabar, R., Komel, T., Fabjan, J., et al., Chemosphere, 2012, vol. 89, no. 3, p. 293.
Remucal, C.K., Environ. Sci.: Processes Impacts, 2014, vol. 16, no. 4, p. 628.
Kete, M., Pavlica, E., Fresno, F., et al., Environ. Sci. Pollut. Res., 2014, vol. 21, no. 19, p. 11238.
Pattard, M. and Moser, H., Luminescent bacteria test, in Ecotoxicological Characterization of Waste, Moser, H. and Römbke, J., Eds., New York: Springer, 2009.
Morrissey, C.A., James, P.M., Devries, H., et al., Environ. Int., 2015, vol. 74, p. 291.
Zhang, P., Ren, Ch., Sun, H., et al., Sci. Total Environ., 2018, vol. 615, p. 59.
Sultana, T., Murray, Cr., Kleywegt, S., et al., Chemosphere, 2018, vol. 202, p. 506.
Lebedev, A.T., Eur. J. Mass Spectrom., 2007, vol. 13, no. 1, p. 51.
Trebse, P., Polyakova, O.V., Baranova, M., et al., Water Res., 2016, vol. 101, p. 95.
Kalister, K., Dolenc, D., Sarakha, M., et al., J. Anal. Chem., 2016, vol. 71, no. 14, p. 1289.
Guzsvány, V., Banić, N., Papp, Z., et al., React. Kinet., Mech. Catal., 2010, vol. 99, no. 1, p. 225.
Wamhoff, H. and Schneider, V., J. Agric. Food. Chem., 1999, vol. 47, no. 4, p. 1730.
Agüera, A., Almansa, E., Malato, S., et al., Analusis, 1998, vol. 26, no. 7, p. 245.
Malato, S., Caceres, J., Aguera, A., et al., Environ. Sci. Technol., 2001, vol. 35, no. 10, p. 4359.
Yokota, T., Mikata, K., Nagasaki, H., et al., J. Agric. Food. Chem., 2003, vol. 51, no. 24, p. 7066.
Ford, K.A. and Casida, J.E., Res. Toxicol., 2006, vol. 19, no.11, p. 1549.
Sánchez-Hernández, L., Hernández-Domínguez, D., Martín, M.T., et al., J. Chromatogr A, 2016, vol. 1428, p. 220.
ACKNOWLEDGMENTS
The work was carried out within the framework of bilateral scientific cooperation between Slovenia and Russia.
Funding
This study was supported by the Slovenian Research Agency, research core funding no. P3-0388, Mechanisms of health protection (Mehanizmi varovanja zsravja) and Scholarship of the Republic of Slovenia scheme Mobility Grant (CMEPIUS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by V. Kudrinskaya
Rights and permissions
About this article
Cite this article
Kralj, M.B., Dilcan, E.G., Salihoğlu, G. et al. Photocatalytic Degradation of Chlothianidin: Effect of Humic Acids, Nitrates, and Oxygen. J Anal Chem 74, 1371–1377 (2019). https://doi.org/10.1134/S1061934819140077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819140077