Abstract
This paper describes the development and utilization of a new nanocomposite consisting of amine-functionalized TiO2/multi-walled carbon nanotubes and sodium dodecylsulfate for glassy carbon electrode modification. The nanocomposite was characterized by Fourier transform infrared spectroscopy, transmission electron microscopy and scanning electron microscopy. The modified electrode was used for electrochemical characterization of olanzapine (OLZ). The efficiency of modified electrode for electrocatalytic oxidation of OLZ was studied by cyclic voltammetry and square wave voltammetry in phosphate buffer solution (pH 7.0). Using square wave voltammetry, the prepared sensor showed good sensitivity and selectivity with low overpotential for the determination of OLZ in the ranges from 0.05 to 0.1 and 0.1 to 10 μM, with a detection limit of 8 nM. The proposed method was employed for the determination of OLZ in tablet and blood serum samples without any pretreatment steps.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Among analytical methods, electrochemical techniques have been the most widely applied because of high sensitivity, simplicity, reproducibility as well as abilities to be miniaturized. Carbon electrodes are widely applied in electroanalysis due to their low background current, broad potential window, chemical inertness, low cost and compatibility for diverse sensing and detection application [1].
Since surface modification is a significant area of study in modern electrochemistry, different application possibilities of the chemically modified electrodes are of concern. Introduction of surface-active agents (surfactants) in this zone of work adds a new and beneficial dimension to the researches. Adsorption of surfactants at the electrode/solution interface as well as solubilization of electrochemically active compounds into micelle aggregates may significantly change and control the attributes of electrode surfaces, heavily affecting the electrochemical process of electroactive species [2]. Analytically, surfactants have also been widely used to improve the sensitivity and selectivity of the voltammetric measurements of organic compounds of environmental and clinical interest [3–9].
On the other hand, the usage of carbon nanotubes (CNTs) modified electrodes is well known due to the fundamental properties of CNTs to mediate electron transfer reactions with electroactive species in solutions [10, 11]. Recent studies have demonstrated that CNTs can increase the electrochemical reactivity of significant biomolecules and can promote the electron-transfer reactions of proteins [12, 13], as well facilitate the determination of important biomolecules [14, 15], pharmacological materials [16] and inorganic ions [17] with the outstanding antifouling properties [18]. To take advantages of the significant properties of CNTs in electrochemical sensing applications, CNTs need to be properly functionalized and immobilized [11, 19]. TiO2 is an important material for electrochemical, photocatalytic, electronic, photovoltaic, biomedical and cosmetic applications [20–28]. There is a growing concern in the development of TiO2–carbon nanotube nanocomposites with improved electronic conductivity for solar cells, sensors and catalytic devices [29, 30].
Olanzapine (Scheme 1) is an antipsychotic drug recently offered in therapy. In fact, it appears to be efficient against both positive (hallucinations, delusions) and negative (poverty of speech, social withdrawal) symptoms of schizophrenia [31, 32], whereas ‘traditional’ antipsychotic drugs (e.g., phenothiazines, butyrophenones) are alone effective in the therapy of positive symptoms of this illness. Due to its far-reaching use, fast, effective and economical procedures for the determination of OLZ in pharmaceutical formulations and biological compounds are required. Therefore, electrochemical techniques appear to be as valuable as chromatographic ones and are a good alternative to the latter.
A survey of literature revealed that no electrochemical data were available concerning the voltammetric behavior of OLZ in the presence of surfactants. Keeping the above knowledge in mind, in this article, as a continuation of our previous study [33], the electrochemical determination of OLZ by a sensitive electrode composed of amine-functionalized TiO2, multi-walled carbon nanotubes, surfactant, paraffin oil, as binder, and glassy carbon electrode (GCE) was investigated. The main purpose was to give special attention on the coupling of adsorptive transfer stripping voltammetry with the unique properties of the in situ surfactant-modified amine-functionalized TiO2/multi-walled carbon nanotubes (ISS-NH2-TiO2-MWCNTs/GCE) for the development and optimization of a sensitive and rapid method for the determination of OLZ in aqueous solutions utilizing the enhancement effect of the anionic surfactant. The practical use of the method was demonstrated by measuring the concentration of OLZ in samples of spiked human serum and tablets.

Scheme 1 . Chemical structure of olanzapine.
EXPERIMENTAL
Reagents and solutions. The MWCNTs (>95%) and 3-aminopropyl-(diethoxy)-methylsilane (97%) were purchased from Nanostartech (Tehran, Iran) and Aldrich (Milwaukee, WI), respectively. The compound OLZ (C17H20N4S) was purchased from Sobhan Darou (Rasht, Iran). Isopropyl alcohol and titanium(IV) isopropoxide were from Fluka (Buchs, Switzerland). The types of surfactants tested were anionic (sodium dodecylsulfate, SDS), cationic (cetyltrimethylammonium bromide, CTAB) and non-ionic (Triton X-100, TX-100). Surfactants, all reagents and solvents were of the highest purity available from Merck (Darmstadt, Germany) and used without further purification. Phosphate buffer solution (PBS, 0.1 M, pH 7.0) was prepared by mixing solutions of 0.1 M Na2HPO4 ⋅ 12H2O and 0.1 M NaH2PO4 ⋅ H2O. Aqueous solutions of OLZ were prepared fresh at the time of experiments in PBS (pH 7.0). All the solutions were prepared using double distilled water.
Apparatus. All voltammetric measurements were carried out using a µAutolab electrochemical system (Eco-Chemie, Utrecht, The Netherlands) equipped with Nova software (Eco-Chemie, Utrecht, The Netherlands). A conventional three-electrode system was used, including a working modified electrode, a saturated Ag/AgCl reference electrode, and a platinum wire counter electrode. All the pH values were measured with a Metrohm pH meter (model 827, Switzerland). The morphology and particle size of NH2-TiO2-MWCNTs were characterized as described previously in [33].
Electrode preparation. 10 mg of graphite powder (~325 mesh, >99.99%, Sigma-Aldrich) was added to a certain amount of NH2-TiO2-MWCNTs and then the mixture was dispersed into 0.4 mL of dimethylformamide with ultrasonic treatment for 20 min to get a homogenous dispersion. Then 5 µL of the suspension was dropped onto the surface of a clean GCE (2 mm diameter, Metrohm) and let dry at room temperature to obtain the modified electrode. In situ modification of the electrode was done by dipping the NH2-TiO2-MWCNTs/GCE in 0.1 mM SDS solution for 180 s.
RESULTS AND DISCUSSION
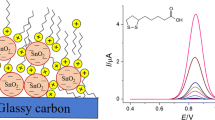
Cyclic voltammetric characterization of modified electrodes. Cyclic voltammograms of OLZ at the bare GCE, NH2-TiO2-MWCNTs/GCE and ISS-NH2-TiO2-MWCNTs/GCE are given in Fig. 1. The anodic and cathodic peak currents of OLZ at the NH2-TiO2-MWCNTs/GCE are much greater than at the GCE. On the other hand, oxidation of OLZ at the NH2-TiO2-MWCNTs/GCE occurs at less positive potentials. The ISS-NH2-TiO2-MWCNTs/GCE also showed a remarkable sensitivity compared to the GCE and NH2-TiO2-MWCNTs/GCE, which implies that the redox reaction of OLZ molecules becomes more facile on the ISS-NH2-TiO2-MWCNTs/GCE (Fig. 1, curve 4 for TX-100, curve 5 for SDS). In addition, the cyclic voltammogram of OLZ shows two anodic peaks at the modified electrodes. The advent of a small peak at more positive potentials is probably due to re-oxidation of oxidized OLZ. The first peak was selected for the rest of the experiments because of lower anodic overpotential for the oxidation of OLZ and higher anodic peak current.
Effect of surfactant. Surfactants have an influence on the electrochemical processes of electroactive species [34, 35] and thus are widely used in electroanalytical chemistry to improve the sensitivity and selectivity [36–38]. Adsorption of surfactant aggregates on the electrode surface through hydrophobic interplay might significantly facilitate the electron transfer, shift the redox potentials or charge transfer coefficients or diffusion factors by stabilizing radical ions and other reaction intermediates and also by modifying the double layer structure [39].
The effects on the peak currents of OLZ using two ionic surfactants, such as CTAB and SDS, and a non-ionic surfactant, such as TX-100 as an in situ modifier, were investigated. Voltammograms obtained at pH 7.0 showed that CTAB decreased the redox peaks of OLZ, whereas the sensitivity of the NH2-TiO2-MWCNTs/GCE was increased using SDS and TX-100 (Fig. 1).
Olanzapine exists as a positively charged form under the studied pH condition (pKa = 7.4) [40]. Therefore, after employing the cationic surfactant, its redox peaks decreased due to the repulsive coulombic forces between the positively charged OLZ and positively charged ammonium group of CTAB that ban the aggregation of the drug molecules at the electrode surface. On the other hand, adsorption of the SDS micelles onto electrode surface shapes a negatively charged hydrophilic film oriented to the water bulk phase. Based on this principle, positively charged OLZ has a trend to accumulate in the negatively charged crown of anionic SDS micelles, which may lower the over potential of the electrode and increase the electron transfer rate. The sensitivity of the SDS-in situ modified electrode was about 5 times higher than that of the NH2-TiO2-MWCNTs/GCE for anodic current. The observed increment in peak currents in the attendance of TX-100 could be due to organization of a thin layer on the electrode surface into which the analyte molecules were preconcentrated. This phenomenon increased the possibility of electron transfer between the electrode and OLZ molecules. However, in this case coulombic forces which lead to efficient aggregation of OLZ with the adsorbed SDS were stronger than any other interactions. Therefore, SDS was selected as the in situ modifier surfactant for further studies (Fig. 1).
Optimization of surfactant solution parameters.Effect of the surfactant amount. The peak currents of OLZ were highly dependent on the concentration of SDS and in situ modification time of the electrode. While gradually enhancing the concentration of SDS from 1 × 10–5 M, the peak currents increased favorably up to the concentration of 1 × 10–4 M; above this concentration, the peak currents were decreased. Therefore, SDS was employed at what was considered the best concentration of 1 × 10–4 M (Fig. 2).
The in situ modification time was investigated in the range of 30–210 s. The anodic peak current intensity was found to increase with increasing in situ modification time up to 180 s. With further increase in the in situ modification time, there was no significant increase in the current response. This is probably due to the saturation of the NH2-TiO2-MWCNTs/GCE surface by adsorption of SDS. Hence, 180 s was chosen as the optimum in situ modification time for the NH2-TiO2-MWCNTs/GCE (data not shown).
The effect of surfactant on the surface area of the modified electrode. The effective surface area of the ISS-NH2-TiO2-MWCNTs/GCE can be determined using the [Fe(CN)6]3–/4– redox system and applying the Randles-Sevcik equation for a reversible process [41]:
where Ip is the redox current peak, n is the total number of electron transferred (n = 1), c* is the bulk concentration of the redox probe (5 mM [Fe(CN)6]3–/4–), ν is the scan rate (V/s), D is the diffusion coefficient of probe (7.6 × 10–6 cm2/s), A is the effective surface area of the electrode (cm2). The electrochemically effective surface areas of the GCE, NH2-TiO2-MWCNTs/ GCE and ISS-NH2-TiO2-MWCNTs/GCE were calculated to be 0.02, 0.09 and 0.159 cm2 respectively.
Effect of solution pH. It is significant to examine the effects of pH on electrochemical systems, because pH is one of the variables which mainly and strongly influences the current and shape of voltammograms. The influence of the solution pH on the electrocatalytic oxidation of OLZ (20 µM) was investigated in the pH range of 4–7.5 employing PBS (Fig. 3). As can be seen, the anodic peak current (Ipa) reached its maximum value at two pHs, 6.5 and 7.0. Because pH 7.0 was the physiological pH value and the oxidation potential was lower, it was employed in this study.
The anodic peak potential (Epa) shifts to less positive potentials when solution pH increases with the equation of Epa(V) = 0.6782 – 0.0649 pH and regression coefficient (R2) of 0.990. The slope of the plot is close to 0.0585 V/pH at the Nernst equation. Therefore, the number of electrons and protons involved in the reaction mechanism is equal. To find the number of involved electrons, surface coverage formula and Laviron equation were used [1]
where F is the Faraday number (C/mol), A is the elec-trode surface area (cm2), R is the gases’ constant (J/(K mol)), n is the number of involved electrons in the oxidation process of OLZ, T is the system temperature (K), and Γ is the surface coverage (mol/cm2) and is calculated by the following formula:
where Q is the peak area (C) and other variables were mentioned before. With the replacement of Eq. (3) in Eq. (2), the electron transfer number was calculated to be 1.92 (ca. 2). Therefore, the electrochemical oxidation of OLZ is a two-proton and two electron transfer process.
Effect of scan rate. The effect of scan rates on the peak currents at the ISS-NH2-TiO2-MWCNTs/GCE in PBS (pH 7.0) was investigated by cyclic voltammetry in the presence of 20 µM OLZ. As shown in Fig. 4, at the scan rates in the range of 0.01 to 0.2 V/s, the anodic peak currents of OLZ increase linearly with the scan rates. The linear equation of OLZ is Ipa(μA) = 98.9(±1.3)V (V/s) – 0.2(±0.0); R2 = 0.991 (Fig. 4, inset). This indicates that the oxidation of OLZ is controlled by adsorption.
In addition, the anodic peak potential shifted to positive potentials as the scan rate was increased. The anodic potentials are found to be linearly dependent on logν at the scan rates between 0.04 and 0.15 V/s. The straight line was obtained with regression equation Epa(V) = 0.0447 log V + 0.2781; R2 = 0.990. According to Laviron’s model [42], plotting the Epa vs. logV yields a straight line with the slope of 2.3RT/(1 − α)nF. From the value of the slope, the electron transfer coefficient (α) was estimated to be 0.34. According to the Laviron equation:
the Ks was calculated to be 0.986 s−1. The results represent that the ISS-NH2-TiO2-MWCNTs as a modifier, considerably facilitating the electron transfer kinetics and assisting the electrochemical oxidation of OLZ.
Effect of accumulation potential and accumulation time. Accumulation step is usually a simple and effective way to raise the determination sensitivity. Therefore, the accumulation time (tacc) and accumulation potential (Eacc) of OLZ in the surface of modified electrode were investigated. When the accumulation time increased from 0 to 60 s, the oxidation peak current of 30 µM OLZ increased gradually and reached the maximum current response at 60 s. With further increase in the accumulation time, there was no considerable increase in the current response. This may be caused by the fact that adsorption of OLZ on the ISS-NH2-TiO2-MWCNTs/GCE surface becomes saturated and the number of active sites on the electrode is reduced. So, the accumulation time of 60 s was used for each voltammetric measurement of OLZ. When accumulation potential varied from –0.5 to 0.0 V, the maximum voltammetric response was obtained at –0.4 V. Herein, –0.4 V was selected as the optimum accumulation potential.
Calibration curve, stability and reproducibility. The electrochemical sensing performance of the ISS-NH2-TiO2-MWCNTs/GCE toward OLZ was investigated by square wave voltammetry (SWV) under the optimized conditions. It can be seen in Fig. 5 that with the increase in the OLZ concentration, the SWV oxidation peak increased. When the concentration of OLZ changes from 0.05 to 10 µM, the calibration curve for OLZ shows two linear working ranges (LWR): the first linear range is from 0.05 to 0.1 μM with the regression equation of Ip(μA) = −0.1134 + 9.2373c (μM) (R2 = 0.984), and the second linear range increases up to 10 μM with the linear regression equation of Ip(μA) = 0.524 + 0.799c (μM) (R2 = 0.995). The limit of detection (LOD) of the proposed modified electrode which is defined as the concentration of the sample yielding a signal identical to the blank signal three times of its standard deviation was estimated to be 0.008 μM. Also, the relative standard deviation (RSD) of the ISS-NH2-TiO2-MWCNTs/ GCE for five measurements of 0.5 μM OLZ was 1.42%, indicating good reproducibility of the modified electrode. Stability of the sensors is an important factor that is a proof of their performance. In order to investigate this factor, when the modified electrode was kept on air at a room temperature for two weeks, no apparent change was found toward OLZ with similar concentration and retained more than 87.3% of the initial response. It was suggested that the proposed sensor possessed acceptable storage stability. A comparison between the analytical efficiency of the present modified electrode and some prior literature electrodes for the determination of OLZ is given in Table 1. As can be seen, wide linear ranges with a very low LOD observed for the proposed electrode are remarkably better than those previously reported for OLZ.
Square wave voltammograms obtained for the oxidation of olanzapine at different concentrations of: 0.05, 0.07, 0.09, 0.1, 0.5, 2, 3, 4, 5, 7, 9, and 10 µM. Inset: The relation between the anodic peak currents and the concentrations of olanzapine. Scan rate: 0.1 V/s; accumulation time: 60 s (–0.4 V); step potential height: 5 mV; modulation amplitude: 50 mV; frequency: 100 Hz.
Method selectivity. Selectivity studies were carried out because psychiatric patients are often subjected to treatment with multiple CNS drugs which can potentially interfere with the analytical determination of olanzapine. The drugs tested for interference were other antipsychotics, antidepressants and benzodiazepines. Eventual interferences of these compounds and some other species in a solution containing 6 µM OLZ in PBS (pH 7.0) were tested by SWV. The tolerance limit was taken as the concentration of the foreign substances that caused an approximately ±10% relative error in the determination of OLZ. The results showed that the presence of K+, Br−, Cu2+, \({\text{NO}}_{3}^{-}{\text{,}}\) Na+, Cl−, glucose, cysteine, uric acid, folic acid, ascorbic acid and tested drugs had no significant influence on the height of the peak current (Table 2).
Application. In order to represent the applicability of the proposed method to the analysis of real samples, determination of OLZ in human blood serum and commercial tablets was demonstrated. The samples were prepared as described in [33]. The oxidation peak of OLZ at the proposed modified electrode was observed at 0.22 V. The human blood sample was then spiked with known concentrations of OLZ. The voltammograms clearly depict that the peak currents increase significantly for the peak at 0.22 V thereby confirming that it corresponds to the oxidation of OLZ. The analytical results are summarized in Table 3. The recovery was from 95.7 to 96.2% and RSD (n = 5) was 2.59% for human blood serum and 2.08% for OLZ tablet. The results show that there is a satisfactory agreement between the declared analyte content and the determined value. Therefore, the ISS-NH2-TiO2-MWCNTs/GCE can be effectively used for the determination of OLZ in commercial and human blood serum samples.
CONCLUSIONS
The results obtained demonstrate the synergistic effect of SDS and NH2-TiO2-MWCNTs modified GCE on the voltammetric determination of OLZ. The modified electrode decreased anodic overpotential for the oxidation of OLZ and increased the anodic peak current. Due to the unique properties of the ISS-NH2-TiO2-MWCNTs/GCE, such as large surface area, numerous active sites and stable electronic properties, the modified electrode showed significant surface enhancement effects on the electrochemical behavior of OLZ. The catalytic peak currents obtained using SWV are linearly dependent on the OLZ concentrations with two linear segments. This electrode presents advantages of easy fabrication, low LOD and high sensitivity. This method can be employed for the determination of OLZ in pharmaceutical formulations and blood serum samples without the necessity for samples pretreatment or any time-consuming extraction prior to the analysis. The lack of need for pretreatment showed an improvement in application features of the procedure to the determination of OLZ compared at least to those reported in the literature using different electrochemical techniques.
REFERENCES
Wang, J., Analytical Electrochemistry, Hoboken, NJ: Wiley, 2006, 3rd ed.
Vittal, R., Gomathi, H., and Kim, K.J., Adv. Colloid Interface, 2006, vol. 119, p. 55.
Li, C., Bioelectrochemistry, 2007, vol. 70, p. 263.
Blanco-López, M.C., Lobo-Castañón, M.J., Miranda-Ordieres, A.J., and Tuñón-Blanco, P., Electroanalysis, 2007, vol. 19, p. 207.
Jain, R., Dwivedi, A., and Mishra, R., Langmuir, 2009, vol. 25, p. 10 364.
Levent, A., Yardim, Y., and Senturk, Z., Electrochim. Acta, 2009, vol. 55, p. 190.
de Araújo, T.A., Marcos Jacques Barbosa, A., Viana, L.H., and Sousa Ferreira, V., Colloids Surf., B, 2010, vol. 79, p. 409.
Jain, R., Yadav, R.K., and Rather, J.A., Colloids Surf., A, 2010, vol. 366, p. 63.
Yi, H. and Li, C., Russ. J. Electrochem., 2007, vol. 43, p. 1377.
Zhao, Q., Gan, Z., and Zhuang, Q., Electroanalysis, 2002, vol. 14, p. 1609.
Wang, J., Electroanalysis, 2005, vol. 17, p. 7.
Gooding, J.J., Wibowo, R., Liu, J.Q., Yang, W., Losic, D., Orbons, S., Mearns, F.J., Shapter, J.G., and Hibbert, D.B., J. Am. Chem. Soc., 2003, vol. 125, p. 9006.
Yu, X., Chattopadhyay, D., Galeska, I., Papadimitrakopoulos, F., and Rusling, J.F., Electrochem. Commun., 2003, vol. 5, p. 408.
Fei, S., Chen, J., Yao, S., Deng, G., He, D., and Kuang, Y., Anal. Biochem., 2005, vol. 39, p. 29.
Jacobs, C.B., Peairs, M.J., and Venton, B.J., Anal. Chim. Acta, 2010, vol. 662, p. 105.
Xiao, F., Zhao, F., Li, J., Yan, R., Yu, J., and Zeng, B., Anal. Chim. Acta, 2007, vol. 596, p. 79.
Guo, M., Chen, J., Li, J., Tao, B., and Yao, S., Anal. Chim. Acta, 2005, vol. 532, p. 71.
Musameh, M., Wang, J., Merkoçi, A., and Lin, Y., Electrochem. Commun., 2002, vol. 4, p. 743.
Qureshi, A., Kang, W.P., Davidson, J.L., and Gurbuz, Y., Diamond Relat. Mater., 2009, vol. 18, p. 1401.
Yang, Z., Choi, D., Kerisit, S., Rosso, K.M., Wang, D., Zhang, J., Graff, G., and Liu, J., J. Power Sources, 2009, vol. 192, p. 588.
Shi, M., Shen, J., Ma, H., Li, Z., Lu, X., Li, N., and Ye, M., Colloids Surf., A, 2012, vol. 405, p. 30.
Ma, F., Shi, T., Gao, J., Chen, L., Guo, W., Guo, Y., and Wang, S., Colloids Surf., A, 2012, vol. 401, p. 116.
Liu, G., Jian, W., Jin, H., Shi, Z., and Qiao, G., Mater. Lett., 2011, vol. 65, p. 3468.
Kolodiazhnyi, T., Annino, G., Spreitzer, M., Taniguchi, T., Freer, R., Azough, F., Panariello, A., and Fitzpatrick, W., Acta Mater., 2009, vol. 57, p. 3402.
Li, Y., Hagen, J., Schaffrath, W., Otschik, P., and Haarer, D., Sol. Energy Mater. Sol. Cells, 1999, vol. 56, p. 167.
Macwan, D.P., Dave, P.N., and Chaturvedi, S., J. Mater. Sci., 2011, vol. 46, p. 3669.
Boccaccini, A.R. and Zhitomirsky, I., Curr. Opin. Solid State Mater. Sci., 2002, vol. 6, p. 251.
Shi, L., Shan, J., Ju, Y., Aikens, P., and Prud’homme, R.K., Colloids Surf., A, 2012, vol. 396, p. 122.
Sampaio, M.J., Silva, C.G., Marques, R.R.N., Silva, A.M.T., and Faria, J.L., Catal. Today, 2011, vol. 161, p. 91.
Liu, H., Ma, H., Zhou, W., Liu, W., Jie, Z., and Li, X., Appl. Surf. Sci., 2012, vol. 258, p. 1991.
Beasley, C.M., Jr., Tollefson, G., Tran, P., Satterlee, W., Sanger, T., and Hamilton, S., Neuropsychopharmacology, 1996, vol. 14, p. 111.
Gerlach, J. and Peacock, L., Int. Clin. Psychopharmacol., 1995, vol. 10, p. 39.
Arvand, M. and Palizkar, B., Mater. Sci. Eng., C, 2013, vol. 33, p. 4876.
Rusling, J.F. and Nassar, A.F., J. Am. Chem. Soc., 1993, vol. 115, p. 11 891.
Jain, R., Radhapyari, K., and Jadon, N., J. Electrochem. Soc., 2008, vol. 155, p. 104.
Jain, R., Mishra, R., and Dwivedi, A., Colloids Surf., A, 2009, vol. 337, p. 74.
Jain, R., Dwivedi, A., and Mishra, R., J. Colloid Interface Sci., 2008, vol. 318, p. 296.
Reis, A.P., Tarley, C.R.T., Maniasso, N., and Kubota, L.T., Talanta, 2005, vol. 67, p. 829.
Atta, N.F., Darwish, S.A., Khalil, S.E., and Galal, A., Talanta, 2007, p. 1438.
Nielsen, M.K. and Johansen, S.S., J. Anal. Toxicol., 2009, vol. 33, p. 212.
Goyal, R.N., Gupta, V.K., and Chatterjee, S., Sens. Actuators, B, 2010, vol. 149, p. 252.
Fan, Y., Huang, K.J., Niu, D.J., Yang, C.P., and Jing, Q.S., Electrochim. Acta, 2011, vol. 56, p. 4685.
Merli, D., Dondi, D., Pesavento, M., and Profumo, A., J. Electroanal. Chem., 2012, vol. 683, p. 103.
Funding
The authors are thankful to the post-graduate office of Guilan University for the support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Majid Arvand, Akram Pourhabib Surfactant-Assisted Voltammetric Determination of Olanzapine at Amine Functionalized TiO2/Multi-Walled Carbon Nanotubes Nanocomposite. J Anal Chem 74, 1096–1103 (2019). https://doi.org/10.1134/S1061934819110030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819110030