Abstract
A model approach is developed, which enables one, using the concentration dependences of the diffusion permeability for individual layers, to estimate the density of a diffusion flux through a bilayer membrane, determine the concentration of a virtual solution at the interface between the membrane layers, and find the thickness of each layer that has constant diffusion characteristics. The approach is verified by the example of a perfluorinated MF-4SC membrane, with its surface being modified with polyaniline under different conditions. The adequacy of the approach is confirmed by a satisfactory coincidence of the modified layer thicknesses calculated in terms of the model and found by the optical examination of membrane sections.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Ion-exchange membranes with asymmetric properties have attracted the attention of researchers, because their transport properties may be controlled by varying the characteristics and thicknesses of individual membrane layers. Polymer films with anisotropic structures are produced both by the joining of two different layers, e.g., by pressing two membranes, and by depositing a modifying layer onto the surface of a commercial membrane [1–4]. Polyaniline is a promising modifier for the production of membranes with anisotropic structures owing to its ability to form a barrier layer on a membrane surface. This leads to a decrease in the diffusion permeability and the appearance of asymmetric properties of a membrane [5], as well as a reduction in its electroosmotic permeability [6]. The aforementioned combination of membrane properties makes it possible to substantially increase the degree of concentrating electrolyte solutions by electrodialysis [7].
The theoretical description of the transport properties of bilayer membranes, including their diffusion permeability, with the purpose of the prediction these properties is an important problem. As a rule, the properties of anisotropic membranes are simulated under the assumption that they have bi- or multilayer structures with distinct or diffuse interlayer interfaces. However, the continuity of the concentration profiles at the interfaces is the necessary condition.
One approach is based on the description of the ion transport through a bi- or multilayer membrane with the use of the Nernst–Planck equation [8, 9]. When one of the layers is rather thin, its effect on the membrane characteristics is taken into account in the Nernst–Planck equation using a phenomenological conductivity coefficient [10]. This approach enables one to estimate not only the transport characteristics of an ion-exchange membrane, but also the concentration profiles in the membrane system.
Another approach is the use of the fine-porous membrane model [11], according to which a membrane consists of two distinctly bounded layers with specified thicknesses, while the asymmetry in the transport characteristics is governed by the different volume charge densities of the layers. The characteristics of the layers are determined by fitting calculated values to experimental data.
An approach proposed in [12] for describing the electrical mass transfer in bilayer ion-exchange membranes is based on the concept of a virtual solution and takes into account structural features of the layers. The authors quantitatively determined the asymmetry effects in the integral diffusion permeability coefficients for bilayer membranes of different structural types and revealed that this approach adequately described these effects for membranes with distinct interfaces between the layers and stable characteristics of the latter, e.g., for reverse-osmosis and ultrafiltration membranes. However, in the case of anisotropic membranes, which were modified with organic surfactants and had no distinct interfaces, the theoretically calculated and experimental asymmetry coefficients of diffusion permeability did not coincide with one another. The authors related this discrepancy to the diffused character of the interfaces between the membrane layers, which causes uncertainty in the choice of the thicknesses of the layers and complicates the formation of fixed concentration profiles in them.
The aim of this study was to develop a model approach that would enable us to use the concentration dependences of the diffusion permeabilities of individual layers for determining not only the value of the diffusion flux through a bilayer membrane and the concentration of a virtual solution at the interlayer interface, but also the thickness of each layer having constant diffusion characteristics.
THEORY
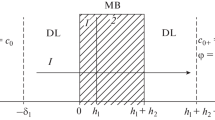
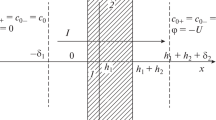
Let us consider a membrane system analogous to that studied in [12] (Fig. 1). In this system, the sides of a bilayer membrane are brought in contact with electrolyte solutions having concentrations C1 and C2 (С1 > С2), with the solutions being intensively stirred to exclude the effects of the diffusion layers at the membrane/solution interfaces on the electrolyte diffusion through the membrane. In the course of the diffusion transfer, the concentration (\(C_{0}^{'}\) and \(C_{0}^{"}\)) of a virtual solution at the interlayer interface is continuous and dependent on the orientation of the membrane relative to the electrolyte flux; therewith, \(C_{0}^{'}\)\( \ne \)\(C_{0}^{"}.\)
The membrane consists of two layers with different properties (parameters bi and βi, where i = 1 or 2) and thicknesses l1 and l2, respectively. In the range of diluted and moderately concentrated solutions (lower than 1 M), the aforementioned parameters are described by the following equation [13]:
where j, which denotes the density of the diffusion flux (mol m–2 s–1), and С, which indicates the dimensionless concentration equal to the ratio between the real concentration of a solution and an auxiliary concentration of 1 M, are constants for a specific membrane. They are determined by processing the concentration dependence of the diffusion flux through the membrane to water (С2 = 0) and represented in bilogarithmic coordinates log j–log C. Parameter β, which is equal to the slope of the aforementioned linear dependence, characterizes the shape of the concentration profile inside the membrane: at β > 1, the profile is convex; at β < 1, it is concave; at β = 1, it is linear [13]. Parameter b (mol m–2 s–1) characterizes the set of membrane properties, which are implicit and affect the diffusion process. This parameter may also be determined by processing the concentration dependences of the diffusion flux density represented in the log j–log C coordinates.
It is known that density j of an electrolyte diffusion flux through a membrane and differential coefficient of its diffusion permeability P* are related to one another by a linear dependence that follows from Fick’s first law:
where l is the membrane thickness.
According to Eq. (2), the densities of the electrolyte fluxes through individual layers of a bilayer membrane oriented with its unmodified side facing the flux (Fig. 1a) are equal to
Since the diffusion transfer in each layer is determined by not only its properties, but also the concentration of the virtual solution at the interlayer interface, for the opposite orientation of the bilayer membrane in a measuring cell (Fig. 1b), j' is replaced by j":
The integral coefficient of diffusion permeability P may be determined from experimental data using the equation
Taking into account the equation relating the differential and integral coefficients of diffusion permeability [14],
and the condition for the continuity of the virtual solution concentration at the interlayer interface, the authors of work [12] obtained an analytical solution of integral equations (3)–(6) in the form of Eqs. (9)–(12):
Under the conditions of a stationary diffusion transfer, the density of the electrolyte flux through a membrane and the densities of the fluxes through its individual layers are equal at a fixed orientation of the membrane relative to the flux direction. Therefore, this approach makes it possible to calculate the electrolyte concentration at the internal interlayer interface from the know electrolyte concentrations on both sides of the membrane and parameters bi and βi for each layer. In the course of solution, concentration C0 of the solution at the interface between the layers is fitted so that the fluxes through both individual layer are equal.
It should be noted that Eqs. (9)–(12) do not contain layer thicknesses l1 and l2 in an explicit form. Their influence is taken into account indirectly when calculating the differential coefficient of diffusion permeability by Eq. (8) from the experimental data on the electrolyte diffusion through the membrane to water.
Figure 2а shows the concentration dependences for the densities of HCl diffusion fluxes through MF-4SC membranes of different thicknesses. It can be seen that the flux density is inversely proportional to the membrane thickness. A similar effect was previously noted by the authors of work [15] in their theoretical analysis of the diffusion permeabilities of membranes for diverse electrolytes. Figure 2b demonstrates the same concentration dependences in bilogarithmic coordinates, which enable us to determine parameters b and β according to Eq. (1). As follows from the equations of the straight lines presented in Fig. 2b, the values of parameter β are close to each other, whereas those of parameter b differ from each other by a factor of nearly 1.5 and amount to 2.26 × 10–9 and 3.77 × 10–9 mol m–2 s–1 for samples with thicknesses of 0.226 and 0.145 mm, respectively.
In this work, we have assumed that the value of parameter b depends on the membrane thickness as follows:
where d is an empirical coefficient, the physical meaning of which corresponds to the density of the diffusion flux through a membrane of unit thickness at unit concentration; hence, this coefficient characterizes the properties of a material. The assessment of the values of d for the samples represented in Fig. 2 has shown that they are close to each other and equal to 5.4 and 5.1 mol m–1 s–1 for the samples with thicknesses of 0.226 and 0.145 mm, respectively, thereby confirming the validity of our assumption.
Substituting Eq. (13) into Eq. (8) and replacing l1 and l2 by (1–q)l and ql, where q is a parameter that characterizes the contribution of the modified layer thickness to total membrane thickness l, we arrive at the following expressions:
In this case, when calculating the density of the diffusion flux through a bilayer membrane, both the concentration of a virtual solution at the interface between the membrane layers and the fraction of the modified layer serve as fitting parameters. Upon diffusion of an electrolyte to water (C2 = 0), Eqs. (14) and (16) are simplified to the following form:
Thus, the developed model approach makes it possible not only to calculate the value of the density of a diffusion flux through a bilayer membrane and estimate concentration C0 of a virtual solution at the internal interface, but also to determine the thicknesses of the individual layers.
EXPERIMENTAL
To verify the proposed approach, a perfluorinated MF-4SC ion-exchange membrane (OAO Plastpolymer, St. Petersburg) modified with polyaniline (PANI) was used as an object of study. PANI was synthesized on one of the membrane surfaces (MF-4SC/PANI-I) using the method of the successive diffusion of concentrated solutions of the monomer (aniline hydrochloride) and an oxidant (ammonium persulfate) through the membrane to water [16]. The synthesis duration was 25 min. To obtain a sample with diffusion characteristics identical to those of the modified layer, a sample modified throughout its volume (MF-4SC/PANI-V) was prepared via the synthesis of PANI under the same conditions on both sides of the MF-4SC membrane.
More diluted working solutions of the monomer and oxidant were used to prepare bilayer membranes with different thicknesses of the modified layer, while varying the modification time from 1 to 3 h [5]. As a result, a series of samples MF-4SC/PANI-II-х, where х is the modification time (h), were obtained. After the modification, the membranes were washed with water and equilibrated with electrolyte solutions of preset concentrations.
The diffusion permeability of the samples was examined in sodium chloride and hydrochloric acid solutions upon the diffusion of the electrolytes with preset concentrations through a membrane to water. The conductometric method was used to monitor an increase in the concentration of a diffusing electrolyte. The diffusion permeabilities of the anisotropic membranes were measured at different orientations relative to the electrolyte flux.
RESULTS AND DISCUSSION
The experimental concentration dependences for the densities of diffusion fluxes of the electrolytes through the membranes to water were used to calculate the integral coefficients of the original MF-4SC membrane and the MF-4SC/PANI-I and MF-4SC/PANI-V composites by the following equation:
The results obtained are shown in Fig. 3. It follows from the figure that the modification of the perfluorinated ion-exchange membrane with PANI significantly decreases its diffusion permeability. The sample modified on both sides has the lowest diffusion permeability; i.e., the modifier deteriorates the diffusion characteristics of the MF-4SC membrane in correspondence with the data obtained previously [5, 6]. The magnitude of the asymmetry in the diffusion properties of MF-4SC/PANI-I is no higher than 10% within the studied concentration range of the electrolyte solutions.
The concentration dependences obtained for the diffusion fluxes through the original and volume-modified (on both sides) membranes were processed according to Eq. (1) in log j–log C (j, mol m–2 s–1; C, mol m–3) coordinates. This enabled us to determine the values of βi and di for each layer (see Table 1).
These values were used to calculate the diffusion flux of NaCl through the MF-4SC/PANI-I membrane to water in terms of the model developed by the authors of [12] with no allowance for the thicknesses of layers in this sample. The calculation results are represented in Fig. 4. It can be seen that the calculated curve (solid line) differs significantly from the experimental data (points). Similar results were obtained by the authors of [12] when describing the diffusion behaviors of anisotropic ion-exchange membranes, the surfaces of which were saturated with large organic ions. Thus, this approach does not exactly describe the diffusion permeability of not only the membranes saturated with large organic ions, but also the membranes modified with PANI.
For the membranes under study, calculation was performed by Eqs. (15) and (17)−(19) in terms of the proposed model, which takes into account both the concentration of the virtual solution at the internal interface between the layers and the ratio between the layer thicknesses. Figure 5 presents the concentration dependences calculated for the diffusion flux densities taking into account different layer thicknesses. In Fig. 5, q values equal to 0 and 1 correspond to MF-4SC and MF-4SC/PANI-V samples, respectively. It is seen that the diffusion fluxes essentially depend on the fraction of the modified layer in the total membrane thickness. The experimental values of the diffusion flux density for the MF-4SC/PANI-I sample lie between the calculated curves corresponding to the fractions of the modified layer equal to 0.25 and 0.5, and the fraction of the modified layer amounts to 0.45 of the total membrane thickness. When the orientation of the sample with respect to the electrolyte flux is changed, the thickness of the modified layer varies within a range of 10%, which is comparable with the experiment error.
(1) Experimental concentration dependences of NaCl diffusion fluxes at MF-4SC/PANI-I membrane orientations with (a) modified and (b) unmodified surfaces facing the electrolyte flux and the dependences calculated at different fractions of the modified layer in the membrane thickness: (2) 0, (3) 0.05, (4) 0.1, (5) 0.25, (6) 0.5, (7) 0.75, and (8) 1.
The study of the optical images taken from sections of composite membranes prepared under the conditions identical to the conditions of modifying the MF-4SC/PANI-I sample [17] has shown that the thickness of the modified layer varies within a range of 30−65% depending on moisture content. Thus, it has been shown that the proposed model makes it possible to estimate adequately the thickness of a modified layer.
Figure 6 presents the data on the concentration of the virtual solution at the internal interlayer interface for different orientations of the MF-4SC/PANI-I membrane with respect to the electrolyte flux at q = 0.45. It is seen that, in complete agreement with the aforementioned assumption, the modified layer has a strong suppressive effect on the diffusion transfer through the bilayer membrane, and the concentration of the virtual solution is always lower, when the membrane is oriented with the modified layer facing the electrolyte flux, as compared with the opposite orientation, irrespective of the concentration of a diffusing electrolyte.
To verify the proposed approach, the layer thicknesses were estimated for a series of perfluorinated MF-4SC/PANI-II-х membranes that were modified with PANI for 1, 2, and 3 h. Figure 7 shows the data on the diffusion permeabilities of the membranes of this series in HCl solutions. In the calculations, we used parameters βi and di determined for the MF-4SC and MF-4SC/PANI-V membranes from the concentration dependences of the diffusion fluxes in HCl solutions (Fig. 7). The results of the calculations are presented in Table 1. A rise in the polymerization time must increase the modified layer thickness, and, as a consequence, decrease the diffusion permeability. This regularity is valid for the samples modified for 1 and 2 h.
Concentration dependences of the diffusion permeability in HCl solutions for (1) the original and modified (3, 3') MF-4SC/PANI-II-1, (4, 4') MF-4SC/PANI-II-2, (2, 2') MF-4SC/PANI-II-3, and (5) MF-4SC/PANI-V membranes orientated with their (2–4) unmodified and (2'–4') modified layers facing the electrolyte flux.
When the modification time is increased to 3 h, both the diffusion and electroosmosis permeabilities grow [5] because of the disjoining action of PANI on the cluster structure of the perfluorinated membrane and the changes in the morphology of the modified layer.
Table 2 lists the layer thicknesses estimated at different orientations of the membranes by our method with allowance for the layer thicknesses of the anisotropic membranes.
As follows from the data in Table 2, the fractions of the modified layer thicknesses at different membrane orientations relative to the electrolyte flux coincide with each other with an accuracy of 20% for all composite samples. This indicates that the model parameters of the MF-4SC/PANI-I composite membrane may be used for the analysis of the properties of the MF-4SC/PANI-II bilayer membranes prepared under different modification conditions. In the cases of MF-4SC/PANI-I and MF-4SC/PANI-II composites, the layer thicknesses are in good agreement with modified layer thickness determined using the optical images taken from the sections of the composite membranes and the model of a bilayer fine-porous membrane [5] (Table 2). The calculations performed by Eqs. (15) and (17)–(19) for the MF-4SC/PANI-II-3 sample have shown that the layer thickness decreases, whereas the optical microscopy data have revealed that it increases. This discrepancy between the experimental and calculated data is caused by changes in the properties of the layers due to (among other reasons) the growth of PANI chains through the membrane, which limits the use of the characteristics of the MF-4SC and MF-4SC/PANI-V membranes for describing the properties of the MF-4SC/PANI-II-3 sample.
CONCLUSIONS
A model approach has been developed for describing the diffusion permeability of bilayer ion-exchange membranes. This approach takes into account not only the properties of individual layers and the concentration of a virtual solution at the interface between the layers, but also their thicknesses. The proposed approach has been verified by the example of perfluorinated MF-4SC membranes modified with polyaniline. A satisfactory coincidence has been shown between the modified layer thicknesses calculated in terms of the proposed model and those determined from the optical images taken from the sections of the membranes. A correlation between the modified layer thickness and the time of PANI synthesis in the membrane has been revealed. The adequacy of using this model for describing the diffusion permeability of perfluorinated membranes modified with PANI under different conditions has been shown.
REFERENCES
Loza, S.A., Zabolotsky, V.I., Loza, N.V., and Fomenko, M.A., Pet. Chem., 2016, vol. 56, p. 1027.
Melnikov, S.S., Sheldeshov, N.V., and Zabolotskii, V.I., Desalin. Water Treat., 2018, vol. 123, p. 1.
Zabolotskii, V., Sheldeshov, N., and Melnikov, S., J. Appl. Electrochem., 2013, vol. 43, p. 1117.
Zhao, Y., Tang, K., Liu, H., Van der Bruggen, B., Sotto Díaz, A., Shen, J., and Gao, C., J. Membr. Sci., 2016, vol. 520, p. 262.
Berezina, N.P., Kononenko, N.A., Filippov, A.N., Shkirskaya, S.A., Falina, I.V., and Sycheva, A.A.-R., Russ. J. Electrochem., 2010, vol. 46, p. 485.
Kononenko, N.A., Loza, N.V., Shkirskaya, S.A., Falina, I.V., and Khanukaeva, D.Yu., J. Solid State Electrochem., 2015, vol. 19, p. 2623.
Protasov, K.V., Shkirskaya, S.A., Berezina, N.P., and Zabolotskii, V.I., Russ. J. Electrochem., 2010, vol. 46, p. 1131.
Moshtarikhah, S., Oppers, N.A.W., De Groot, M.T., Keurentjes, J.T.F., Schouten, J.C., and Van der Schaaf, J., J. Appl. Electrochem., 2017, vol. 47, p. 213.
Lebedev, K., Ramırez, P., Mafe, S., and Pellicer, J., Langmuir, 2000, vol. 16, p. 9941.
Nikonenko, V.V., Zabolotskii, V.I., and Lebedev, K.A., Elektrokhimiya, 1996, vol. 32, p. 234.
Filippov, A.N., Starov, V.M., Kononenko, N.A., and Berezina, N.P., Adv. Colloid Interface Sci., 2008, vol. 139, p. 29.
Kononenko, N.A., Gnusin, N.P., Berezina, N.P., and Parshikov, S.B., Russ. J. Electrochem., 2002, vol. 38, p. 828.
Gnusin, N.P., Berezina, N.P., Shudrenko, A.A., and Ivina, O.P., Zh. Fiz. Khim., 1994, vol. 68, p. 565.
Demina, O.A., Kononenko, N.A., Falina, I.V., and Demin, A.V., Colloid J., 2017, vol. 79, p. 317.
Filippov, A.N., Kononenko, N.A., and Demina, O.A., Colloid J., 2017, vol. 79, p. 556.
Shkirskaya, S.A., Sycheva, A.A., Berezina, N.P., Timofeev, C.B., and Krishtopa, M.V., RF Patent No. 2411070 (2009).
Berezina, N.P., Shkirskaya, S.A., Kolechko, M.V., Popova, O.V., Senchikhin, I.N., and Roldughin, V.I., Russ. J. Electrochem., 2011, vol. 47, p. 995.
Shkirskaya, S.A., Extended Abstract of Cand. Sci. (Chem.) Dissertation, Krasnodar: Kuban State Univ., 2008.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 18-38-20069.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of in-terest.
Additional information
Translated by E. Khozina
Rights and permissions
About this article
Cite this article
Falina, I.V., Demina, O.A., Kononenko, N.A. et al. A Model Description of Diffusion Permeability of Bilayer Ion-Exchange Membranes. Colloid J 82, 200–207 (2020). https://doi.org/10.1134/S1061933X20010044
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X20010044