Abstract
The electrokinetic properties and selectivity of an acetyl cellulose membrane with respect to 0.0001 mol/L sodium chloride solutions in water–ethanol mixtures have been studied. The electrical conductivity, streaming potential, and filtration and selectivity characteristics of the membrane have been measured. It has been found that, in solutions with alcohol contents of 4 and 12%, the membrane selectivity with respect to sodium chloride is increased and decreased relative to that in an aqueous solution, respectively. No correlation between the membrane selectivity and its surface charge has been observed. The membrane has been found to possess a slight selectivity (20–26%) with respect to ethanol. It has been hypothesized that the solvation enthalpy of electrolyte ions changes differently in a free solution and membrane pores at different contents of ethanol in the mixtures, thereby affecting the membrane selectivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The study of the mechanism of reverse-osmosis membrane (ROM) selectivity is of great scientific interest, because the knowledge of factors affecting ion retention by a membrane will result in the prediction of results and a more targeted and selective approach to the synthesis of membranes and the choice of reagents.
As a rule, contemporary theories describing the mechanisms of ion retention by ROMs involve the notions of the “nondissolving volume,” which is related to a reduction in the solvation ability of water due to a decrease in its dielectric permittivity (DP) in the pores of a selective layer of a membrane (a change in the Born energy of ions) and the “dielectric exclusion of ions” caused by the low DP of a membrane matrix. These factors lead to an increase in the free energy and standard chemical potential (SCP) of ions transported from a bulk solution into a membrane and, as a consequence, to a decrease in their concentration inside of membrane pores [1–4]. In addition, some authors have focused attention on the possibility of changes in the transport characteristics of ions due to the frictional interaction with the polymer matrix of a membrane [5–7]. As a whole, the ion transfer through a membrane under the nonequilibrium conditions of reverse osmosis is realized due to diffusion, solvent convection, and electromigration. A decrease in the concentration and mobility of ions upon their transport through a membrane reduces their flux density and promotes their retention.
The ionic selectivity of nanofiltration membranes (NFMs) is commonly interpreted within the framework of the charge-related mechanism of ion retention [8–10] (Donnan’s exclusion of coions from a pore space) and a decreased DP of water in the pores of a selective layer, as well as a low DP of a membrane matrix [3, 4] (dielectric exclusion). Surface charge density, electrolyte concentration, and pore sizes are the criteria that determine the Donnan exclusion. Recently, the combined model and its different versions that take into account the charge-related mechanism, the effect of a decrease in the ion concentration in pores due to a decreased DP of water, and the steric factor have become widely used as applied to NFMs and ROMs [11–17].
The first attempt to perform a model calculation of the thermodynamic parameters of ion solvation was made by Born [18], who proposed the following equation for free energy of solvation ΔGs:
where z is the ion charge, e is the electron charge, ri is the ion radius, and E is the solvent dielectric permittivity.
However, a substantial difference was observed between the ΔGs values obtained experimentally and calculated by Eq. (1). Different authors have tried to eliminate this discrepancy by introducing a number of additional terms into Eq. (1). In this respect, the Stokes model [19] is considered to be the most successful attempt. The author introduced the effective dielectric permittivity, the thickness of the water layer interacting with an ion, and the ion radius equal to the inert gas radius. The results obtained in terms of this model agree with the experimental data within an error of ±13%. The authors of study [20], which was based on the notions of nonlocal electrostatics, have introduced the characteristic correlation radius of fluctuations in the orientational polarization, with this radius having different values in a free solution and in fine pores of an ROM. They have presented an equation for calculating a change in the Born energy upon the transfer of an ion from a free solution into a pore space. However, this electrostatic approach to the solvation process suffers from two basic drawbacks: it ignores the covalent character of the ion–dipole interaction and the energy of the dipole–dipole interactions.
Simultaneously with the evolution of the Born equation, another approach was developed, in which the authors rejected Born’s “sphere-in-continuum” model. This concept was based on ideas reported in the classic work by Bernal and Fowler [21]. The essence of their model consists in allowance for the structure of water molecules adjacent to an ion. Krestov [22] improved this method by employing semiempirical expressions for the following integrals of the intermolecular interactions:
(1) the intrinsic energy of a solvate complex in a liquid;
(2) the energy that solvate complex molecules had in a pure liquid;
(3) the energy of the interaction of solvate complex molecules with ambient molecules; and
(4) the enthalpies of polarization (ΔUpol) outside of the first solvation sell determined by the Born–Bjerrum equation.
The calculations have shown that a decisive contribution is made by the energy of solvate complex formation to the ΔH value of alkaline ion solvation. However, ΔUpol increases with the ion radius.
Two approaches to the analysis of the bond nature in solvates are used when considering the energy of a solvate complex in a liquid, i.e., the electrostatic and donor–acceptor approaches. However, according to the mass spectrometry data, neither of them can, in full measure, describe separately the interaction of cations with molecules of hydroxyl-containing compounds. Most likely, it is necessary to take into account both the classical and the quantum effects. Their contributions alter with variations in the radius, charge, and electron configuration of an ion. Ignoring the question about the structures of a solvate complex in a free solution and in a membrane pore, it may be stated that the energies of solvate complex formation in the free solution and in a membrane pore are different.
The strengthening of the hydrogen bonds under the action of surface forces in membrane pores leads to the fact that the energy consumed for the reorientation of dipoles in the immediate environment of an ion is higher than that in bulk, because the polarization of elastically bonded polar molecules am depends on both electric moment µ0 of each molecule and energy U0 of intermolecular bonds [23]:
The two opposite positions of the dipole axis correspond to two minima of dipole potential energy. To transfer a dipole from one position to the other (rotation by 180°), it is necessary to spend the work of dipole detachment from other dipoles. The value of this work is determined by the potential barrier that separates these two positions.
The aforementioned contribution must grow with an increase in the ability of ions (Li+ Mg2+, F–) to form their own hydrate complexes without distortions of water structure. The consideration of other components of the solvation energy shows that, for a hypothetical liquid that possesses the properties of a solvent in membrane pores, their contributions will differ from those in a free solution. The interaction energy of solvate complex particles plays the key role in quasi-molecular models. In electrostatic models, the energy of particles in vacuum is actually calculated; thereafter, it is assumed that the formation energy of an isolated solvate coincides with the interparticle interaction energy in a solution. This approach is also transferred to the calculation of a change in the intrinsic energy of an ion upon its passage from a free solution into a membrane pore. Indeed, nonempirical calculations of ion hydrates [24] have shown that the filling of the second and subsequent layers has almost no effect on the distance between an ion and the molecules of the hydration layer; however, the formation energies of the layers will be different, which is natural for associated liquids. Therefore, the enthalpy of ion transfer from a free solution into a membrane pore will be a more correct parameter characterizing the selective properties of ROMs. A change in the entropic component cannot also be ignored. The rather approximate calculation of the energy by the Born procedure actually reflects the polarization component of the enthalpy of ion transfer from a free solution into a membrane pore. Taking into account the real pore sizes (1–2 nm) of reverse-osmosis membranes and the fact that, for many ions, the formation of a hydrate complex is not confined to the first and, even, second sphere, it becomes evident that the potential energy of the ion-molecular and intermolecular interactions will be of great importance for the change in the enthalpy of hydration of ions upon their transfer from a free solution into a membrane pore.
It has been found [25, 26] that the transfer energy of cobaltocene and cobaltocinium ions in aprotic solvents with different DPs is adequately described by the Born equation. These experiments represent a good model for verifying the Born equation. Solvent molecules are not bonded to each other; hence, intermolecular interactions are absent. Cobaltocene and cobaltocinium ions are organometallic compounds, in which a cobalt ion is surrounded with two cyclopentadienyl rings, which reliably protect it from the direct contact with solvent ions. In this case, the ambient medium of an ion may be considered to be a continual one, while the ion has only a polarizing effect on solvent molecules; i.e., Born’s electrostatic “sphere-in-continuum” approach is completely instantiated. The authors of these works have also revealed a difference between experimental data and those calculated by the Born equation, when calculating the energy of ion transfer in water–alcohol solutions. This difference has been related to the ignorance of specific interactions. Moreover, the influence of the solvophobic effects must not be ignored in this case.
The authors of [27] used NMR data to calculate the standard Gibbs free energy of Li+ transfer from POCl3 to SOCl2. A linear correlation has been revealed between the SCP of an ion and the donor number of a solvent. In this case, the mechanism of solvation by oxyhalides is similar to that in dipolar aprotic solvents. In [28], a correlation has been found between the enthalpy of the ion transfer and the acid–base properties of binary solutions, with this finding corresponding to the donor–acceptor mechanism of the ion–solvent interaction. This suggests an important role of the ion–dipole interaction potential in the alteration of ion SCP. It is evident from all that has been stated above that the use of the Born equation for the interpretation of the selectivity mechanism of ROMs has essential limitations imposed by the following factors:
(1) a change in the molecular field of a liquid in a membrane pore relative to that of a free solution;
(2) the specificity of ion–dipole interactions relevant to the electron density redistribution;
(3) difference between the energies of hydrate complex formation in a free solution and a membrane pore;
(4) anisotropy of the molecular field of a liquid subjected to the action of surface forces in a membrane pore; and
(5) assumption of a “rigid” solvate complex without taking into account its molecular-kinetic transformations [29].
Molecular-dynamic calculations performed by the authors of [30] have shown that, in pores of polyamide ROMs, Na+ and Cl– ions are partly dehydrated, while the amount of hydrogen bonds between water molecules is decreased.
The authors of [31] tried to formulate a statistical thermodynamic model of water in fine pores using experimental values of the density (nearly 3% lower than the density of bulk water) and increased values of the specific heat (25%) and viscosity of water bound in fine-pore glasses. The model has appeared to correlate with some thermodynamic and dynamic properties of water in fine pores. According to this model, water bound in fine pores turns out to be similar to water occurring in a supercooled state or under a negative pressure. In other words, hydrogen bonds are stronger near a solid surface.
In the case of acetyl cellulose membranes (ACMs), which are moderately hydrophilic, it follows from the IR and Raman spectroscopy data on the state of water in ACMs at low moisture contents that the interaction of water molecules with the polymer matrix is much weaker than their interaction with each other [32]. As was found in [33], ACMs contain unfrozen water at ‒40°C. The authors have determined the cluster number of water (1.8–2.4) and concluded that it has a low degree of association, while, in a bulk solution, the water cluster number is, as a rule, close to 6 [34]. It was inferred from adsorption measurements that the water–membrane interaction was weaker than the interaction of water molecules with each other.
The authors of [35] used the vapor–liquid metastable equilibrium method to study the stability limit of liquid water as depending on temperature in a range from 15 to 0°C for a nanoporous silicon membrane with pore sizes of 3–8 nm. They have also inferred that the state of water in membrane pores is similar to its state under a high negative pressure (up to –30 MPa).
Amphiphilic ethanol molecules exhibit the properties of a nonionic surfactant and are adsorbed on acidic hydrophilic sites via the oxygen atoms of hydroxyl groups [36]. The addition of the alcohol to aqueous electrolyte solutions can have a marked effect on ion solvation. Even at a low molar fraction of ethanol (0.05), an increase is observed in the energy of ion solvation [37–39]. This increase has been related to the “solvophodic effect” [40]. Alcohol molecules are incorporated between water molecules, thereby strengthening the hydrogen bonds between them and, as a consequence, increasing the energy of ion solvation.
At high concentrations of ethanol in water, the negative potential of a quartz surface decreases and, then, even becomes positive at very high alcohol concentrations [41]. Analogous results were also obtained for a polished silicon surface; therewith, the authors noted that low ethanol concentrations in water had a very weak effect on the surface properties [42]. For polyamide NFMs of the OPMN (Vladipor, Russia) and NF200 (Filmtec) brands, a decrease in the negative surface potential down to its sign reversal was found [43] at very high ethanol concentrations in aqueous–ethanol electrolyte solutions. The authors of [44] have related this effect to an increase in the solvation energy of salt cations in aqueous–alcohol solutions. As alcohol concentration increases, this effect is enhanced, and the potential of cation adsorption on the surface increases, thereby leading to positive values of the surface potential at a very high content of ethanol in the water–alcohol mixture (higher than 80%).
The study of the effect of ethanol concentration on the membrane selectivity with respect to electrolyte solutions is of great interest, because the addition of ethanol not only changes (reduces) the DP of the aqueous solutions, but also alters their physicochemical properties, such as polarity, acid–base and donor–acceptor properties, and capacity for association.
EXPERIMENTAL
Aqueous and aqueous–alcohol solutions of NaCl with ethanol concentrations of 4 and 12 wt %, which corresponded to its molar fractions of 0.016 and 0.051, were used in the experiments. The NaCl concentration was 10–4 mol/L in all cases. An aqueous 10–4 mol/L NaCl solution was used as the reference.
The concentrations of Na+ ions in filtrates of aqueous and aqueous–alcohol solutions were measured with an OP-263 Na+ ion meter (Radelkis, Hungary). Ethanol concentrations in the filtrates were determined by chromatography using a GC 8700 gas chromatograph (Perkin-Elmer, United States).
MGA-80 acetyl cellulose membrane (NPO Vladipor), sodium chloride (reagent grade), and ethanol (rectificate) were used in the experiments. Solutions were prepared in triply distilled water with an electrical conductivity of 1.2 × 10–6 Ω–1 cm–1 and pH 6.3. The measurements were performed at 20°С.
Membrane selectivity with respect to sodium chloride was studied with a laboratory dynamic setup [45], which enabled us to measure the streaming potential, electrical resistance, and permeability of the membrane directly in the course of separation. The separation was carried out at pressure drop ΔP across the membrane up to 3 MPa.
Membrane charge σ was calculated using the Schmidt–Schwarz approach [9],
where ΔE is the streaming potential, R is the electrical resistance of the membrane active layer, Kf is the filtration coefficient of the membrane, and r is the average pore radius of the membrane active layer. The values of R and r were calculated by the procedure developed previously [46]. The average pore radius of the membrane active layer calculated with respect to distilled water was 1.4 nm.
Using the data on filtration coefficient Kf and viscosity η of water–alcohol mixtures and taking into account the membrane selectivity with respect to alcohol, variations in the pore radius of the membrane, through which aqueous–alcohol NaCl solutions are flowing, may be monitored employing the Poiseuille equation,
where r is the average effective radius of membrane pores, N is the number of pores per 1 cm2 of the membrane, and h is the thickness of the membrane selective layer.
Assuming that the values of N and h remain unchanged in all experiments, the relative change in the pore radius is found from the following relation:
RESULTS AND DISCUSSION
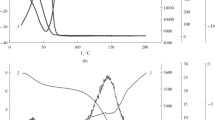
Figure 1 shows the dependences of membrane selectivity φ with respect to Na+ ions on the pressure drop (Fig. 1a) and the solution flow velocity (Fig. 1b). It can be seen that, for curves 2, which correspond to an ethanol content of 4%, the maximum values of the selectivity coefficient in the plateau region are about 0.85. These values exceed the corresponding values of φ for an aqueous 10–4 М NaCl solution, which attain 0.79 (curves 1). When ethanol fraction is increased to 12%, the retention coefficient of Na+ ions decreases and amounts to 0.60 in the plateau region (curves 3).
Since the viscosities of the solutions changed from one experiment to another, the dependences of the membrane selectivity on the solution flow velocity must be more correct and informative in our case.
Figure 2 depicts the curves describing the dependences of the membrane selectivity with respect to ethanol on the applied pressure (Fig. 2a) and the solution flow velocity (Fig. 2b). In this case, the pattern of the curves is somewhat different. It is seen that the membrane has low selectivity values with respect to ethanol of 0.26 and 0.20 in the plateau region for solutions with alcohol concentrations of 4 and 12%, respectively.
It should be noted that the flow velocity of the aqueous–ethanol NaCl solution with the alcohol content of 4% is dramatically decreased relative to that of the aqueous salt solution. The decrease is reversible for both aqueous–alcohol solutions, with the reversibility being confirmed by the reproducible unchangeable value of the filtration coefficient of distilled water after each performed experiment. Since the membrane selectivity values with respect to alcohol for 4 and 12% ethanol solutions were 0.26 and 0.20, respectively, ethanol concentration in membrane pores was decreased to 3 and 9.6% in the first and second cases, respectively. Therefore, in the calculations, we used the viscosity values corresponding to the ethanol concentrations in the filtrates, assuming that its concentration in a pore solution was equal to that in a filtrate. For 4 and 12% alcohol solutions, these values were 1.16 and 1.5 mPa s, respectively. The viscosity of an aqueous 10–4 М NaCl solution was taken to be 1 mPa s.
The calculations have shown that, when using the aforementioned viscosity values for water–alcohol mixtures and passing from the aqueous to the aqueous–alcohol 10–4 M NaCl solution with the alcohol content of 4%, the calculated pore radius of the membrane appears to be decreased by 4%. This is, most probably, due to the adsorption of alcohol molecules on the surface of membrane pores and an increase in the viscosity of boundary (adsorbed) layers of ethanol [47].
The authors of [41] have shown that, in water–ethanol mixtures, alcohol molecules displace water molecules from the solid surface into the bulk solution. As a result, the average apparent viscosity of the solution may appear to be somewhat higher than the values that we use.
The comparative analysis performed on the basis of Eq. (5) has shown that, when filtering the aqueous–alcohol 10–4 M NaCl solution with an ethanol content of 12%, the membrane pore radius remains equal to that in the case of filtering the aqueous–alcohol NaCl solution containing 4% ethanol. However, curves 3 in Figs. 1a and 1b show that, when the solution with an alcohol content of 12% is separated, the membrane selectivity with respect to sodium ions markedly decreases.
The dependences of the membrane surface charge on the pressure drop for the flows of aqueous and aqueous–alcohol solutions of sodium chloride through the membrane are presented in Fig. 3. These dependences were calculated by Eq. (3). The analysis of these dependences shows no correlation between the selectivity and the surface charge of the membrane. For example, in the solution with the ethanol content of 12%, the membrane acquires a higher charge than that obtained in the 4% alcohol solution and an aqueous solution; however, its selectivity with respect to sodium ions is minimum in all three cases. It should be recognized that the MGA-80 membrane cannot be attributed to nanofiltration membranes, for which the dependences of the selectivity on the surface charge of a membrane and the sign and concentration of electrolyte ions have a more pronounced character.
CONCLUSIONS
The data obtained have shown that the addition of even very small amounts of ethanol (0.016 and 0.051 molar fractions) to an aqueous 10–4 mol/L NaCl solution have opposite effects on the selectivity of an acetyl cellulose membrane with respect to sodium ions. In our opinion, this is due to the differences in the changes of ion solvation enthalpy upon its transport from a bulk solution into a membrane pore. At an ethanol molar fraction of 0.016, this change appears to be much larger than that at a molar fraction of 0.051. No correlation has been revealed between the membrane selectivity with respect to sodium ions and the membrane surface charge in water–alcohol mixtures.
REFERENCES
Martynov, G.A., Starov, V.M., and Churaev, N.V., Kolloidn. Zh., 1980, vol. 42, p. 489.
Derjaguin, B.V., Churaev, N.D., and Martynov, G.A., J. Colloid Interface Sci., 1980, vol. 75, p. 419.
Churaev, N.V. and Derjaguin, B.V., Zh. Vses. Khim. O-va.im.D.I. Mendeleeva, 1987, vol. 32, p. 614.
Yaroshchuk, A.E., Adv. Colloid Interface. Sci., 2000, vol. 85, p. 193.
Spiegler, K.S., Trans. Faraday Soc., 1958, vol. 54, p. 1408.
Merten, U., Desalination by Reverse Osmosis, Cambridge: MIT, 1966.
Manning, G.S., J. Phys. Chem., 1972, vol. 76, p. 393.
Donnan, F.G., J. Membr. Sci., 1995, vol. 100, p. 45.
Schmid, G. and Schwars, H., Z. Elektrochem., 1952, vol. 52, p. 35.
Hoffer, E. and Kedem, O., J. Phys. Chem., 1972, vol. 76, p. 3638.
Bowen, W.R. and Mukhtar, H., J. Membr. Sci., 1996, vol. 112, p. 263.
Bowen, W.R. and Welfoot, J.S., Chem. Eng. Sci., 2002, vol. 57, p. 1121.
Vezzani, D. and Bandini, S., Desalination, 2002, vol. 149, p. 477.
Bandini, S. and Vezzani, D., Chem. Eng. Sci., 2003, vol. 58, p. 3303.
Bandini, S., J. Membr. Sci., 2005, vol. 264, p. 75.
Bruni, L. and Bandini, S., J. Membr. Sci., 2008, vol. 308, p. 136.
Szymczyk, A. and Fievet, P., J. Membr. Sci., 2005, vol. 252, p. 77.
Born, M., Z. Phys., 1920, vol. 1, p. 45.
Stokes, R.H., J. Am. Chem. Soc., 1964, vol. 64, p. 979.
Kornyshev, A.A., Tsitsuashvili, G.I., and Yaroshchuk, A.E., Elektrokhimiya, 1989, vol. 25, p. 1037.
Bernal, J.D. and Fowler, R.H., J. Chem. Phys., 1933, vol. 1, p. 515.
Krestov, G.A., Ionnaya sol’vatatsiya (Ion Solvation), Moscow: Nauka, 1987.
Skanavi, G.I., Fizika dielektrikov (Physics of Dielectrics), Moscow: Gos. Izd. Tekh.-Teor. Lit., 1949.
Ontaki, H., Yamaguchi, T., and Maeda, M., Bull. Chem. Soc. Jpn., 1976, vol. 49, p. 701.
Krishtalik, L.I., Alpatova, N.M., and Ovsyannikova, E.V., Elektrokhimiya, 1990, vol. 26, p. 429.
Krishtalik, L.I., Alpatova, N.M., and Ovsyannikova, E.V., Elektrokhimiya, 1990, vol. 26, p. 436.
Mishustin, A.I., Mozalevskaya, V.A., and Ponomarev, V.P., Zh. Fiz. Khim., 1989, vol. 63, p. 1345.
Manin, N.G. and Korolev, V.P., Russ. J. Phys. Chem., 2002, vol. 76, p. 177.
Samoilov, O.Ya., Struktura vodnykh rastvorov elektrolitov i gidratatsiya ionov (The Structure of Electrolyte Aqueous Solutions and Ion Hydration), Moscow: Akad. Nauk SSSR, 1957.
Ding, M., Szymczyk, A., and Ghouf, A., Desalination, 2015, vol. 368, p. 76.
Etzler, F.M., J. Colloid Interface Sci., 1983, vol. 92, p. 43.
Ovchinnikov, A.L., Timashev, S.F., and Belyi, F.L., Kinetika diffuzionno-kontroliruemykh protsessov (Kinetics of Diffusion Controlled Processes), Moscow: Khimiya, 1986.
Atamanenko, I.D. and Bryk, Yu.I., Kolloidn. Zh., 1991, vol. 53, p. 336.
Sun, Q. and Zheng, H.-F., Chin. Phys. Lett., 2006, vol. 23, p. 3022.
Chen, I.T., Sessoms, D.A., Sherman, Z., Choi, E., Vincent, O., and Stroock, A.D., J. Phys. Chem. B, 2016, vol. 120, p. 5209.
Ivanova, N.I., Vakar, N.T., and Pertsov, N.V., Kolloidn. Zh., 1987, vol. 49, p. 1348.
Chankina, T.I. and Parfenyuk, V.I., Russ. J. Phys. Chem., 2011, vol. 85, p. 1307.
Chankina, T.I. and Parfenyuk, V.I., Russ. J. Electrochem., 2008, vol. 44, p. 1162.
Parfenyuk, V.I., Russ. J. Phys. Chem., 2005, vol. 79, p. 898.
Kessler, Yu.M. and Zaitsev, A.L., Sol’vofobnye effekty. Teoriya, eksperiment, praktika (Solvophobic Effects. Theory, Experiment, Practice), Leningrad: Khimiya, 1989.
Zhukov, A.N. and Fedorova, I.L., Colloid J., 2004, vol. 66, p. 292.
Kosmulski, M. and Matijevic, E., Langmuir, 1992, vol. 8, p. 1060.
Pihlajamaki, A., Laakso, T., and Manttari, M., Proc. Eng., 2012, vol. 44, p. 1502.
Zhukov, A.N. and Fedorova, I.L., Kolloidn. Zh., 1990, vol. 52, p. 781.
Sabbatovskii, K.G., Sobolev, V.D., and Churaev, N.V., Kolloidn. Zh., 1991, vol. 53, p. 74.
Sabbatovskii, K.G., Sobolev, V.D., and Churaev, N.V., Kolloidn. Zh., 1991, vol. 53, p. 403.
Rodionova, I.A., Shkol’nikov, E.I., and Volkov, V.V., Colloid J., 2005, vol. 67, p. 469.
Funding
This work was performed within the framework of a state order to the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Sabbatovskii, K.G., Sergeeva, I.P. & Sobolev, V.D. The Effect of Aqueous–Ethanol Sodium Chloride Solutions on the Selectivity and Electrosurface Properties of an Acetyl Cellulose Membrane. Colloid J 81, 747–753 (2019). https://doi.org/10.1134/S1061933X19060164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19060164