Abstract
Polyvinyl chloride-based heterogeneous cation exchange membranes were modified by embedding carboxy methyl cellulose in ionic transfer channels of membrane. The effect of CMC to PVC blend ratios on properties of membranes was studied. SOM images showed uniform distribution and surfaces for prepared membranes relatively. The SEM images showed uniform and dense structure for the membranes. The XRD pattern also demonstrated amorphous structure for the membranes. Membrane water content was improved from 25 to 39 % by increase of CMC concentration up to 32 %wt. Similar trend was found for membrane surface hydrophilicity. The membrane ion exchange capacity, fixed ion concentration, membrane potential, charge density, transport number, permselectivity, and ionic flux were enhanced initially by increase of CMC ratio up to 16 %wt and then began to decrease by increase in CMC concentration from 16 to 32 %wt. The membrane oxidative stability and areal electrical resistance showed decreasing trends by utilizing of carboxy methyl cellulose in the membrane matrix. Membrane transport number and selectivity were also increased by increase of electrolyte concentration. Similar trend was found for the membrane electrical conductivity by increase of electrolyte concentration. Also prepared membranes showed higher transport number, selectivity, and areal electrical resistance at pH 7 compared to other pH values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ion exchange membranes play important parts in environmental protection, treating industrial effluents, desalting brackish waters, reconcentrating brine from seawater, production of table salt, recovery of valuable metals, and food and pharmacy processing as well as manufacturing of basic chemical products. In this kind of membrane, charged groups attached to polymer backbone [1–9]. Knowledge of the electro-kinetic and structural properties of ion exchange membranes are major factors behind decisions about their applicability in specific processes [4, 10–12]. So, preparing inexpensive membranes with special physico-chemical characteristics may be a vital step in future applications [4, 13–16]. Functional groups’ variation [17], selection of different polymeric matrices [18, 19], polymers blending [20, 21], use of various additives such as nanoparticles [22–24], alteration of cross-link density [25, 26], plasma treatment [27], polymer coating [6, 25], and use of various solvents [28] are important ways to obtain superior ion exchange membranes. It is well known that each slight change in membrane fabrication process can affect the membrane performance [23]. Blending is an inexpensive and advantageous method to obtain new structural materials. The effect of polymer blending such as ABS/PS [20], PC/PVC [21], CA/PVA [29], PES/CAP [30], and PVC/CA [31] on membrane separation performance and morphology has been examined.
Nowadays, numerous new functional materials from cellulose are being developed for a broad range of applications because of the increasing demand for environmentally friendly and biocompatible products. Carboxy methyl cellulose having abundant hydroxyl and carboxyl groups can be used to prepare membranes and hydro gels easily with fascinating structures and properties [29].
In the current study, polyvinyl chloride-based heterogeneous cation exchange membrane was prepared by solution casting techniques. The membranes were modified by embedding carboxy methyl cellulose in ionic transfer channels of membrane matrix for the application in electrodialysis processes related to water recovery and water treatment.
Polyvinyl chloride is a flexible and durable polymer with suitable biological and chemical resistance [32–34]. Carboxy methyl cellulose is also one of the most applicable functional polymers in fabrication of membranes due to its unique features such as high hydrophilicity, ion exchange ability, and low price [29]. Utilizing of these polymers can dedicate special characteristics into the membranes.
Currently, no reports have considered fabrication of (polyvinyl chloride/carboxy methyl cellulose) heterogeneous cation exchange membranes and the literature is silent on the characteristics and functionality of electrodialysis ion exchange membranes modified by embedding CMC in ionic transfer channels of electrodialysis membrane.
The effect of carboxy methyl cellulose (CMC) to polyvinyl chloride (PVC) blend ratio on physico-chemical characteristics of homemade cation exchange membranes was studied. The obtained results are valuable in the electro-membrane processes especially electrodialysis.
Materials and methods
Materials
PVC (grade S-7054; density, 490 g/l) supplied by BIPC, Iran, and CMC (average MW 90,000, sodium form) supplied by Sigma-Aldrich, USA, were used in membrane fabrication. Tetrahydrofuran (THF, solvent) and cation exchange resin (Ion exchanger Amberlyst® 15, strongly acidic cation exchanger, H+ form—more than 1.7 meq/g dry, spec. density 0.6 g/cm3), by Merck Inc., Germany, were also used as solvent and functional groups agent, respectively. All other chemicals were supplied by Merck. Throughout the experiment, distilled water was used. The chemical structure of used polymers is shown in Table 1.
Fabrication of homemade membranes
The heterogeneous cation exchange membranes were prepared by casting solution technique and phase inversion method. For membrane preparation, resin particles were dried in oven (SANEE. V. S. Co) at 30 °C for 48 h and then pulverized into fine particles in a ball mill (Pulverisette 5, Fritisch Co.) and sieved to the desired mesh size (−300 +400 mesh). The preparation proceeded by dissolving the PVC into THF solvent in a glass reactor equipped with a mechanical stirrer (Model, Velp Sientifica Multi 6 stirrer) for more than 5 h. This was followed by dispersing a specific quantity of CMC and grind resin particle in the solution. The mixture was mixed vigorously at room temperature to obtain uniform particle distribution in the polymeric solution. In addition, for better dispersion of particles and breaking up their aggregates, the solution was sonicated for 1 h using a sonication bath instrument. The mixing process was repeated for another 30 min using the mechanical stirrer. The mixture was then cast onto a clean and dry glass plate at 25 °C with 400 μm thickness. The membranes were dried at ambient temperature (25 °C) for 1 h and immersed in distilled water for 1 day. As the final stage, the membranes were pretreated by immersing in NaCl solution for 2 days. The membrane thickness was also measured by a digital caliper device (electronic outside micrometer, IP54 model OLR) around 80 μm. The composition of casting solution is depicted in Table 2.
Test cell
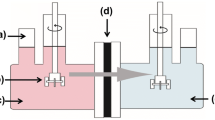
The electrochemical properties measurements for the prepared membranes were carried out using the test cell (Fig. 1). The cell consists of two cylindrical compartments made of Pyrex glass which are separated by membrane. One side of each vessel was closed by Pt electrode supported with a piece of Teflon and the other side was equipped with a piece of porous medium. In order to minimize the effect of boundary layer during experiments and to establish the concentration polarization on the vicinity of membrane’s surface, both sections were stirred vigorously.
Membrane characterization
Morphological studies
The behavior of prepared membranes is closely related to their structure, especially the spatial distribution of ionic site [16]. The structures of prepared membranes were examined by scanning optical microscopy (SOM, Olympus, model IX 70, in transmission mode) and scanning electron microscopy (SEM, Philips-X130 and Cambridge SEM). For the SOM analysis, samples were cut in small pieces and then mounting between lamellas, observation was made by the optical microscope. For the scanning by SEM device, the membranes were frozen in liquid nitrogen, fractured, and sputtered with gold; observation was undertaken using the electron microscope.
X-ray diffraction (XRD)
For micro-structural studies of prepared composite membranes, X-ray diffraction patterns were carried out by an X-ray diffractometer (XRD, model X’Pert Pw 3373, k α = 1.54 A°, Philips, Holland).
Water content
The water content was measured as the weight difference between the dried and swollen membranes. The wet membranes was weighed (OHAUS, Pioneer TM; readability, 10−4 g, OHAUS Corp.) and then dried in oven until the constant weight was reached. The following equation [7, 16, 35] can be used in water content calculations:
Where W wet and W dry are the wet and dry membranes weight (g), respectively. To minimize the experimental errors, measurements were carried out three times for each sample and then their average value was reported.
Water contact angle measurements
The water contact angle measurement was carried out between water and the membrane surface using contact angle measuring instrument to evaluate the membranes surface hydrophilicity and wetting characteristic. De-ionized water was used as the probe liquid in all measurements. To minimize the experimental error, the contact angle was measured in five random locations for each sample and then their average was reported. All experiments were carried out in the ambient conditions.
Ion exchange capacity (IEC) and fixed ion concentration (FIC)
The IEC determination was performed using titration method. For the IEC measurements, the membranes in acid form (H+) was converted to Na+ form by immersing in 1 M NaCl solution to liberate the H+ ions. The H+ ions in solution were then titrated with 0.01 M NaOH and phenolphthalein indicator. The IEC is calculated from the following equation [6, 16, 25, 35]:
Where a is the milli-equivalent of ion exchange group in membrane and W dry is the weight of dry membrane (g).
The fixed ion concentration also (F.I.C) can be calculated by:
Membrane potential, transport number, and permselectivity
The membrane potential is an algebraic sum of Donnan and diffusion potentials determined by the partition of ions into the pores as well as the mobilities of ions within the membrane phase compared with the external phase [17, 18]. This parameter was evaluated for the equilibrated membrane with unequal concentrations of electrolyte solution (NaCl, C1 = 0.1 M, C2 = 0.01 M at ambient temperature) on either sides of membrane using two-cell glassy apparatus shown in Fig. 1. The developed potential across the membrane was measured by connecting both compartments and using saturated calomel electrode (through KCl bridges) and digital auto multi-meter (DEC, model DEC 330FC, Digital Multimeter, China). The measurement was repeated until a constant value was obtained. The membrane potential (E Measure) is expressed using Nernst equation [16, 18, 35–37] as follows:
Where t i m is transport number of counter ions in membrane phase, R is gas constant, T is temperature, n is the electrovalence of counter-ion, and a 1, a 2 are solutions electrolyte activities in contact membrane surfaces.
The ionic permselectivity of membranes also is quantitatively expressed based on the migration of counter-ion through the IEMs [18, 36, 37]:
Where t 0 is transport number of counter ions in the solution [38].
Concentration of fixed charge on membrane surface
The excessive homogeneity, concentration, and uniform distribution of functional groups on the surface of membranes provide more conducting regions in the membranes and improve their electrochemical properties. Also, the existence of greater conducting regions on the membrane surface can strengthen the intensity of uniform electrical field around the membrane and decreases the concentration polarization phenomenon [39]. The concentration of fixed charge on the membrane surface (Y) has been expressed in terms of permselectivity as follows [10, 18, 36]:
Where P s is the permselectivity and C Mean is the mean concentration of electrolytes.
Ionic permeability and flux of ions
The measurements of ionic permeability and flux were carried out using the test cell. A 0.1 M NaCl solution was placed on one side of the cell and a 0.01 M solution on its other side. A DC electrical potential (Dazheng, DC power supply, Model PS-302D) with an optimal constant voltage was applied across the cell with stable platinum electrodes. The cations pass through the membrane to cathodic section. According to anodic and cathodic reactions, the produced hydroxide ions remain in cathodic section and increase the pH of this region.
Also based on the first Fick’s law, the flux of ions through the membrane can be expressed as follows [15, 16]:
Where P is coefficient diffusion of ions, d is membrane thickness, N is ionic flux, and C is the cations concentration in the compartments.
Where A is the membrane surface area. Integrating of Eq. (8) was as follows:
The diffusion coefficient of cations in membrane phase is calculated from Eq. (10) considering pH changes measurements (Digital pH-meter, Jenway, Model: 3510) in cathodic section.
Electrical resistance
The electrical resistance of equilibrated membrane was measured in NaCl solution with 0.5 M concentration (at 25 °C) following the procedures described earlier [25, 35]. Measurement was carried out by an alternating current bridge with 1500 Hz frequency (Audio signal generator, Electronic Afzar Azma Co. P.J.S). The membrane resistance is calculated using difference resistance between the cell (R 1) and electrolyte solution (R 2) (R m = R 1 − R 2). The areal resistance was expressed as follows:
Where r is areal resistance and A is the surface area of membrane.
Membrane oxidative stability
The prepared membranes were immersed into 3 % H2O2 aqueous solution containing 4 ppm Fe3+ at 25 °C for up to 60 h. The weights of dried samples before and after the experiment were compared. Drying was done at 50 °C for 4 h [19].
Results and discussion
Morphological studies
SOM studies have been carried out to evaluate the spatial distribution of resin particles and CMC in the membrane matrix. The SOM images with ×4 and ×10 magnifications are shown in Figs. 2 and 3. The polymer binder and particles are clearly seen in the images. The particles are observed as dark spots. Images show uniform surface for the prepared membranes relatively. The uniform distribution of particles (resin/CMC) provides more conducting regions for the membrane and generates easy flow channels for counter ions transportation. This also strengthens the intensity of uniform electrical field around the membrane and decreases the concentration polarization phenomenon [39]. The uniform distribution of solid particles in the casting solution also improves the polymer chains relaxation/conformation with particles surfaces which enhances the membrane selectivity [40].
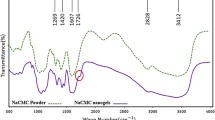
The cross-sectional SEM images of unmodified and PVC/CMC membranes are given in Fig. 4. The SEM images showed uniform and dense structure for the prepared membranes. The structures of membranes were also studied by X-ray diffraction. As shown in Fig. 5, there is no peak in XRD pattern for virgin membrane and PVC/CMC modified ones. The results demonstrated amorphous structure for prepared membranes.
Water content and contact angle measurements
Results (Fig. 6) demonstrated that increases of carboxy methyl cellulose ratio in the casting solution led to increase of water content in prepared membranes. This is due to hydrophilic characteristic of CMC which increases the amount of absorbed water in the membranes matrix. Moreover, gelatinous behavior of CMC in aqueous solution accommodates more water molecules in the membrane body. In general, high water content can provide more and wider transfer channels for the co/counter ions transportation and decrease the ion selectivity.
Moreover, Fig. 6 shows the effect of CMC blend ratio on contact angle and wettability of surface of prepared membranes. The results exhibited that increase of CMC percentage ratio in the membrane matrix led to decrease of water contact angle for the prepared membranes. This is due to abundant hydrophilic hydroxyl and carboxyl groups of CMC which is hydrated by water molecules and enhances the surface hydrophilicity.
Ion exchange capacity (IEC) and ionic concentration (FIC)
The use of CMC up to 16 %wt in membrane matrix initially led to an improvement in ion exchange capacity for the prepared membranes (Fig. 7). This is attributed to the fact that carboxyl groups of CMC provide more ionic functional groups for the membrane and enhance the ion exchange possibilities. The ion exchange capacity was decreased again by more increase of CMC blend ratio from 16 to 32 %wt. This is due to resin particles surrounded by the CMC particles at its high concentration which restricts the activity of sulfonate groups in resin particles and so declines the membrane IEC slightly.
There is a relationship between the IEC and water content and so their effects during the process are open to optimization. For the purpose, FIC or equivalent of functional group per absorbed water content can be used. The membrane FIC is shown in Fig. 7. The high fixed ion concentration can have better control on the pathways of counter ions traffic in the matrix of membrane and increases the ionic selectivity.
Membrane potential, charge density, permselectivity, and transport number
When both surfaces of an ion exchange membrane are in contact with a solution which has different concentrations, an electrical potential would be developed across the membrane. Magnitude of this parameter depends on the electrochemical characteristic of the membrane along with nature and concentration of electrolyte solution [17, 18]. Figures 8 and 9 showed that membrane potential, transport number, and permselectivity were increased initially by increase of CMC ratio up to 16 %wt in prepared membranes. This is attributed to increase of membrane fixed ionic concentration (Fig. 7) and membrane charge density (Fig. 8) which enhances the Donnan exclusion [17, 18, 41]. The increase of conducting regions on the surface and in the bulk of membrane matrix also enhances the intensity of uniform electrical field around the membrane which declines the concentration polarization phenomenon. The potential, transport number, and permselectivity were decreased again by more increase of CMC concentration from 16 to 32 %wt. This is due to decrease of membrane FIC and surface charge density which facilitate the co-ions percolation through the membrane. In addition, increase of membrane water content and its swelling by increase of CMC ratio make wide ionic transfer pathways for the membrane and reduce the ionic sites domination on ions traffic which decline membrane selectivity/transport number.
The effect of concentration and pH on transport number and selectivity
Table 3 exhibited that membrane potential, transport number, and selectivity all were improved by increase of electrolyte concentration. This may be attributed to high concentration of counter ions in electrolyte environment at high electrolyte concentration which increases the possibility of counter ions interaction with membrane surface. This leads to enhanced Donnan exclusion. The obtained results are significantly in contrast with Donnan equilibrium theory [3, 25]. Also obtained results (Table 4) revealed that prepared membranes have higher potential, transport number, and selectivity at pH 7 compared to other pH values. This is because of the difference in dissociation of membrane functional groups at various pH values which has important impact on the charge nature of membrane matrix [17] and enforces the ionic sites domination on ions traffic. At suitable electrolyte pH, more dissociation and higher activation of ion exchange functional groups in the membrane matrix increase the membrane charge density and strengthen the dominations of ion exchange functional groups on ions traffic.
Ionic permeability and flux of ions
The ionic permeability and flux (Fig. 10) were enhanced by increase of CMC blend ratio up to 16 %wt in the casting solution. This is attributed to formation of suitable ionic pathways in membrane matrix by utilizing of CMC which provides wide transfer channels in membrane matrix by its gelatinous behavior. The increase of membrane IEC, FIC, and surface charge density due to abundant hydroxyl and carboxyl groups of CMC facilitates the ions transportation through the membrane matrix. Moreover, hydrolysis phenomenon of CMC at high pH values increases the negative charge of membrane matrix [42, 43] and improves the ionic permeability and flux.
The ionic permeability and flux were decreased again by more increase of CMC concentration because of decrease on membrane IEC, FIC, and also surface charge density which reduces the counter ions interactions with membrane and so declines the ionic permeability and flux.
Electrical resistance
The electrical resistance is practically important due to its relation with energy consumption in the process [35]. The areal electrical resistance of the prepared membranes (Fig. 11) was decreased sharply by utilizing of CMC in casting solution. This is because of increase in membrane water content, membrane IEC, and also membrane swelling by using of CMC which provides suitable ionic transfer channels in membrane matrix and declines the areal electrical resistance.
The effect of concentration and pH on membrane electrical resistance
The effects of electrolyte concentration and pH variations on electrical resistance of prepared membranes are given in Table 5. The significant increase in membrane resistance at lower salt concentration is attributed to the diffusion boundary layer resistance which is more significant at lower salt concentration. Moreover, at high electrolyte concentration, the membrane swelling decreases the electrical resistance. The obtained results also showed that membrane electrical resistance was increased initially by increase of pH value and then began to decrease. Variations of membrane conductance may be explained with respect to membrane selectivity at the given electrolyte environment. In general, less selective membranes have lower membrane resistances but this is not always true and depends on the membrane structure and its properties [17, 44].
A comparison between the electrochemical properties of prepared membranes in this study and some commercial/studied membranes is given in Table 6. Results show that modified membrane in the present study is comparable with that of other reported ones.
Membrane oxidative stability
The obtained results (Fig. 12) indicated that oxidative stability of prepared membranes was declined by increase of CMC content ratio in membrane matrix. This may be due to hydrophilic characteristic of CMC which facilitates the diffusion of oxidant solution into the membrane matrix. The slight increase of oxidative stability at high additive concentration is attributed to gelatinous behavior of CMC in aqueous solution which restricts the oxidant in the membrane body.
Conclusion
In this study, PVC-based heterogeneous cation exchange membranes were modified by embedding CMC in ionic transfer channels of the membranes. Also membranes were fabricated by solution casting technique using THF as solvent and cation exchange resin powder as functional groups agents. SOM images showed uniform particles distribution and uniform surface for the prepared membranes. The SEM images showed uniform and dense structure for the prepared membranes. The XRD pattern also demonstrated amorphous structure for the membranes. The increases of CMC ratio in the membrane matrix led to increase of membrane water content and decrease in water contact angle obviously. The membrane ion exchange capacity and fixed ion concentration were enhanced initially by increase of CMC ratio up to 16 %wt and then declined by more concentration of CMC from 16 to 32 %wt. It was found that membrane potential and charge density, permselectivity and transport number, ionic permeability, and flux all were increased by using of CMC up to 16 %wt in membranes body and then decreased again by more CMC percentage ratio. Additionally, membrane oxidative stability and electrical resistance were decreased by increase of CMC concentration in the homemade membranes. Membrane transport number, selectivity, and membrane electrical conductivity were enhanced by increase of electrolyte concentration. In addition, prepared membranes showed higher transport number, selectivity, and areal electrical resistance at neutral pH compared to other pH values. The obtained results are valuable in the electro-membrane processes especially, in the electrodialysis process related to water recovery and treatment.
References
Volodina E, Pismenskaya N, Nikonenko V, Larchet C, Pourcelly G (2005) Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J Colloid Interface Sci 285:247–58
Vyas PV, Ray P, Adhikary SK, Shah BG, Rangarajan R (2003) Studies of the effect of variation of blend ratio on permselectivity and heterogeneity of ion-exchange membranes. J Colloid Interface Sci 257:127–34
Baker RW (2004) Membrane technology and applications, 2nd edn. Wiley, England
Elattar A, Elmidaoui A, Pismenskaia N, Gavach C, Pourcelly G (1998) Comparison of transport properties of monovalent anions through anion-exchange membranes. J Membr Sci 143:249–61
Nagarale RK, Gohil GS, Shahi VK, Trivedi GS, Rangarajan R (2004) Preparation and electrochemical characterization of cation- and anion-exchange/polyaniline composite membranes. J Colloid Interface Sci 277:162–71
Nagarale RK, Shahi VK, Schubert R, Rangarajan R, Mehnert R (2004) Development of urethane acrylate composite ion-exchange membranes and their electrochemical characterization. J Colloid Interface Sci 270:446–54
Hwang GJ, Ohya H, Nagai T (1999) Ion exchange membrane based on block copolymers. Part III. Preparation of cation exchange membrane. J Membr Sci 156:61–5
M’ Bareck CO, Nguyen QT, Alexandre S, Zimmerlin I (2006) Fabrication of ion exchange ultrafiltration membranes for water treatment. I. Semi-interpenetrating polymer networks of polysulfone and poly (acrylic acid). J Membr Sci 278:10–8
Schauer J, Brozova L (2005) Heterogeneous ion-exchange membranes based on sulfonated poly (1,4-phenylene sulfide) and linear polyethylene: preparation, oxidation stability, methanol permeability and electrochemical properties. J Membr Sci 250:151–7
Shahi VK, Trivedi GS, Thampy SK, Rangarajan R (2003) Studies on the electrochemical and permeation characteristic of asymmetric charged porous membranes. J Colloid Interface Sci 262:566–73
Dlugolecki P, Anet B, Metz SJ, Nijmeijer K, Wessling M (2010) Transport limitations in ion exchange membranes at low salt concentrations. J Membr Sci 346:163–71
Gohil GS, Shahi VK, Rangarajan R (2004) Comparative studies on electrochemical characterization of homogeneous and heterogeneous type of ion-exchange membranes. J Membr Sci 240:211–9
Kariduraganavar MY, Nagarale RK, Kittur AA, Kulkarni SS (2006) Ion-exchange membranes: preparative methods for electro-dialysis and fuel cell application. Desalination 197:225–46
Nagarale RK, Gohil GS, Shahi VK (2006) Recent developments on ion-exchange membranes and electro-membrane processes. Adv Colloid Interface Sci 119:97–130
Kerres J, Cui W, Disson R, Neubrand W (1998) Development and characterization of crosslinked ionomer membranes based upon sulfinated and sulfonated PSU crosslinked PSU blend membranes by disproportionation of sulfinic acid groups. J Membr Sci 139:211–25
Li X, Wang Z, Lu H, Zhao C, Na H, Zhao C (2005) Electrochemical properties of sulfonated PEEK used for ion exchange membranes. J Membr Sci 254:147–55
Nagarale RK, Gohil GS, Shahi VK, Rangarajan R (2004) Preparation and electrochemical characterization of cation-exchange membranes with different functional groups. Colloids Surf A 251:133–40
Nagarale RK, Shahi VK, Thampy SK, Rangarajan R (2004) Studies on electrochemical characterization of polycarbonate and polysulfone based heterogeneous cation-exchange membranes. React Funct Polym 61:131–8
Hosseini SM, Madaeni SS, Khodabakhshi AR (2011) Preparation and characterization of heterogeneous cation exchange membranes based on S-poly vinyl chloride and polycarbonate cations. Sep Sci Technol 46:794–808
Hosseini SM, Madaeni SS, Khodabakhshi AR (2010) Preparation and characterization of ABS/HIPS heterogeneous cation exchange membranes with various blend ratios of polymer binder. J Membr Sci 351:178–88
Khodabakhshi AR, Madaeni SS, Hosseini SM (2011) Effect of polymers blend ratio binder on electrochemical and morphological properties of PC/S-PVC-based heterogeneous cation-exchange membranes. J Appl Polym Sci 120:644–56
Hosseini SM, Madaeni SS, Heidari AR, Amirimehr A (2012) Preparation and characterization of ion-selective polyvinyl chloride based heterogeneous cation exchange membrane modified by magnetic iron–nickel oxide nanoparticles. Desalination 284:191–9
Khodabakhshi AR, Madaeni SS, Hosseini SM (2011) Investigation of electrochemical and morphological properties of S-PVC based heterogeneous cation-exchange membranes modified by sodium dodecyl sulphate. Sep Pur Tech 77:220–9
Hosseini SM, Madaeni SS, Heidari AR, Moghadassi AR (2011) Preparation and characterization of polyvinyl chloride/styrene butadiene rubber blend heterogeneous cation exchange membrane modified by potassium perchlorate. Desalination 279:306–14
Sata T (2004) Ion exchange membranes: preparation, characterization, modification and application. The Royal Society of Chemistry, Cambridge
Shah BG, Shahi VK, Thampy SK, Rangarajan R, Ghosh PK (2005) Comparative studies on performance of inter polymer and heterogeneous ion-exchange membranes for water desalination by electrodialysis. Desalination 172:257–65
Hosseini SM, Madaeni SS, Khodabakhshi AR (2010) Preparation and surface modification of PVC/SBR heterogeneous cation exchange membrane with silver nanoparticles by plasma treatment. J Membr Sci 365:438–46
Hosseini SM, Madaeni SS, Heidari AR, Khodabakhshi AR (2012) Preparation and characterization of poly (vinyl chloride)-blend-poly (carbonate) heterogeneous cation exchange membrane: investigation of solvent type and ratio effects. Desalination 285:253–62
Ibrahim MM, Koschella A, Kadry G, Heinze T (2013) Evaluation of cellulose and carboxy methyl cellulose/poly (vinyl alcohol) membranes. Carbohydr Polym 95:414–20
Rahimpour A, Madaeni SS (2007) Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: preparation, morphology, performance and antifouling properties. J Membr Sci 305:299–312
Hosseini SM, Gholami A, Madaeni SS, Moghadassi AR, Hamidi AR (2012) Fabrication of (polyvinyl chloride/cellulose acetate) electrodialysis heterogeneous cation exchange membrane: characterization and performance in desalination process. Desalination 306:51–9
Wiks ES (2001) Industrial polymers handbook: products, processes, application. Wiley-VCH, Germany
Mark JE (1999) Polymer data handbook. Oxford University Press Inc, New York
Harper CA (1975) Handbook of plastic and elastomers. McGraw-Hill, New York
Tanaka Y (2007) Ion exchange membranes: fundamentals and applications. Membrane science and technology series. Elsevier, Netherlands
Nagarale RK, Shahi VK, Rangarajan R (2005) Preparation of polyvinyl alcohol-silica hybrid heterogeneous anion-exchange membranes by sol–gel method and their characterization. J Membr Sci 248:37–44
Gohil GS, Binsu VV, Shahi VK (2006) Preparation and characterization of mono-valent ion selective polypyrrole composite ion-exchange membranes. J Membr Sci 280:210–8
Lide DR (2006) CRC handbook of chemistry and physics, 87th edn. CRC, Boca Raton
Kang MS, Choi YJ, Choi IJ, Yoon TH, Moon SH (2003) Electrochemical characterization of sulfonated poly(arylene ether sulphone) (S-PES) cation-exchange membranes. J Membr Sci 216:39–53
Powell CE, Qiao GG (2006) Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J Membr Sci 279:1–49
Shahi VK, Thampy SK, Rangarajan R (1999) Studies on transport properties of surfactant immobilized anion-exchange membrane. J Membr Sci 158:77–83
Ghaemi N, Madaeni SS, Alizadeh A, Daraei P, Vatanpour V, Falsafi M (2012) Fabrication of cellulose acetate/sodium dodecyl sulfate nanofiltration membrane: characterization and performance in rejection of pesticides. Desalination 290:99–106
Ghaemi N, Madaeni SS, Alizadeh A, Daraei P, Zinatizadeh AA, Rahimpour F (2012) Separation of nitrophenols using cellulose acetate nanofiltration membrane: influence of surfactant additives. Sep Pur Tech 85:147–56
Długolecki P, Nymeijer K, Metz S, Wessling M (2008) Current status of ion exchange membranes for power generation from salinity gradients. J Membr Sci 319:214–22
Acknowledgments
The authors gratefully acknowledge Arak University for the financial support during this research (No. 94/2108- 22/4/1394). Authors are also grateful to Mr. Alireza Hamidi for his valuable helps during this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, S.M., Andani, S.M.J.M. & Jafari, M.R. Tailoring the ionic transfer characteristics of polyvinyl chloride-based heterogeneous ion exchange membranes by embedding carboxy methyl cellulose in membrane channels. J Polym Res 23, 160 (2016). https://doi.org/10.1007/s10965-016-1053-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-1053-y