Abstract
In this work, a crystal chemical analysis of the known experimentally deciphered crystal structures of tetrahedrite group minerals was carried out in order to reveal the relationships between the occupancies of anion crystallographic sites in the structures, their effective sizes and unit cell parameters. To achieve this aim, we analyzed the effective sizes of the 24g and 2a anion sites in 68 deciphered crystal structures of tetrahedrite group minerals according to the published data. The analysis was carried out using the TOPOSPro software package by partitioning the space into Voronoi–Dirichlet polyhedra (VDP). It has been shown theoretically for the first time that the content of a large sulfur ion and its deficiency affect the unit cell parameter. A linear correlation between the VDP volume of the anionic site of S2– (24g) and the unit cell parameter in minerals of the tetrahedrite group was established, which shows that the anionic substructure dictates the structural motif in this class of compounds. It was found that the change in the VDP volumes of sulfur anions is associated with different occupancies of anionic sites. It is found that the formula (unit cell) of the compound contains fewer than 13 sulfur ions in almost all deciphered crystal structures of tetrahedrite group minerals. It was concluded that the calculated VDP volume of the 24g anionic position less than 22 Å3 indicates a significant deficit in the anionic substructure. It was shown that, using information about the VDP volumes of all anionic and cationic sites in the structure, it is possible to predict the unit cell parameters of minerals of the tetrahedrite group with an accuracy of 0.01 Å.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Minerals of the tetrahedrite group (fahlore) that belong to the cubic syngony with the general structural formula \(_{{}}^{{M(2)}}\)A\(_{6}^{{M(1)}}\)(B4C2)\(_{{}}^{{X(3)}}\)D\(_{4}^{{{\text{S}}(1)}}\)Y\(_{{12}}^{{{\text{S}}(2)}}\)Z, where A = Cu+, Ag+, \(\square \) (vacancy), and (Ag6)4+ clusters; B = Cu+ and Ag+; C = Zn2+, Fe2+, Hg2+, Cd2+, Mn2+, Cu2+, Cu+, and Fe3+; D = Sb3+, As3+, Bi3+, and Te4+; Y = S2– and Se2–; Z =S2–, Se2–, and \(\square \) (vacancy), are the most common sulfosalts found in many hydrothermal deposits [1]. As is seen from the formula, the characteristic feature of the minerals in this group is the realization of various substitutions of isovalent and heterovalent isomorphisms, which complicates their elemental analysis and assessment of the contribution made by each component to the value of the unit cell parameter. Furthermore, fahlores were found to have large variations in the ratios of ΣMe : S, ΣMe : ΣSemiMe, and ΣMe+ : ΣMe++ and showed deviations from the stoichiometric ratios in the idealized formula, which was detected in both natural and synthetic fahlores [2]. For instance, the generalized empirical formula for the fahlore from the Darasun deposit according to 459 electron probe microanalyses (EPMA) is Me\(_{{(9.38--10.56)}}^{ + }\)Me\(_{{(1.76--2.63)}}^{{ + + }}\)SemiМе(3.42–4.41)(S, Se)(12.38–13.47) [3]. As is seen from the formula, the coefficients of the elements vary within one atom per formula, exceeding the analytical uncertainty of the method for determining the chemical composition of the mineral. However, the variations in the ranges of the above ratios in natural fahlore and their synthetic analogs differ: the variations in natural fahlores are somewhat shifted towards a higher sulfur concentration (or a lower metal content) [2, 4, 5]. Taking into account the possible nonstoichiometry of fahlore, it is necessary to identify the correlation between it and its crystal structural parameters, which will enable us to interpret the experimental data on the chemical composition and powder diffraction more qualitatively.
In order to consider the whole variety of the chemical variability and atomic sites in the unit cell of fahlore, the Commission on New Minerals Nomenclature and Classification of the International Mineralogical Association (IMA-CNMNC) proposed a new nomenclature [1]. Today, the official IMA-CNMNC list of mineral names of the tetrahedrite group includes ten subgroups (series) under the name of minerals in the group for tetrahedrite, in which 45 mineral species (hypothetical endmembers), of which 40 are approved and 5 are predicted, but not yet found in nature and require official approval by the IMA-CNMNC (https://www.mindat.org/min-29338.html). Subgroups are separated by the dominant (>50%) chemical element in positions A, B, D, and Y, while the names of mineral species are given by the dominant chemical element in positions C and Z (component C indicates the charge-compensating constituent and is added after the root name of the group by a hyphen in parentheses; if there is a vacancy in position Z, the prefix “keno-” is added to the name of the mineral species).
The parameters of the unit cell of the tetrahedrite group minerals depend strongly on the chemical composition [2]. It was found that the parameter of the unit cell decreases from 10.32 to 10.19 Å at a decrease in the Sb content and a simultaneous increase in the As content. The most common isomorphic divalent metals, Zn and Fe, which have similar ionic radii, have the same effect on the fahlore cell: they slightly expand it (by 0.031 Å per atom of Zn(Fe) in the formula). The inclusion of the large Hg cation increases the size of the unit cell in direct proportion. The Bi content in fahlore also increases the size of its unit cell, whereas the Te content reduces it slightly. At an increase in the Ag content, the fahlore structure shows two trends: (1) the unit cell parameters increase linearly from 10.3 to 11.0 Å; (2) the unit cell parameters increase from 10.3 to 10.6 Å (until the concentration reaches approximately four atomsFootnote 1 per formula unit) and then decrease to 10.4 Å, which is related to the formation of (Ag6)4+ clusters [1].
Attempts were made to identify the quantitative relationship between the unit cell parameter, the composition, and nonstoichiometry of fahlore [2, 4, 6]. To derive equations connecting these values, the authors took into account the influence of the As, Fe, Zn, Hg, and Ag cations and the ΣMe : S ratio on the parameter a0 of pure tetrahedrite, which is equal to 10.319 ÅFootnote 2. Differences were found in the influence of nonstoichiometry (ΣMe:S) of fahlore on its unit cell parameter: for synthetic fahlores, the contribution of nonstoichiometry to the parameter a0 was significant (0.027 and 0.075) [4, 6], while for natural fahlores, this contribution was unappreciable (0.007) [2].
Despite the variety of works aimed at detecting the relationship between the value of the unit cell parameter and the main isomorphic elements in the fahlore structure, the researchers took into account only the cationic sites and did not consider the influence of the anionic sites on the parameter a0.
The “long-lived” question in the crystal chemistry of tetrahedrite is about the number of sulfur ions in its formula/unit cell. N.V. Belov stated that tetrahedrite formula contains twelve sulfur ions, while most researchers believed that there were thirteen sulfur ions in the formula [7]. The new IMA-CNMC nomenclature and subsequent works reported that a sulfur vacancy can appear only at the site of octahedrally coordinated sulfur ([1, 8–11], etc.). However, recent studies revealed a sulfur deficit in the freibergite series in the argentotetrahedrite–kenoargentotetrahedrite series: up to two or more sulfur vacancies per formula unit according to the EPMA data, which is due to the presence of vacancies in both the octahedral and tetrahedral sites [12].
Nevertheless, in most cases, to calculate the atomic contents of elements in the chemical formula and to decipher the crystal structures of tetrahedrite group minerals, a formula is used in which 13 is the total number of anions in the Y and Z positions. However, some experimental data on the deciphering of tetrahedrite crystal structures [13] demonstrate noninteger occupancies of the anionic Y and Z positions are almost always observed in these crystal structures. Therefore, tools need to be developed that allow for the correct processing and interpretation of elemental analysis and powder diffraction data in order to establish relevant formulas of fahlore group minerals. In this work, we conducted a crystal chemical analysis of experimentally deciphered crystal structures of tetrahedrite group minerals in order to reveal the relationships between the occupancy of anion crystallographic sites in the structures, their effective sizes, and the unit cell parameters.
RESULTS AND DISCUSSION
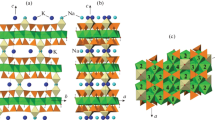
In the structural type of tetrahedrite, there are two crystallographically independent Wyckoff positions identified in the anionic substructure, which is mostly comprised of sulfur ions, 24g and 2a (Fig. 1а). According to the structural analyses of tetrahedrite group minerals, 2a site is partially occupied in many cases, while 24g site is fully occupied in most structural interpretations. Since the large anions dictate the structural metrics, it is logical to assume that the occupancy of anionic sites affects the parameters of the unit cell in the structures considered. In order to reveal the correlation between the unit cell parameters and sulfur site occupancy, the effective sizes of the 24g and 2a anion sites (Fig. 1b) were analyzed in 68 deciphered crystal structures based on the published data. The analysis was conducted using the TOPOSPro software package [14] with the Voronoi–Dirichlet polyhedra (VDP) method of space partitioning. The VDP volumes correspond to the effective volumes of ions in crystal structures, and the radii of the spheres, the volume of which corresponds to the volume of the corresponding VDP, represent the effective radii of the ions in a particular crystallographic site. Thus, by analyzing the VDP characteristics for the same ions in the crystal structures, we can compare their effective sizes in different compounds. Figure 1a shows the crystal structure of tetrahedrite in the classical polyhedral representation, while Fig. 1b shows VDP for sulfur anions. The average effective radius of S2– in the 24g site is 1.75 Å, which is quite close to the ionic radius of S2– according to ionic radii table (1.84 Å), while the average radius of S2– in the 2а site is significantly smaller and equals 1.45 Å, which indicates its partial occupancy.
As a result of analyzing the VDP volumes for various crystallographic sites in 68 experimentally deciphered structures of tetrahedrite group minerals, a linear correlation was found between the unit cell parameters and the VDP volume for the 24g anion site (Fig. 2а): as the VDP volume increases from 17 to 22 Å3, the unit cell parameters increase from 10.2 to 11.0 Å, respectively. We note that no reliable correlations with VDP volumes were found for the 2a anion site (Fig. 2b) and the 12e, 12d, and 8c cation sites.
We assume that the size of the 24g site depends on its occupancy by sulfur ions in the crystal structure: the higher the sulfur occupancy, the larger the unit cell parameter, and vice versa. Judging by the scattering of the unit cell parameters and the volumes of the 24g site, most tetrahedrite structures deciphered experimentally contain fewer than 13 sulfur atoms in the formula. However, it is currently not provable by the available experimental data since the formula for most structures is calculated for 13 sulfur atoms. Only in three deciphered structures [13] did the formula contain fewer than 13 sulfur atoms. We also note that it seems impossible to construct a reliable plot of the dependence between the cell parameter and the sulfur content since almost all analyses are calculated for 13 sulfur atoms. However, we conclude that a 24g site volume less than 22 Å3 indicates a sulfur deficit in the anionic substructure and the need to normalize the formula to a lesser amount of sulfur in the structure.
The use of a machine learning algorithm showed that the unit cell parameters of various minerals of the tetrahedrite group can be predicted just by using information about the VDP volumes calculated for all crystallographically inequivalent sites of anions (2a and 24g) and cations (12e, 12d, 8c) in the crystal structures. To perform such an analysis, the VDP volume data were normalized, and weight coefficients were set in the function depending on the VDP volumes. Built-in tools of the Python programing language were used to build a decision-making algorithm for successful prediction of the unit cell parameter based on the experimental data. All available crystallographic data about 68 crystal structures were divided into the testing and training sets at a ratio of 80% to 20%. The training set was used to train the machine learning algorithm (selection of nonlinear function criteria), and the testing set was used to verify the correctness of the algorithm operation. Figure 3 shows the comparison of the experimentally determined parameters of the unit cells of tetrahedrite group minerals of different compositions (the x-axis) and the predicted values of the unit cell parameters by an automatic algorithm with machine learning elements (the y-axis) for the test data set. The root-mean-square error of the algorithm in predicting the unit cell parameter was 0.97, and the mean absolute error was 0.01 Å. Thus, for the sample of tetrahedrite group mineral selected particularly, nonintegral occupancies may occur in both cationic and anionic substructures. Consequently, the conventional normalization of the fahlore formula to 13 sulfur atoms is incorrect in most cases.
Comparison of the unit cell parameter predicted by the machine learning model and from the experimental data. The plot shows structures from the test set used to evaluate the accuracy of the algorithm operation. R2 is the coefficient of determination, MAE is the mean absolute error, and RMSE is the root-mean-square error.
CONCLUSIONS
Based on the available crystallographic data on the structures of the tetrahedrite group minerals and data from the crystal chemical analysis of the Voronoi–Dirichlet polyhedra conducted in this study, we make the following conclusions.
(1) It was shown theoretically for the first time that the contents of the large ion of sulfur and its deficit affect the unit cell parameter. There is a linear correlation between the VDP volume of the 24g site of S2– anion and the unit cell parameter in the minerals of the tetrahedrite group, which indicates that the anionic substructure dictates the structural framework in this class of compounds. The change in the VDP volumes of sulfur anions is associated with different occupancies of the anion sites.
(2) In almost all deciphered crystal structures of tetrahedrite group minerals, the formula of the compound has fewer than 13 sulfur ions (from 12 to 13 atoms per formula); i.e., there is a certain deficit in the number of sulfur ions in the unit cell.
(3) Calculation of the VDP volume of the 24g anion site makes it possible to determine whether the normalization of the compound formula to 13 sulfur atoms is correct: a value less than 22 Å3 indicates a significant deficit in the anionic substructure and the need for recalculation of the formula.
(4) Given only the unit cell parameters of tetrahedrite group minerals, today it is hardly possible to determine the number of vacancies in sulfur sites due to the lack of correct structural interpretations with crystallographic models taking into account noninteger occupancies of anion sites.
(5) The unit cell parameters of tetrahedrite group minerals can be predicted with an accuracy of up to 0.01 Å by using information about the VDP volumes of all anion and cation sites in the structure.
Notes
According to the data of Mozgovaya and Tsepin [2], the inflection in the dependence of parameter a0 on the Ag content is when the Ag content = 3.7 Ag atoms in the formula.
a0 (Å) = 10.319 – 0.059KAs + 0.075(ΣMe:S) [6], where Ki are formula coefficients of the respective elements, the ΣMe:S ratio is the nonstoichiometry measure. a0 (Å) = 10.319 + 0.017KFe + 0.027(ΣMe:S) [4]; a0 (Å) = 10.319 + 0.031KFe + 0.028KZn + 0.096KHg + 0.007(ΣMe:S) – 0.040KAs + “Ag” [2], where “Ag” = KAg/(21.9-1.01KAg), when the Ag content is <3.7 atoms in the formula and “Ag” = 1/(1.66KAg – 1.28) when the Ag concentration is >3.7 atoms in the formula.
REFERENCES
C. Biagioni, L. L. George, N. J. Cook, E. Makovicky, Y. Moëlo, M. Pasero, J. Sejkora, C. J. Stanley, M. D. Welch, and F. Bosi, Am. Mineral. 105 (1), 109–122 (2020). https://doi.org/10.2138/am-2020-7128
N. N. Mozgova and A. I. Tsepin, Fahlores: Features of Chemical Composition and Properties (Nauka, Moscow, 1983) [in Russian].
N. G. Lyubimtseva, N. S. Bortnikov, S. E. Borisovsky, V. Yu. Prokofiev, and O. V. Vikent’eva, Geol. Ore Deposits 60 (2), 93–120 (2018). https://doi.org/10.1134/S1075701518020034
K. Tatsuka and N. Morimoto, Am. Mineral. 62 (11-12), 1101–1109 (1977).
N. S. Bortnikov and I. Ya. Nekrasov, Dokl. Akad. Nauk SSSR 297 (2), 449–451 (1987).
F. D. Luce, C. L. Tuttle, and B. J. Skinner, Econ. Geol. 72 (2), 271–289 (1977). https://doi.org/10.2113/gsecongeo.72.2.271
N. V. Belov and E. A. Pobedimskaya, Mineral. Sb. L’vov. Gos. Univ. im. I. Franko, Iss. 1 (27), 3–9 (1973).
M. D. Welch, C. J. Stanley, J. Spratt, and S. J. Mills, Eur. J. Mineral. 30 (6), 1163–1172 (2018). https://doi.org/10.1127/ejm/2018/0030-2773
C. Biagioni, J. Sejkora, Y. Moëlo, E. Makovicky, M. Pasero, and Z. Dolniček, Mineral. Mag. 84, 971−975 (2020). https://doi.org/10.1180/mgm.2020.93
K. Qu, X. Sima, X. Gu, W. Sun, G. Fan, Z. Hou, P. Ni, D. Wang, Z. Yang, and Y. Wang, Mineral. Mag. 85, 278−281 (2021). https://doi.org/10.1180/mgm.2021.5
Z. Shu, C. Shen, A. Lu, and X. Gu, Crystals 12 (4), 467 (2022). https://doi.org/10.3390/cryst12040467
R. O. Sack, N. G. Lyubimtseva, N. S. Bortnikov, E. Y. Anikina, and S. E. Borisovsky, Contrib. Mineral. Petrol. 177 (8), 82 (2022). https://doi.org/10.1007/s00410-022-01942-5
I. V. Rozhdestvenskaya, N. V. Zayakina, and V. P. Samusikov, Mineral. Zh. 15, 9 (1993).
V. A. Blatov, A. P. Shevchenko, and D. M. Proserpio, Cryst. Growth Des. Am. Chem. Soc. 14 (7), 3576–3586 (2014). https://doi.org/10.1021/cg500498k
Funding
This work was carried out under project no. 121041500220-0 “Structural and Chemical Inhomogeneities and Paragenetic Mineral Associations as a Reflection of the Petro- and Ore Genesis” of the Institute of Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflicts of interest.
Additional information
Translated by L. Mukhortova
Rights and permissions
About this article
Cite this article
Lyubimtseva, N.G., Marchenko, E.I., Eremin, N.N. et al. To a Question of Sulfur Sites in Crystal Structures of Tetrahedrite Group Minerals: Relationships between Occupancy, Effective Ion Sizes, and Unit Cell Parameters. Dokl. Earth Sc. 512, 824–828 (2023). https://doi.org/10.1134/S1028334X23601189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X23601189