Abstract
The implantation of \({\text{O}}_{2}^{ + }\) ions in Si with an energy of E0 = 1 keV followed by annealing at 850–900 K yielded nanocluster phases and SiO2 films. Using Auger-electron spectroscopy, spectroscopy of elastically reflected slow electrons, and ultraviolet photoelectron spectroscopy, the composition, formation depths, and electronic structure of nanocluster phases and SiO2 layers are studied. It is found that nanocluster phases and layers of SiO2 at doses of D ≤ 5 × 1015 cm–2 are formed in the surface layer at a depth of 25–30 Å. With an increase in the ion dose, the SiO2-oxide layer shifts towards the surface, and at D ≈ 6 × 1016 cm–2, a continuous SiO2 film with a thickness of ~25–30 Å is formed. The band gap of this film is 7.9 eV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Nanoscale structures and layers based on Si, Ge and their oxides are promising for fabricating nanoelectronic and optoelectronic devices. In particular, SiO2/Si heterostructures with various nanoinclusions serve as the basis for the development of new types of high-frequency transistors, integrated circuits, optical converters, and solar cells [1–4]. In this regard, special attention is paid to improving the technology of preparing and studying the regularities of the formation of nanoscale structures with new physical properties. Si nanoclusters in a silicon-oxide matrix are mainly produced by thermal evaporation, laser ablation, and chemical vapor deposition [5, 6]. The passivation of dangling surface bonds in the case of amorphous nanocluster phases requires subsequent annealing at T = 623 K in an oxygen-containing atmosphere. The ion-bombardment method is often used to manufacture nanoscale structures on the surface of semiconductors and dielectric films [7–10]. In [10], the implantation of \({\text{O}}_{2}^{ + }\) ions into Si was used to fabricate nanoscale SiO2 films with a thickness of 30–90 Å and their composition, surface morphology, emission and optical properties were studied. However, the regularities and mechanisms of the formation of SiO2 nanofilms have not been practically studied, and the change in the density of state of valence electrons during the implantation of \({\text{O}}_{2}^{ + }\) ions into Si and subsequent annealing has also not been studied. This work is devoted to studying the regularities of the formation of nanoscale SiO2 phases and SiO2 films during the implantation of \({\text{O}}_{2}^{ + }\) into Si and the study of their composition and electronic structure.
EXPERIMENTAL
The targets were single-crystal Si(111) samples. After grinding, the samples were polished with diamond pastes until a mirror smooth surface was obtained, and then they were subjected to electropolishing in a sulfuric-methanol solution. After washing, the samples were placed in an ultrahigh vacuum device, which consists of two compartments. Annealing and ion bombardment were carried out in the first compartment. In the second compartment, the composition, electronic and optical properties were studied using a set of methods: Auger-electron spectroscopy, spectroscopy of elastically reflected slow electrons and ultraviolet photoelectron spectroscopy.
Before the implantation of \({\text{O}}_{2}^{ + }\) ions, the Si samples were degassed under ultrahigh vacuum conditions (P = 10–6 Pa), first, during long-term annealing at 1200 K for 4–5 h and then, short-term annealing at 1500 K combined with soft etching of the surface with Ar+ ions of E0 = 1 keV at an angle of 10°–15° with respect to the surface. To study the regularities of the formation of nanoscale phases and films of SiO2, we prepared identical Si samples implanted with \({\text{O}}_{2}^{ + }\) ions of Е0 ≈ 1 keV at doses of D = 0, 5 × 1013, 5 × 1014, and 6 × 1016 cm–2. After each cycle of ion implantation, the sample was annealed at the temperature of formation of a SiO2 type compound of ~850–900 K for 40 min [11]. First, the Auger spectra of the Si surface, and then of the surface region at a depth of 25–30 Å were measured. The surface was etched by bombardment with Ar+ ions.
RESULTS AND DISCUSSION
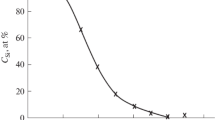
Figure 1 shows the dependence of the intensity I of the Auger peak of О (Е ≈ 506 eV) on the irradiation dose for Si implanted with \({\text{O}}_{2}^{ + }\) ions of Е0 ≈ 1 keV. Curve 1 refers to the variation of I on the surface, and curve 2 refers to that at a depth of ~25 Å, which corresponds to the maximum of the distribution of oxygen atoms near the surface. Figure 1 shows that the oxygen concentration in the surface layer is much higher than that on the surface. At an ion-irradiation dose of D ≥ 5 × 1016 cm–2 both dependences reach a plateau, i.e., the oxygen concentration on the surface and in the surface layer up to a depth of 25–30 Å is distributed almost equally. A SiO2 film with a thickness of ~25–30 Å is formed after annealing at Т ≈ 850–900 K. Based on this, it can be concluded that nanocluster phases and layers of silicon oxide are formed first in the surface layer at a depth where the oxygen distribution maximum is observed. As the ion dose increases, the oxide film grows towards the surface [12–15].
Figure 2 shows the initial part of the spectrum of Auger electrons of Si implanted with \({\text{O}}_{2}^{ + }\) ions of Е0 = 1 keV at D = 6 × 1016 cm–2 measured before and after annealing at 900 K for 40 min. It can be seen that after the implantation of oxygen ions with Е0 = 1 keV the intensity of the Si Auger peak L23VV (ЕAuger = 92 keV) decreases sharply, and peaks at an energy of 84 and 80.5 eV, typical for Si suboxides (SiО, SiО0.5), and a peak at Е = 76 eV for SiО2 appear near it. Analysis of the complete Auger-electron spectrum showed that the Si surface contains ~30–35 at % of SiО2, ~40–45 at % of Si suboxides, and also unbound Si (~10–15 at %) and О (5–10 at %) atoms [16].
The concentration of atoms on the surface and in the surface layer was determined from the change in the intensities of the main high-energy peaks of O (506 eV) and Si (1614 eV) according to the well-known formula:
where I is the peak intensity of the corresponding element, and S is the Auger sensitivity coefficient of this element.
The concentration of SiO2, Si suboxides and excess Si atoms was estimated by the change in the area under the Si peak L23VV at 91 eV before and after ion implantation. After ion implantation, the area under the pure Si peak decreases by about seven times (Fig. 2, curve 2), i.e., it can be assumed that the concentration of pure Si is ~10–15 at %, and Si in a concentration of ~75–80 at % forms a chemical bond with oxygen. The ratio of the areas under the Si + O peaks was used to estimate the concentrations of SiO2, SiO and SiO0.5. After annealing at 900 K the Auger peaks of Si, О and Si suboxides disappear completely, and the intensity of the Auger peak of SiО2 at Е = 76 eV increases to the greatest extent (curve 3).
Figure 3 shows the photoelectron spectra of single-crystal Si(111) implanted with \({\text{O}}_{2}^{ + }\) ions of Е0 = 1 keV at D = 6 × 1016 cm–2 obtained at hν = 21.2 eV before and after annealing at 900 K. The Si(111) spectrum exhibits peaks due to the excitation of electrons from the surface states and the 3p, 3s + 3p, 3s states of valence electrons. After ion implantation, the shape and structure of the energy distribution curve of single-crystal Si(111) changes dramatically; the density of electron states of the valence band and the parameters of the energy bands of pure and ion-implanted Si differ significantly from each other. As shown above, these changes are related to disordering of the surface layers, the formation of various silicon oxides, and the presence of unbound Si and O atoms [10, 16–18]. Therefore, the photoelectron spectrum of ion-implanted Si has a very complex structure, and features associated with the presence of various compounds and unbound atoms are observed in it (Fig. 3, curve 2). The positions of some peaks approximately coincide with those of Si (3.6 eV) and SiО2 (7.2 eV). A continuous SiO2 film with a thickness of d ≈ 25–30 Å is formed after annealing. Two maxima are clearly distinguished in the spectrum of the SiO2 film. The maximum at Еb = 7.2 eV is apparently due to hybridization of the Si3p and O2p states, and the maximum at Еb ≈ 13.2 eV is associated with the hybridization of Si3s and O2p states. Based on analysis of the spectra of photoelectrons and elastically reflected electrons, the energy parameters of the electron bands of ion-implanted Si before and after annealing at 900 K were determined (Table 1).
It can be seen from Table 1 that the energy parameters of the Si(111) bands change sharply after ion implantation. In particular, the Еg value is doubled, which may be due to the presence of non-stoichiometric SiOx oxide in the ion-doped layer. Post-implantation annealing at 900 K leads to the formation of a continuous layer of SiO2 oxide; therefore, the Еg value increases to 7.9 eV [19].
CONCLUSIONS
It has been established that SiO0.5, SiO and SiO2 oxides are formed in the ion-doped layer after the implantation of \({\text{O}}_{2}^{ + }\) ions with Е0 = 1 keV at D = 6 × 1016 cm–2. A uniform continuous SiO2 film 25–30 Å thick was formed after annealing at 900 K. It has been shown that nanocluster SiO2 phases are formed at ion implantation with a dose of D ≤ 5 × 1015 cm–2 after annealing at a depth of 25–30 Å. Starting with a dose of D ≈ 5 × 1015 cm–2, the cluster phases, the boundaries of which overlap, form the SiO2 layers. At a further increase in the ion dose, the oxide film grows towards the surface, and at D ≥ 5 × 1016 cm–2 the thickness reaches a plateau. The density of state of electrons in the valence band has been studied for the first time and the band-energy parameters of Si implanted with \({\text{O}}_{2}^{ + }\) ions of a dose of ~6 × 1016 cm–2 were determined before and after annealing at 900 K.
REFERENCES
E. S. Demidov, A. N. Mikhaylov, A. I. Belov, M. V. Karzanova, N. E. Demidova, Yu. I. Chigirinskii, A. N. Shushunov, D. I. Tetelbaum, O. N. Gorshkov, and E. A. Evropeitsev, Phys. Solid State 53, 2415 (2011). https://doi.org/10.1134/S1063783411120067
D. G. Gromov, O. V. Pyatilova, S. V. Bulyarskii, A. N. Belov, and A. A. Raskin, Phys. Solid State 55, 619 (2013). https://doi.org/10.1134/S106378341303013X
K. Hoppe, W. R. Fahrner, D. Fink, S. Dhamodoran, A. Petrov, A. Chandra, A. Saad, F. Faupel, V. S. K. Chakravadhanula, and V. Zaporotchenko, Nucl. Instrum. Methods Phys. Res., Sect. B 266, 1642 (2008). https://www.doi.org/10.1016/j.nimb.2007.12.069
S. B. Donaev, A. K. Tashatov, and B. E. Umirzakov, J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 9, 406 (2015). https://www.doi.org/10.1134/S1027451015020263
F. Rochet, G. Dufour, H. Roulet, B. Pelloie, J. Perriere, E. Fogarassy, A. Slaoui, and M. Froment, Phys. Rev. B 37, 6468 (1988). https://www.doi.org/10.1103/PhysRevB.37.6468
B. E. Umirzakov, D. A. Tashmukhamedova, M. A. Tursunov, Y. S. Ergashov, and G. Kh. Allayarova, Tech. Phys. 64, 1051 (2019). https://doi.org/10.1134/S1063784219070260
B. E. Umirzakov, R. K. Ashurov, and S. B. Donaev, Tech. Phys. 64, 232 (2019). http://www.doi.org/10.1134/S1063784219020269
S. Takeoka, M. Fujii, and S. Hayashi, Phys. Rev. B 62, 16820 (2000). https://doi.org/10.1103/PhysRevB.62.16820
R. Krishnan, Q. Xie, J. Kulik, X. D. Wang, S. Lu, M. Molinari, Y. Gao, T. D. Krauss, and P. M. Fauchet, J. Appl. Phys. 96, 654 (2004). https://www.doi.org/10.1063/1.1751632
D. A. Tashmukhamedova, M. B. Yusupjanova, G. Kh. Allayarova, and B. E. Umirzakov, Tech. Phys. Lett. 46, 972 (2020). https://doi.org/10.1134/S1063785020100144
Yu. K. Undalov and E. I. Terukov, Semiconductors 49, 867 (2015). https://doi.org/10.1134/S1063782615070222
D. A. Tashmuhameva, M. B. Yusupjanova, and B. E. Umirzakov, Tech. Phys. 61, 627 (2016).
V. F. Zinchenko, K. V. Lavrent’ev, V. V. Emel’yanov, and A. S. Vatuev, Tech. Phys. 61, 187 (2016).
Yu. K. Undalov and E. I. Terukov, Semiconductors 49, 867 (2015). https://doi.org/10.1134/S1063782615070222
D. A. Tashmukhamedova and M. B. Yusupjanova, J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 10, 1273 (2016).
E. V. Parinova, Candidate’s Dissertation in Mathematics and Physics (Voronezh State Univ., Voronezh, 2016).
B. E. Umirzakov, D. A. Tashmukhamedova, G. Kh. Allayarova, and Zh. Sh. Sodikzhanov, Tech. Phys. Lett. 45, 356 (2019). https://doi.org/10.1134/S1063785019040175
Y. S. Ergashov, D. A. Tashmukhamedova, and E. Rabbimov, J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 9, 350 (2015). https://doi.org/10.1134/S1027451015020287
Y. S. Ergashov, D. A. Tashmukhamedova, and B. E. Umirzakov, J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 11, 480 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
I declare that I have no conflicts of interest.
Additional information
Translated by L. Mosina
Rights and permissions
About this article
Cite this article
Allayarova, G.K. Regularities of the Formation of SiO2 Nanophases and Nanofilms on a Si Surface during \({\text{O}}_{2}^{ + }\)-Ion Implantation. J. Surf. Investig. 16, 1171–1174 (2022). https://doi.org/10.1134/S1027451022060039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451022060039