Abstract
Carbon films 50–180 nm thick on nickel substrates are fabricated by the ion sputtering of graphite and the deposition of heavy hydrocarbons from the gas phase with simultaneous electron irradiation. Irradiation results in the formation of bonds in carbon films due to the sp and sp3 hybridization of orbitals (sp and sp3 bonds), mainly, sp3 bonds. A fraction of these bonds does not change with growth in the electron energy; it increases three-fold with a reduction in the temperature and an increase in the electron current density. Electron irradiation enhances the film microhardness which exceeds 12 GPa. The films, prepared by heavy hydrocarbon deposition, contain CHn bonds and a small fraction of sp3 bonds. The maximum value of the microhardness of the hydrocarbon films is no more than 4.5 GPa. The analysis of the proposed model of the kinetics of forming different allotropic phases in a carbon film to be deposited shows that a temperature reduction changes the specific volume of an atom in the lattice, while under conditions of simultaneous electron irradiation, it appreciably increases the content of the phase with sp3 bonds. The effect of spi-bond breakage during electron-beam-assisted deposition weakly depends on the electron energy. The weak excitations of electrons of carbon atoms can also result in the formation of sp3 bonds and increases their concentration with growth in the electron current density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The application of coatings is an efficient method for creating new materials with specified operating properties. Among the techniques for application, material deposition with concurrent ion-beam processing is widely used. Ion irradiation makes it possible to considerably improve the properties of coatings; in particular, increase their density. These processes are caused mainly by elastic collisions in the coating under ion irradiation [1]. At present, of considerable interest are coatings in the form of thin carbon films of various structural modifications with different ratios of carbon bonds, arising due to sp, sp2, and sp3hybridization of orbitals [2–6]. Interest in films with a high content of carbon atoms with sp3 bonds is explained by the unconventional combination of such physicochemical properties as high hardness, wear resistance, chemical inertness, a wide band gap, low friction factor, biocompatibility, etc. Significant advances in the growth and search for the optimal formation modes of carbon films of specified structural modifications are achieved with the use of vacuum growth processes, based on the sputtering of graphite by an ion beam and on exposure of the carbon-condensate structure to either an ion or high-power electron beam [7, 8].
In [9] it was found that with the simultaneous irradiation of nickel by 30-keV C+ ions and 1–5-keV electrons, a carbon film with a thickness of a few tens of nanometers, mainly consisting of amorphous diamond with sp3 bonds, grows on its surface. Studies of carbon films [10], applied on nickel substrates during the ion sputtering of graphite under conditions of concurrent electron irradiation and subsequent ion irradiation, have shown that ion irradiation, following film deposition, induces the formation of sp bonds in them, while the concurrent electron irradiation contributes to a growth in the number of sp3 bonds. Thus it has been shown that the electron irradiation of a carbon film to be deposited must shift the equilibrium concentrations of allotropic phases in the direction of an increase in the concentration of phases with sp3 bonds. The ion beam impacts in a similar manner. Ion irradiation of an already formed film enhances its density [1], increasing the probability of the formation of a shorter allotropic sp bond.
Based on these concepts and for the purpose of optimizing the parameters of the electron-beam–assisted process, it is decided that the processes of carbon structure modification under electron irradiation and at different deposition temperatures, electron energy, and electron density will be studied. In addition, of certain interest is investigation of the structure of hydrocarbon films, deposited from the gas phase with heavy hydrocarbon evaporation under conditions of concurrent electron irradiation, and their comparison with carbon films prepared by the sputtering of graphite.
EXPERIMENTAL
Deposition of films, electron irradiation of samples, and direct ion implantation were carried out at the ILU ion-beam accelerator with the separation of ions by mass [11], in the receiving chamber of which an electron gun was arranged. The electron gun was fastened to the flange of the ion receiver at an angle of 30° to the ion-beam axis, perpendicular to the sample surface. The substrate samples were installed in the holder, which was attached directly to the furnace device with an ohmic heater. The ion-current density was recorded using a special reference plate, located on a diaphragm in front of the sample, while the electron current density was recorded using a Faraday cup, located near the sample. The sample temperature was measured using a platinum–rhodium thermocouple within the range of 470–1270 K. A carbon film was deposited due to the sputtering a graphite target, which was set to an angle of 60° with respect to the sample surface, by a narrow beam of C+ ions [10]. In the experiments for carbon-film deposition using ion sputtering of the graphite target with simultaneous electron irradiation, the following combination of electron and ion beams was used: preliminary implantation of C+ ions (Е = 40 keV, f = 2 × 1021 m–2, j = 0.5 A/m2) into the substrate, then the sputtering of graphite by C+ ions (Е = 40 keV, f = (4–8) × 1022 m–2, j = 2 A/m2), and simultaneous irradiation by electrons (Е = 1–5 keV, f = (1–6) × 1023 m–2, j = 5–30 A/m2).
A schematic of film deposition from the gas phase using the electron-beam-assisted evaporation of heavy hydrocarbons is presented in Fig. 1. As the working medium, heavy hydrocarbons of two types—naphthalene С10Н8 and polyethylene (С2Н4)n—were used, which were loaded into the evaporator crucible and heated up to temperatures of 405 and 498 K, respectively. Metallic substrate samples were installed on a copper receiver, cooled with water. During film deposition (tdepos = 40 min), the substrate temperature, measured by a platinum–rhodium thermocouple, did not exceed 345 K. To separate the droplet phase and decrease the flow of particles of the material to be deposited, a collimator in the form of a disc with a slot is used, which has a collimation coefficient of 1/100. To prevent the deposited substance from being overheated by the electron beam, an insignificant current density of 0.5-keV electrons was chosen which was varied from 1 to 3 A/m2.
For the deposition of carbon films, planar samples with a size of 15 × 15 × 0.5 mm made of Ni of the NP-1 trademark (99.9%) were used as substrates after preannealing (1170 K, 1 h) in a vacuum chamber with a residual pressure of 10–3 Pa. The surface of the Ni substrates was prepared using standard techniques of mechanical and electrolytic polishing.
During studies of carbon films, techniques of profilometry (Alpha-Step-200 profilometer), the optical (Nikon МА100) and electron (JSM-35CF) microscopy, and X-ray photoelectron spectroscopy (XPS) were used [12, 13]. The XPS measurements (panoramic spectrum, the C1s, O1s, N1s, and Ni2p spectra) of thin carbon films were performed on the ESCA module (SPECS company) at the NANOPhES station on the Siberia-2 synchrotron-radiation source. The ESCA module includes an AlKα source of 100-W power (1486.6 eV), from which the focused X-ray beam exits (after the monochromator) at an angle of 45° with respect to the sample, and the PHOIBOS-150 electron analyzer which records the photoelectron yield along the normal to the sample. The size of the monochromator input slot is 1 × 25 mm, the beam illumination region on the sample is 3 × 1 mm, the calibration is implemented along the Au4f line (84.02 eV); the full width at half maximum is 0.62 eV with the analyzer pass energy PE = 120 eV. In the module a provision is made for layer-by-layer surface etching by Ar+ ions (Е = 1.5 keV, I = 0.01 mA), incident at an angle of 45° to the surface. The etching area amounts to 4 × 4 mm, the etching rate with 1.5 keV is about 0.4–0.5 nm/min. The processing of experimental XPS spectra is performed using the UNIFIT 2006 software, allowing different hybridization fractions to be determined in the observed peaks [9]. To study the hardening of deposited carbon films, microhardness tests were conducted, as a result of which profiles of the microhardness variation over depth were obtained. The microhardness tests were carried out using a PMT-3 microhardness tester using a Vickers pyramid and the load P = 0.005–2 N. The microhardness of the thin (to 10–6 m) near-surface layer was determined under indentation loads of 0.005–0.05 N. The microhardness was calculated from the formula HV = 1.854 × 103P/d2, where P is the indentation load, d is the diagonal of the impression. The depth of indentor penetration in the material was calculated from the relation h = d/7. The spread of the values did not exceed 5% [14].
DISCUSSION OF RESULTS
The carbon films on nickel substrates, prepared by depositing carbon atoms via the ion sputtering of graphite targets and simultaneous electron irradiation, had a thickness from 50 to 150 nm. The thickness of the hydrocarbon films on nickel, deposited from the gas phase with the evaporation of heavy hydrocarbons, varied within the range of 140–180 nm. The XPS spectra presented in this work demonstrate diamond-like structures in the films, deposited during the electron-beam-assisted ion sputtering of graphite and show that different modes of electron irradiation stipulate different fractions of diamond- and graphite-like bonds. It should be noted that the initial carbon film, fabricated only by carbon deposition without concurrent electron irradiation, is characterized exclusively by graphite sp2 bonds and by an insignificant fraction of С–О bonds.
The specific features of the structural modification of carbon films deposited in the course of the ion sputtering of graphite and concurrent electron irradiation are presented in Fig. 2 as functions of the substrate temperature. An increase in the fraction of sp3 bonds \(\left( {P_{{s{{p}^{3}}}}^{{}} = {\text{ }}{{N_{{s{{p}^{3}}}}^{{}}} \mathord{\left/ {\vphantom {{N_{{s{{p}^{3}}}}^{{}}} {\left( {N_{{s{{p}^{3}}}}^{{}} + N_{{s{{p}^{2}}}}^{{}} + {{N}_{{sp}}}} \right)}}} \right. \kern-0em} {\left( {N_{{s{{p}^{3}}}}^{{}} + N_{{s{{p}^{2}}}}^{{}} + {{N}_{{sp}}}} \right)}}} \right)\) is observed with a decrease in the substrate temperature from 1070 to 470 K. At high temperatures (T ≥ 1070 K), a carbon film with sp, sp2, and sp3 bonds is not formed. The carbide (NinC) bonds and the C–O-, C=O-bonds reveal themselves well in the C1s XPS spectra. It is likely that the carbon atoms deposited in the process of ion sputtering remain for too long on the nickel-substrate surface: one part of the atoms evaporates from the substrate surface; another part diffuses to the surface layer, creating chemical compounds with substrate atoms which are stable at the given temperatures. With temperatures of T < 870 K, peaks, corresponding to the sp,sp2, and sp3 bonds, start to manifest themselves. So, the fraction of sp3 bonds \(\left( {P_{{s{{p}^{3}}}}^{{}}} \right)\) increases from 24 to 75% with a temperature reduction from 870 to 470 K, respectively. For example, Fig. 3 presents the experimental C1s spectra of the carbon films, formed via sputtering of a graphite target by 40-keV C+ ions and with concurrent electron irradiation (Е = 4 keV, j = 10 A/m2) at temperatures of 870 and 470 K. The carbon bonds, forming as a result of the sp,sp2, and sp3 hybridization of orbitals, are characterized by an energy of 283.5, 284.4, and 285.2 eV, respectively [15]. Apart from the sp, sp2, and sp3 bonds, extended “tails”, corresponding to the C–O, С=O, and C–OH bonds, are seen in the spectra. Oxygen is present in all films, while a fraction of its bonds may reach 15% of the entire number of all detected bonds, especially at high temperatures.
Decomposition of the C1s spectrum (experiment) into components, corresponding to sp, sp2, sp3, C–O, С=O, and C–OH bonds (calculation), for a nickel substrate with a carbon film, deposited due to the sputtering of graphite by 40-keV C+ ions and simultaneous irradiation by 4-keV electrons with current density of 10 A/m2 at temperatures of (a) 470 and (b) 870 K.
A variation in the energy of concurrent electrons barely changes the degree of structural modification of the carbon films deposited via ion sputtering. With an electron-energy variation from 1 to 4 keV, the average fraction of sp3 bonds \(\left( {P_{{s{{p}^{3}}}}^{{}}} \right)\) is 70 ± 5% for fixed values of the electron current density of 10 A/m2 and a substrate temperature of 470 K.
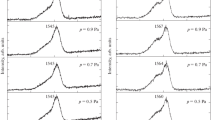
The impact of the electron current density (i.e., density of the flow of concurrent electrons) on the degree of carbon-film modification was studied under the following conditions: the electron energy was 3 keV, the electron current density was varied from 5 to 30 A/m2, and the substrate temperature was maintained at a level of 670 K. It is found that the fraction of sp3 bonds almost continuously increases from 35 to 94% with a change in the electron current density from 5 to 30 A/m2 (Fig. 4). Only within the range of low electron current densities of 5–10 A/m2 does the \(P_{{s{{p}^{3}}}}^{{}}\) value remain unchanged within the limits of measurement error. Some of the experimental C1s spectra of the carbon films deposited with sputtering of the graphite target by 40-keV C+ ions and with simultaneous electron irradiation (Е = 3 keV, j = 10 and 30 A/m2) at a temperature of 670 K are shown in Fig. 5. Comparison of the C1s spectrum components, corresponding to the sp, sp2, sp3, C–O, C=O, and C–OH bonds, with electron current densities of 10 and 30 A/m2 clearly demonstrates the transformation of peaks, related to the sp, sp2, and sp3 bonds. With an electron current density of 10 A/m2, a structure with sp2 bonds noticeably prevails in the film (the area under the sp2 peak more than twice exceeds the total area under the sp and sp3 peaks). With an electron current density of 30 A/m2, a structure with sp3 bonds dominates in the film (the relative area under the sp3 peak approaches 100%). The “tails” of the distributions corresponding to C–O, С=O, and C–OH bonds are clearly seen in the spectra.
Decomposition of the C1s spectrum (experiment) into components, corresponding to sp, sp2, sp3, C–O, С=O, and C–OH bonds (calculation), for the nickel substrate with a carbon film, deposited due to the sputtering of graphite by 40-keV C+ ions and simultaneous irradiation by 4-keV electrons with a current density of 10 A/m2 at temperatures of (a) 470 and (b) 870 K.
The effect of structural modification, substantiated by a growth in the fraction of diamond-like structures \(\left( {P_{{s{{p}^{3}}}}^{{}}} \right)\) in the carbon films, deposited and simultaneously irradiated by electrons, is confirmed indirectly by the results of studying the microhardness of the thin-film–substrate system. Profiles of the microhardness variation over the depth of nickel with the applied carbon films, prepared as a result of the ion sputtering of graphite and electron irradiation at different substrate temperatures and electron current densities, are presented in Fig. 6. The test results show that noticeable material hardening is observed in a layer with a thickness of up to 0.4 μm, while the maximum hardening is observed in a layer less than 0.2 μm thick. As can be seen from Fig. 6a, in a layer with a depth of less than 0.2 μm, the microhardness values of the carbon films, deposited simultaneously with electron irradiation at temperatures of 870, 670, and 470 K, reach 4, 6, and 12 GPa, respectively. These values correlate well with sp3-bond fractions \(P_{{s{{p}^{3}}}}^{{}}\) [ of 24, 35, and 75% and considerably exceed the microhardness of the substrate material. The maximum increase in the microhardness (almost by an order of magnitude) is detected in the surface layer of all nickel substrates with an applied carbon film at 470 K regardless of the energy of concurrent electrons. Figure 6b presents profiles of the microhardness variation over the depth of nickel with deposited carbon films, prepared as a result of the ion sputtering of graphite and simultaneous electron irradiation (Е = 3 keV) at a temperature of 670 K and electron current densities of 5–30 A/m2. The maximum microhardness values of the nickel substrate with a carbon film continuously increase from 4.7 to 12.3 GPa as the electron current density grows from 5 to 30 A/m2, respectively.
Microhardness variation over the depth of a nickel substrate with a carbon film, deposited due to the sputtering of graphite by 40-keV C+ ions and simultaneous irradiation by 4-keV electrons: (a) with a current density of 10 A/m2 at a temperature of (1) the initial substrate, (2) 870, (3) 670, and (4) 470 K; (b) at a temperature of 670 K and with an electron current density of (1) the initial substrate, (2) 5, (3) 10, (4) 20, and (5) 30 A/m2.
Thus, studying the microhardness of the surface layers of the film–substrate system provides important information on structural changes in the deposited carbon films, in particular, on the development of diamond-like structures with sp3 bonds in them.
The preparation of films from the gas phase due to the evaporation of heavy hydrocarbons—naphthalene С10Н8 or polyethylene (С2Н4)n—differs from the method of film application with the ion sputtering of graphite in that large molecules (С10Н8 or (С2Н4)n) or their conglomerates predominate in the flow of the deposited substance. The deposition of these molecules or their conglomerates onto the substrate leads to the formation of “soft” hydrocarbon films. Concurrent electron irradiation may destroy the large hydrocarbon molecules deposited and contribute to the creation of hard carbon films with diamond-like structures.
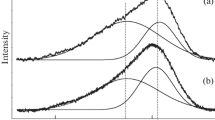
The typical experimental C1s spectra of hydrocarbon films, prepared from the gas phase due to the evaporation of heavy hydrocarbons (naphthalene С10Н8 or polyethylene (С2Н4)n) with simultaneous electron irradiation at a temperature of 345 K, are given in Fig. 7. For all investigated films, decomposition into components of C1s XPS-spectra showed discernible peaks, corresponding to the sp, sp2, sp3, C–O, C=O, and C–OH bonds. The fraction of sp3 bonds \(P_{{s{{p}^{3}}}}^{{}}\) has a maximum value and varies within the range from 87 to 95%. The maximum energy of the photoelectron sp3 bond, Ebond, is within the limits of 284.9–285.4 eV, i.е., the spread is about 0.5 eV. It should be noted that aliphatic or aromatic carbon (–СНn–) and different polymer groups (СmНn) [16], which cannot be excluded during the deposition of large molecules С10Н8, (С2Н4)n or their conglomerates, fall into this range of energies Ebond. It turns to be impossible to separate the sp3 peak of diamond-like carbon with an energy of Ebond. = 285.2 eV and the above indicated peaks of СНn and СmНn. Since hardening is ensured due to the presence of diamond-like sp3 bonds, then measuring the microhardness of films may be helpful in revealing these bonds and even in qualitatively estimating their relative fraction. Figure 8 shows profiles of the microhardness variation over the depth of the nickel substrate with a hydrocarbon film, deposited from the gas phase due to the evaporation of polyethylene (С2Н4)n and naphthalene С10Н8 with concurrent irradiation by 0.5-keV electrons at 345 K. It can be seen that with identical fractions of sp3 bonds (\(P_{{s{{p}^{3}}}}^{{}}\) ~ 90%), the hydrocarbon films have a much smaller (3–8 times smaller) microhardness in comparison to the carbon films prepared with the sputtering of graphite. Probably, films, fabricated by heavy hydrocarbon deposition, contain, mainly, hydrocarbon СНn bonds and a small fraction of diamond-like bonds. Based on the above detected correlation between the relative amount of diamond-like sp3 bonds and the microhardness value of the films, the fraction of diamond-like bonds in the hydrocarbon films can be assumed to vary from 5 to 40%. The films prepared during naphthalene evaporation are almost twice harder than the films of polyethylene, which can be due to a difference in the Н/С ratios in these substances: Н/С = 2 for polyethylene while for naphthalene, Н/С = 0.8.
Decomposition of the C1s spectrum (experiment) into components, corresponding to the sp, sp2, sp3, C–O, С=O, and C–OH bonds (calculation), for the nickel substrate with the hydrocarbon film, deposited from the gas phase due to the evaporation of (a) naphthalene С10Н8 and (b) polyethylene (С2Н4)n and simultaneous irradiation with 0.5-keV electrons with an current density of 10 A/m2 at a temperature of 345 K.
Microhardness variation over the depth of a nickel substrate with a hydrocarbon film, deposited from the gas phase due to heavy hydrocarbon evaporation and simultaneous irradiation by 0.5-keV electrons: at a temperature of (1) the initial substrate, (2) (С2Н4)n, je = 1 A/m2; (3) (С2Н4)n, je = 2 A/m2; (4) С10Н8, je = 1 A/m2; (5) С10Н8, je = 2 A/m2; (6) С10Н8, je = 3 A/m2.
The previously proposed model [10] of the kinetics of creating allotropic forms of carbon in the film deposited considers the formation and breakage of bonds with the given type of hybridization of orbitals. From the system of equations for the equilibrium concentration of the sp, sp2, and sp3 bonds, the following ratios of spi bond fractions result:
where ni is the relative concentration of spi bonds, ki is the probability of the formation of i bonds, τi is the time in which they break. Hybridization of one kind or another is induced by neighboring atoms, ki depends on the carbon-material density and is the even function f(\(v\) – vi), where vi = M/ρi is the specific volume of an atom in the lattice of the film and in the ith modification, and М is the mass of a carbon atom. Concurrent electron irradiation breaks, first and foremost, the low-energy bonds and leads to an increase in the diamond phase and a decrease in the graphite phase. The effect of breaking bonds by electrons slightly (logarithmically) depends on the current density and energy of electrons [10]. However the bond-formation probability ki also depends on electron irradiation. For example, weak excitations of carbon-atom electrons can lead to a change in the type of hybridization of orbitals rather than to a breakage of bonds (ionization). In this case, it can be assumed that ki = ki0(1 + Bij), where Bi is the constant, j is the electron current density. Since v3 < v2 (Table 1) and this excitation is more probable for denser packing, then B2 < B3 and the ratio P32 = sp3/sp2 = k3τ3/k2τ2 grows with an increase in the electron current density j: dP32/dj ~ (B3 – B2) > 0. An increase in the density of the deposited film increases the probability of shorter-bond formation. Cooling of the film during deposition contributes to forming the denser phase. Indeed, the ratio P32 = k3τ3/k2τ2 depends on the thermal expansion of the deposited film. However, it should be emphasized that the issue here is the distance between atoms in the film beyond the pores rather than the average film density, which can decrease with decreasing temperature due to degradation of the mobility of deposited atoms and by virtue of pore formation in the film. In accordance with the previous assumption, the probability of ith-bond creation depends on the volume \({{v}_{i}}\) = M/ρi as
where vi is the volume, corresponding to a single atom in the ith phase, and Ai is a constant. Considering that the volume v depends on the temperature as \(v\) = \(v\)0(1 + 3αT), where α is the coefficient of linear thermal expansion, we derive

Based on the graphite–diamond diagram, it can be assumed that k3/k2\( \ll \) 1, while А2 ≈ А3. At the same time, the specific volume v of the film is larger than the specific volumes v2 and v3, while v2 > v3 (see Table 1) and Δv3 > Δv2, therefore dP32/dT < 0. The ratio P32 increases with cooling. The effect value can be judged by comparing a reduction in the specific volume with cooling by ΔT and with compression under the pressure Р. The pressure P, which is equivalent to cooling by ΔT, is written as
where K is the compression modulus, K = 10.3 GPa for graphite, while for diamond, K = 435 GPa. Thus, cooling by ΔT = 100 K is equivalent to compression under a pressure of P ≈ 100 atm. This pressure is significantly smaller than the pressure needed for the phase transition of graphite to diamond without electron irradiation. The quantity ∂(k3/k2)/∂T is small, but the quantity ∂(k3τ3/k2τ2)/∂T is no longer small. This implies that the electron irradiation increases P32 due to the predominant breakage of sp2 bonds. So, a change in the specific volume of an atom in the lattice of the film with cooling by ΔT ~ 100 K under conditions of simultaneous electron irradiation leads to an appreciable increase in the concentration of the phase with sp3 bonds.
CONCLUSIONS
Studies of carbon films, applied on nickel substrates in the process of the ion sputtering of graphite under conditions of concurrent electron irradiation, have shown that electron irradiation induces the formation of sp3 bonds in the films. The fraction of sp3 bonds \(\left( {P_{{s{{p}^{3}}}}^{{}}} \right)\) grows from 24 to 75% with a decrease in the nickel-substrate temperature from 870 to 470 K and grows from 35 to 94% with an increase in the electron current density from 5 to 30 A/m2. The variation in the electron energies from 1 to 4 keV barely changes the degree of structural modification of the deposited carbon films (\(P_{{s{{p}^{3}}}}^{{}}\) ≈ 70%) at a substrate temperature of 470 K. A decrease in temperature and increase in the electron current density enhance the microhardness of the substrate with the applied film (the greatest hardening is observed in a layer up to 0.2 μm deep). The maximum microhardness, exceeding a value of 12 GPa, is detected at a minimum deposition temperature of 470 K and with a maximum electron current density of 30 A/m2. The microhardness value of the film–substrate system can serve as a good indicator for the fraction of diamond-like sp3 bonds.
The films, prepared by the electron-beam-assisted (0.5 keV) deposition of heavy hydrocarbons (С2Н4)n and С10Н8 at 345 K, contain predominantly the hydrocarbon СНn bonds and a small fraction of diamond-like sp3 bonds. The hydrocarbon-film microhardness is 3–8 times smaller in comparison with the films deposited due to the sputtering of graphite. The films produced during С10Н8 evaporation are almost twice as hard as the films prepared from (С2Н4)n, since the ratio Н/C for (С2Н4)n is 2.5 times larger than that for С10Н8. The maximum microhardness value of the hydrocarbon films is no more than 4.5 GPa.
The analysis of the kinetics of forming different allotropic phases in the deposited carbon film showed that concurrent electron irradiation must lead to an increase in the diamond-phase concentration and to a decrease in the graphite-phase fraction. As a result of lowering the temperature of film deposition, the specific atom volume in the lattice of the film changes, while under the condition of simultaneous electron irradiation, the concentration of the phase with sp3 hybridization increases appreciably. The effect of the breakage of bonds with i-type hybridization during electron-beam-assisted deposition weakly depends on the current density and energy of electrons. Weak excitations of electrons of carbon atoms may lead to a change in the type of hybridization, increasing the probability of sp3 bond formation and augmenting the relative concentration of sp3 bonds with a growth in the electron current density.
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research (project no. 16-08-01144a).
REFERENCES
Yu. V. Martynenko and G. Carter, Radiat. Eff. Defects Solids 132, 103 (1994).
Yu. P. Kudryavtsev, S. E. Evsyukov, M. B. Guseva, et al., Izv. Ross. Akad. Nauk, Ser. Fiz., No. 3, 450 (1993).
Yu. P. Kudryavtsev, S. Evsyukov, M. Guseva, et al., in Chemistry and Physics of Carbon, Ed. by P. A. Thrower (Marcel Dekker, New York, 1997), Vol. 25, p. 1.
V. G. Babaev and M. B. Guseva, in Carbyne and Carbynoid Structures, Ed. by R. B. Heimann, (Kluwer Academic Publ., Dordrecht, Boston, London, 1999), p. 159.
J. Robertson, Mater. Sci. Eng., R 37, 129 (2002).
V. G. Babaev, M. B. Guseva, N. F. Savchenko, et al., Poverkhnost, No. 3, 16 (2004).
A. P. Semyonov, A. F. Belyanin, I. A. Semyonova, et al., Tech. Phys. 49 (5), 619 (2004).
A. P. Semenov, I. A. Semenova, and N. N. Smirnyagina, Tech. Phys. 60 (3), 461 (2015).
Yu. V. Martynenko, S. N. Korshunov, N. E. Belova, and I. D. Skorlupkin, JETP Lett. 97 (10), 588 (2013).
S. N. Korshunov, Yu. V. Martynenko, N. E. Belova, and I. D. Skorlupkin, J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 11 (4), 807 (2017).
V. M. Gusev, N. P. Busharov, and S. M. Naftulin, Prib. Tekh. Eksp., No. 4, 19 (1969).
N. Yu. Svechnikov, V. G. Stankevich, I. I. Arkhipov, et al., Vopr. At. Nauki Tekh., Ser.: Termoyad. Sint., No. 3, 3 (2012).
N. Yu. Svechnikov, V. G. Stankevich, I. I. Arkhipov, et al., J. Surf. Invest.: X-ray, Synchrotron Neutron Tech. 7 (5), 863 (2013).
S. N. Zvonkov, S. N. Korshunov, Yu. V. Martynenko, and I. D. Skorlupkin, JETP Lett. 94 (2), 112 (2011).
A. Hu, M. Rybachuk, Q.-B. Lu, and W. W. Duley, Appl. Phys. Lett. 91, 131906 (2007).
G. Beamson and D. Briggs, High Resolution XPS of Organic Polymers–The Scienta ESCA 300 Database (John Wiley and Sons, Chichester, 1992).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by M. Samokhina
Rights and permissions
About this article
Cite this article
Korshunov, S.N., Lebedev, A.M., Martynenko, Y.V. et al. Structure Changes in Carbon Films Prepared by Electron-Beam-Assisted Deposition. J. Surf. Investig. 13, 317–325 (2019). https://doi.org/10.1134/S1027451019020307
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451019020307