Abstract
Heating of chloride electrolyte up to 70°С ensures the normal codeposition of components of the Co–Ni–Fe alloy as a result of the discharge of iron, cobalt, and nickel ions at the high cathodic current density. The chloride electrolyte with filtration and pH correction by hydrochloric acid at the concentration CСо : CNi : CFe = 1 : 1 : 1 provides the electrochemical deposition of Co–Ni–Fe films. The mechanism of anomalous deposition of Co, Fe, and Ni takes place due to the incomplete ionization of atoms and the different mobility of ions. The Co–Ni–Fe films lacking mechanical stresses with the uniform structure and the high magnetic properties are synthesized without using high annealing temperatures. The electrochemical deposition allows reproducible Co–Ni–Fe films to be obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The electrochemical deposition of Co–Ni–Fe coatings is used in many technological fields to decrease corrosion and deterioration, for magnetic and electric devises, and also for the synthesis of electrocatalytic materials. As compared with “dry” processes, the electrochemical deposition produces the more uniform coatings with the smaller number of defects and also makes it possible to increase, if necessary, the film thickness without inducing mechanical stresses.
In this study, we show the results on the electrodeposition of metal films based on the ternary system Co–Ni–Fe from the chloride electrolyte with the equal molar concentrations of components and the ratio Со : Ni : Fe = 1 : 1 : 1 at the temperature of 70°С with regard to the experience in the congruent deposition of the Ni–Fe permalloy [1] and the dependence of the deposition process on the ion charge [2].The goal of this study is to determine how the concentration of electrolyte components affects the composition, mechanical stresses, and magnetic properties of Co–Ni–Fe films.
1 THEORETICAL ANALYSIS
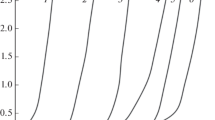
The alloy formation is determined by the peculiarities of the discharge of each electrolyte component, the specific features of the surface composition and structure of the cathode, the electric double layer structure, and the surface charge [3]. On the cathode, iron always evolves simultaneously with hydrogen. The temperature has a strong effect on the coevolution of iron and hydrogen. At the low temperature (20–25°С), the discharge rate of iron ions exceeds the discharge rate of hydrogen ions only at the low current densities (up to 100 A/m2–10 mA/cm2). To obtain the high current efficiencies, the temperature and the current density should be high: 85–100°С (Fig. 1) and 50–100 mA/cm2, respectively.
Furthermore, the current efficiency decreases with the decrease in the hydrochloric acid concentration. However, at the low concentration of hydrochloric acid, hydroxides are formed. To avoid their formation, pH 2–3 is maintained. The current efficiency also depends on the reaction of Fe3+ reduction to Fe2+. These peculiarities of iron electrodeposition should be taken into account.
The book published relatively recently and devoted to the theory and practice of metal electrodeposition [4] shows the typical solution compositions used in the deposition of soft magnetic Ni–Fe alloys; however, it contains no information on the deposition of the ternary system Co–Ni–Fe.
As was shown in [5], for the 1 M solution concentration, the NiCl2 solution has рН 5.8, the CoCl2 solution has рН 4.7, and the FeCl2 solution has рН 2.7, i.e., the hydrolysis of iron chloride increases more strongly the concentration of protons in the electrolyte.
The relative contents of metals in the electrolyte used in deposition of Co–Ni–Fe films and in the resulting deposits are the closest in a weak electrolyte containing no additives. The addition of ammonium hydrate, boric acid, and saccharine to the electrolyte affects the film composition by changing the balance of ions.
Comparing the magnetization of the Co–Ni–Fe and Ni–Fe films as a function of the applied magnetic field showed that the slope of the magnetization line for the ternary system was higher by a factor of 1.7, which allowed considering the ternary system as the more promising for the use in magnetic field transducers. The possibility of optimization of magnetic parameters was demonstrated for the film composition Co43.1Ni35.3Fe21.6.
In [6], the kinetics of electrodeposition of the Co–Ni–Fe alloy was considered. The experimentally found peculiarities of electrodeposition of the Co–Ni–Fe alloy were described by a sequence of chemical and electrochemical reactions proceeding in the electrolyte bulk, on the anode, and on the cathode.
In [7], the results are shown on the electrodeposition of films in the ternary system Co–Ni–Fe from the chloride electrolyte with equal molar concentrations of components and the molar ratio Со : Ni : Fe = 1 : 1 : 1 carried out at 70°С. A decrease in the electrolyte concentration led to the stronger potential drop in the interelectrode space on retention of the current value. The growth rate of the Co–Ni–Fe films increased with the increase in the current density but depended weakly on the concentrations of components at their 77-fold difference and was half the value calculated based on the Faraday law. The relative content of components Co, Ni, Fe in the films differed from their equal content in the electrolyte and depended strongly on the current density. At the high current density, cobalt and iron were deposited preferentially and the film composition was stabilized. The increase in the interelectrode space and the more active stirring of the electrolyte improved the uniformity and homogeneity of films. Moreover, the iron content in films decreased and the nickel content increased. The coercive force of Co–Ni–Fe films was low at the iron content in the interval of 15–30%, similar to permalloy, but the greater part of nickel was replaced by cobalt.
In [8], the dependence of the pH of chloride electrolytes with equal contents of components on the temperature and also the electrochemical deposition of Co–Ni–Fe films at 70°С were studied. The pH of the mixed electrolyte at all concentration of salts CoCl2, NiCl2, FeCl2 was determined by the hydrolysis of iron chloride. The deposition from the electrolyte with 0.083 М concentration showed that the relative content of Co, Ni, and Fe in the film changed insignificantly at the high current densities, which was also true for the concentration of 0.48 М. The preferential deposition of cobalt was observed for all electrolyte concentrations.
EXPERIMENTAL
For the deposition of Co–Ni–Fe films, we used the chloride electrolyte with the equal molar content of salts CoCl2·6Н2О, FeCl2·4H2O, and NiCl2·6H2O in deionized water. The following chemicals were added: boric acid H3BO3 20 g/L, sodium saccharinate C7H4NaNO3S·2Н2О 1.5 g/L, and hydrochloric acid 3 mL/L. The film was deposited from this electrolyte in an electrochemical setup with a galvanic bath of 2 L volume and a graphite anode [5]. The electrolyte had the temperature of 70°C maintained by a submersible heater and was stirred by a magnetic stirrer. The process was carried out in the galvanostatic mode. The following parameters of Co–Ni–Fe films were studied as a function of the electrodeposition conditions: composition, thickness, mechanical stresses, and magnetic properties.
The film parameters were measured with the use of instruments of the Center of Collective Use “Functional Control and Diagnostics of Micro and Nanosystem Equipment” at the Research and Industrial Center “Technological Center”. The thickness of the films of magnetic field concentrators was measured by means of the MSA-500 analyzer of microsystems. The magnetic characteristics, namely, the coercive force and the magnetization were studied on the setup for controlling the magnetic parameters of films. The film composition was studied by means of the energy-dispersive X-ray microanalyzer PhilipsXL 40. The bending of plates was analyzed on the optical profilometer FRT MicroProb 100.
RESULTS AND DISCUSSION
1. Congruent Electrodeposition of Co‒Ni‒Fe Films
For electrodeposition of Co–Ni–Fe films under galvanostatic conditions, we used the electrolyte with the equal 0.00625 М concentrations of chlorides CoCl2, NiCl2, FeCl2 in deionized water with the addition of 0.3 mL/L of 30% hydrochloric acid. Table 1 shows the results of deposition from the similar electrolytes but with addition of saccharine or boric acid.
Table 1 shows the parameters of the process: electrolyte pH, voltage between anode and cathode U, electric current I, deposition time t, film growth rate V, and also the additives to electrolyte, namely, boric acid Н3ВО3 and sodium saccharinate hydrate C7H4NaNO3S·2Н2О.
Figure 2 shows the results of studying the film composition as a function of current density. As seen, the film composition depended on the current density and, at the current density of 12.1 mA/cm2, the Co–Ni–Fe film composition was close to the electrolyte composition, namely, 33 mol % of each of salts CoCl2, NiCl2, and FeCl2, i.e., the congruent electrodeposition of the ternary alloy Co–Ni–Fe was observed.
The percentage of components C(Co; Ni; Fe) in the films deposited from the electrolyte with the equal content (33 mol %) of salts CoCl2, NiCl2, FeCl2 and the concentration of each salt of 0.00625 M as a function of the current density J in the interval of 10‒12.8 mA/cm2. The compositions of Co–Ni–Fe films without additives are shown by symbols which are connected by lines depending on the current density. The Co content is shown by squares and the dashed line. The Ni content is shown by triangles and the dash-and-dot line. The Fe content is shown by circles and the solid line. The composition of films obtained in electrolyte with additions of saccharine 3 g/L (at 10 mA/cm2) and 1.5 g/L (at 10.7 mA/cm2), boric acid 20 g/L (at 11.4 mA/cm2) are shown by individual points. The arrows show how the film composition changes in the presence of the corresponding additive at the chosen current density.
The additions to the electrolyte changed the film composition and disturbed the congruency of deposition in different ways. The addition of saccharine increased the content of Со and Ni and decreased the content of Fe. Boric acid (20 g/L) increased the Co and Fe content and decreased the Ni content. The simultaneous addition of 1.5 g/L saccharine and 20 g/L boric acid increased the Co content, slightly decreased the Ni content, and strongly decreased the content of Fe.
The electrolyte with the relatively low concentration (0.00625 М) of chlorides CoCl2, NiCl2, and FeCl2 is promising for studying the congruency of the deposition of Co–Ni–Fe films with the thickness less than 1 µm but cannot be used in practice due to its exhaustion when thick films are deposited.
2. The Effect of Additions to Electrolyte on the Bending of Silicon Plates with Electroplated Films of the Co‒Ni‒Fe Alloy
To obtain thick films, it is expedient to use the electrolyte composition with the equal concentrations (0.083 М) of each chloride CoCl2, NiCl2, and FeCl2. Boric acid, saccharine, and hydrochloric acid were added to the electrolyte [8]. Boric acid is a complexing agent and decreases the mobility of metal ions. Hydrochloric acid stabilizes the electrolyte and rules out the formation of the precipitate of metal hydroxides. Saccharine is adsorbed on the cathode and thus limits the discharge of ions [9]. These additives improved the morphology of Co–Ni–Fe films, weakened the mechanical stresses, but decreased the deposition rate.
Table 2 shows the data on the thickness of Co–Ni–Fe films and the bending of silicon plates
Figure 3 shows the bending of silicon plates (solid bars, D0) before and (patterned bars, D1) after the electrodeposition of films of the ternary alloy Co–Ni–Fe from the electrolytes containing (1–2) saccharine, (3–4) boric acid, (5–6) inhibitor catapin, (7–8) glycolic acid, (9, 10) a mixture of glycolic acid and catapin.
Bending of silicon plates (solid bars) before D0 and (patterned bars) after D1 the electrochemical deposition of films of the ternary alloy Co–Ni–Fe from electrolytes with additions of (2) saccharine, (4) boric acid, (6) inhibitor catapin, (8) glycolic acid, and (9, 10) the mixture of catapin and glycolic acid.
The addition of boric acid and saccharine and also the individual additions of catapin and glycolic acid decreased the plate bending, whereas the mixture of catapin and glycolic acid totally ruled out bending of films with deposited Co–Ni–Fe films and strongly decreased the deposition rate.
Figure 4 shows how the composition of Co–Ni–Fe films and the deposition rate change with the concentration of boric acid and saccharine in the electrolyte. The film composition changed insignificantly, whereas the deposition rate decreased 1.5 fold when the additions of saccharine and boric acid were doubled. Saccharine deposited on the film surface and forming a white deposit.
Dependence of the composition С and the deposition rate V of the Co–Ni–Fe alloy films deposited at 70°С, the deposition time 150 min from the chloride electrolyte with the concentration of each component of 0.083 M in the presence of the following additives, at the current density of 12.1 mA/cm2: I. 10 g/L H3BO3, 1.5 g/L saccharine, 2 mL/L HCl; II. 10 g/L H3BO3, 3.0 g/L saccharine, 2 mL/L НСl; III. 20 g/L H3BO3, 3.0 g/L saccharine, 2 mL/L HСl.
3. Mechanical Stresses in Electroplated Co‒Ni‒Fe Films
The high mechanical stresses in thin magnetic films can cause the deformation and failure of devices due to exfoliation of films from silicon supports.
The thin Fe70Co29Ni1 films with the high saturation of magnetic induction of 2.2 Torr at the coercive force of 60 Oe and the low mechanical stresses were prepared [12] by electrodeposition from chloride electrolyte. The mechanical stresses in the electroplated Co‒Ni–Fe films decreased as the electrolyte temperature increased from 20 to 70°С at рН 0.3 and J = 5 mA/cm2, increased as the nickel content in the deposit increased, and were inversely proportional to the grain size. The addition of ascorbic acid to the electrolyte stabilized the solution and allowed carrying out the deposition only at the high pH 2 which favored the low mechanical stresses.
The films of ternary alloy Co–Ni–Fe were deposited in [13] from a sulfate electrolyte with the varying content of saccharine. The lowest mechanical stresses of 61 MPa were observed at the saccharine concentration 0.004 М (0.9 g/L). As the electrolyte temperature increased from 25 to 50°С, the mechanical stresses decreased to 32 MPa.
To reveal the factors responsible for the appearance of mechanical stresses in Co–Ni–Fe films, we studied the bending of silicon plates with deposited films. The plate bending was analyzed on its back side. Figure 5 shows the measured geometrical parameters of plates.
According to Fig. 5, the bending was the maximum of 370 µm for the film 30 µm thick and the minimum for the film thickness of 2 µm. The support edges rose above plate’s center on its front side, i.e., the film was contracted. The bending was determined only by the thickness of films of the ternary alloy Co–Ni–Fe. No exfoliation was observed.
According to the formula of Stoney [14], the bending of the support is proportional to the mechanical stresses in the film
where DSi is the bending of the silicon plate; MSi is the Young modulus of a silicon single crystal 1.09 × 1011 Pa [9]; hSi is the thickness of the silicon support 450 µm; rSi is the radius of silicon plate 50 mm; hf is the magnetic film thickness.
For the film thickness of 15 µm and the bending of 180 µm, the mechanical stresses in the film of the ternary Co–Ni–Fe alloy is 17.7 MPa. This value agrees by the order of magnitude with the mechanical stresses of 270–32 MPa found by means of an analyzer of mechanical stresses in [11].
The mechanical stresses in Co–Ni–Fe films appear due to the peculiarities of the electrochemical process of their deposition. Water dissociates to ions Н2О ↔ Н+ + ОН–. The dissolution of cobalt, nickel, and iron chlorides is accompanied by their hydrolysis. The salts FeCl2, NiCl2, and CoCl2 are formed by the weak bases Fe(OH)2, Ni(OH)2, Co(OH)2 and the strong acid—hydrochloric acid HCl. At the hydrolysis of salts formed by a weak base and a strong acid, the reaction proceeds via the cation and the solution becomes acidic [15].
Hydrogen ions evolve on the cathode together with metal ions. Being adsorbed on the cathode, a part of protons lose their charge and form hydrogen molecules Н2. The molecules form gas bubbles observed at the metal electrodeposition.
It was found [16] that hydrogen dissolves in octo- and tetrapores of the metal lattice in its ionized state, accumulates in the pores and other defects in its molecular form, enters into chemical reactions with various elements and phases present in metals and alloys, and also adsorbs inside the metal on the surface of microvoids, pores, and microcracks, and segregates on lattice imperfections and grain boundaries.
In the end of electrochemical processes, hydrogen evolves from metal deposits giving rise to compressive stresses in the deposited film. For thin Co–Ni–Fe and Ni–Fe films, the relatively low mechanical stresses are almost the same, have the linear D(H) dependence, but pass to saturation for Co–Ni–Fe films with the thickness larger as compared with Ni–Fe films. In the Co–Ni–Fe films, the mechanical stresses reach the higher values as compared with the Ni–Fe films.
4. Magnetization and Coercive Force of the Permalloy and the Ternary Co‒Ni‒Fe Alloy
The magnetic properties, viz., the magnetization and the coercive force of films of the permalloy and the ternary Co‒Ni‒Fe alloy were studied based on the hysteresis loop of the magnetic field flux by means of an analyzer of magnetic properties of films. The composition of films on the plates was determined by the energy-dispersive X-ray microanalyzer.
Table 3 and Fig. 6 show the results of measurements of magnetic parameters: specific magnetization B/h, coercive force Hc, and the composition of the ternary Co–Ni–Fe films in at %.
The magnetization of Co–Ni–Fe films was 135 ± 5 nWb/µm for the Fe content from 13 to 23 at %. The coercive force Hc of Co–Ni–Fe films had the minimum of 1.25 Oe for the Fe content of 14 at %.
The X-ray diffraction analysis showed [17] that in the studied region of compositions, the Co–Ni–Fe films have the face-centered cubic lattice.
Figure 7 shows the magnetization curve of the Co–Ni–Fe film.
By optimizing the deposition mode by choosing the current density of 12.1 mA/cm2 and varying the electrolyte composition, the high values of magnetic induction of magnetization of 4000 nWb was obtained for the film thickness of 30 µm and the low values of coercive force of about 1 Oe.
5. Discussion
The following questions can be posed.
Why can the congruent electrodeposition of films based on metals of the ternary system Co‒Ni‒Fe be achieved by decreasing the electrolyte concentration?
Why does the composition of the Co–Ni–Fe film depend on the current density and stop to change at the high current density?
How do the additions to electrolyte change the film composition and disturb the congruency of deposition?
Assessing the deposition rate based on electrochemical potentials predicts the preferential deposition of nickel as normal; however, there are many factors that can affect the metal deposition.
The flowing current disturbs the thermodynamic equilibrium giving rise to overvoltage, i.e., the shift of the electrode potential. The diffusion overvoltage is caused by the slow stage of substance transport through the stagnant layer adjacent to the cathode. The electrochemical overvoltage is associated with the slow stage of complex dissociation.
Iron, cobalt, and nickel are virtually undissolved in water. The existence of metal ions in water is associated with the presence of their salts. The deposition of a metal on the cathode is accompanied of the discharge of ions and decomposition of salt molecules. In the process, a layer of residues forms near the cathode, changing the electrolyte composition. The changes in the electrode potential as a result of changes in the reagent concentration in the space between the electrodes when current flows are called the concentration polarization associated with the slow supply of reagents and removal of products of electrochemical reaction [9].
The phenomena occurring in the electric double layer formed by charges near the cathode surface do not take into account the existence of ions with different charge and mobility in the electrolyte volume, the mass transfer, the stirring of electrolyte and its viscosity. The latter phenomena pertain to the electrolyte bulk properties.
The concentration polarization makes it possible to explain the dependence of the content of components in the Co–Ni–Fe film on the current density by the difference in the diffusion coefficients or mobilities of ions, the partial ionic currents that determine the mass transfer of electroactive ions to the electrode. The lower the electrolyte concentration, the smaller is the deviation of the film composition from the equilibrium state and the closer is the film composition to the electrolyte composition. This is why the congruent electrochemical deposition of the Co–Ni–Fe alloy proceeds at the low concentration of the main electrolyte components even without additions of boric acid and saccharine.
By the probe measurements of the pH it was shown [10] that near the cathode, the region of 0.6 mm with the enhanced pH 7 was formed at polarization with the current density of 5 mA/cm2, as compared with the рН 2.7 observed at the current density of 1 mA/cm2 in the solution of 0.2 M FeSO4 and 0.8 M NiSO4. The higher the current density, the wider is the deviation of the electrolyte composition near the electrodes from the uniform composition corresponding to the thermodynamic equilibrium. A sufficiently large region of concentration polarization is formed.
The peculiarities of the ionic current flowing in the electrolyte were clearly demonstrated at the working electrode potential from –1.3 to 0.6 V in a three-electrode electrochemical cell with the standard hydrogen electrode as the reference electrode by potentiodynamic measurements at a rate of 10 mV/s with monitoring of the current through the electrode [11]. In the CoSO4 solution, the anodic peak and a minimum were observed at the potential of 0.5 V. In the FeSO4 the current peak was observed at –0.49 V. In the NiSO4 solution where the conductivity was very low, the peak and the minimum were not observed. In the ternary electrolyte with concentrations the same as in individual solutions, namely, 0.2 M Ni, 0.005 M Fe, and 0.15 M Со, the anodic peak and the minimum were observed at the potential of 0.12 V, being determined by CoSO4.
During the dynamic variation of the cathode potential, the transport of ions in the electrolyte leads to the heterogeneous distribution of the ion charge. The positive ions moving to the cathode form the electric double layer the internal field in which is directed oppositely to the external field between cathode and anode, thus compensating the latter. This results in a decrease in the current to the working electrode and gives rise to the current peak. A substantial difference between partial currents is observed, which is determined by the concentrations and mobilities of ions. The presence of the peak during dynamic measurements is associated with the kinetic properties of ions. The difference in the mobility of cobalt, iron and nickel ions determines the time and the difference in amplitudes of current peaks. The above results clearly demonstrate the difference in the dynamic characteristics of Co, Ni, and Fe ions and allows us to consider them as the factor determining the peculiarities of the electrochemical deposition of films based on the ternary system Co–Ni–Fe.
The increase in the current density extends the region of concentration polarization to the value where balance in observed between the reagent delivery and the removal of electrochemical reaction products. At the higher current densities, the composition stops to change.
The complexing agents and inhibitors added to electrolyte change the electrolyte composition in the region of concentration polarization, thus changing the mobility and the partial currents of ions. The film composition depends on the additions to electrolyte.
The electrolyte composition optimal for producing thick Co–Ni–Fe films with soft magnetic properties is 0.083 M for each chloride CoCl2, NiCl2, FeCl2 at the appropriate choice of the current of 12.1 mA/cm2. The electrolyte contains also boric acid, saccharine, and hydrochloric acid. These additions decrease the deposition rate, lower down the mechanical stresses, and improve the morphology of Co–Ni–Fe films.
CONCLUSIONS
The thick Co–Ni–Fe films with the high magnetic permeability and the low mechanical stresses were obtained by electrodeposition from the chloride electrolyte with the component ratio СCo : СNi : СFe = 1 : 1 : 1 with filtration, at the deposition temperature of 70°С. Studying the electrochemical deposition of films based on metals of the ternary system Co–Ni–Fe when varying the electrolyte concentration made it possible to find that the congruent deposition occurred at the current density of 12.1 mA/cm2 in the electrolyte containing 33 mol % of each salt CoCl2, NiCl2, FeCl2 and the concentration of 0.00625 М and also with the addition of 0.3 mL/L 30% hydrochloric acid at the temperature of 70°С.
The mechanical stresses in the films were associated with hydrogen absorption. The optimization of the deposition mode and the electrolyte composition allowed the soft magnetic Co–Ni–Fe films with the high magnetization and the low coercive force to be synthesized. The results obtained are explained by the effect of the concentration polarization. The survey of the literature [17–54] showed the absence of analogous studies.
REFRENCES
Tikhonov, R., Congruent Electrochemical Deposition of Ni–Fe Alloy, Lambert Acad., 2019.
Tikhonov, R.D., Electrochemical deposition of NiFe alloy at a temperature of 70°C, Russ. J. Electrochem., 2020, vol. 56, p. 611]
Fedot’ev, N.P., Alabyshev, A.F., Rotinyan, A.L., Vyacheslavov, P.M., Zhivotinskii, P.B., and Gal’nbek, Prikladnaya Elektrokhimiya, (Applied Electrochemistry), Goskhimizdat, 1962.
Gamburg, Yu.D. and Zangari, G., Theory and Practice of Metal Electrodeposition, New York: Springer, 2011.
Tikhonov, R.D., Cheremisinov, A.A., Gorelov, D.V., and Kazakov, Yu. V., Magnetic properties of Co–Ni–Fe films synthesized by electrochemical deposition by the Tikhonov method, Nano- Mikrosist. Tekh., 2020, vol. 22, p. 123.
Tikhonov, R.D., Features of electrochemical deposition of films of the triple system of the Co-Ni-Fe, EJERS, 2021, vol. 6, p. 19.
Tikhonov, R.D., Cheremisinov, A.A., and Tikhonov, M.R., Ion discharge in electrochemical deposition of Co–Ni–Fe films, Russ. J. Electrochem., 2021, vol. 57, p. 1151.
Tikhonov, R.D., Polomoshnov, S.A., Amelichev, V.V., Cheremisinov, A.A., and Kovalev, A.M., Formation of films in the ternary system Co–Ni–Fe by electrodeposition, Izv. Vyssh. Uchebn. Zaved., Elektron., 2021, vol. 26, p. 246.
Damaskin., B.B., Petrii, O.A., and Tsirlina, O.A., Elektrokhimiya (Electrochemistry), St. Petersburg: Lan’, 2015.
Nakano, H., Matsuno, M., Oue, S., Yano, M., Kobayashi, Sh., and Fukushima, H., Mechanism of anomalous type electrodeposition of Fe-Ni alloys from sulfate solutions, Trans. Jpn. Inst. Met., 2004, vol. 45, p. 3130.
Hanafi, I., Daud, A.R., and Radiman, Sh., Potentiostatic electrodeposition of Co–Ni–Fe thin films from sulfate medium, J. Chem. Technol. Metall., 2016, vol. 51, p. 547.
Park, D.Y., Yoo, B.Y., Kelcher, S., and Myung, N.V., Electrodeposition of low-stress high magnetic moment Fe-rich Fe–Co–Ni thin films, Electrochim. Acta, 2006, vol. 51, p. 2523.
Kim, Jin-Soo, Kwak, Jun-Ho, Na, Seong-Hun, Lim, Seung-Kyu, and Suh, Su-Jeong, The deposit stress behavior and magnetic properties of electrodeposited Ni‒Co–Fe ternary alloy films, J. Korean Phys. Soc., 2012, vol. 61, p. 21.
Chason, E., Measurement of stress evolution in thin films using real-time in situ wafer curvature(k-SpaceMOS), https//www.k-space.com›wp-content/uploads/MOSforThinFilms.
Korovin, N.V., Obshchaya Khimiya (General Chemistry), Moscow: Vysshaya Shkola, 1998.
Beloglazov, S.M., Elektrokhimicheskii vodorod i metally (Electrochemical Hydrogen and Metals), Kaliningrad: KGU, 2004.
Osaka, T., Sawaguchi, T., Mizutani, F., Yokoshima, T., Takai, M., and Okinaka, Y., Effects of saccharin and thiourea on sulfur inclusion and coercivity of electroplated soft magnetic Co-Ni-Fe film, J. Electrochem. Soc., 1999, vol. 146, p. 3295.
Zech, N., Podlaha, E.J., and Landolt, D., Anomalous codeposition of iron group metals I. Experimental results, J. Electrochem. Soc., 1999, vol. 146, p. 2886.
Tabakovic, I., Riemer, S., Inturi, V., Jallen, P., and Thayer, A., Organic additives in the electrochemical preparation of soft magnetic Co-Ni-Fe films, J. Electrochem. Soc., 2000, vol. 147, p. 219.
Tobakovic, I., Inturi, V., and Riemer, S., Composition, structure, stress, and coercivity of electrodeposited soft magnetic Co–Ni–Fe films, J. Electrochem. Soc., 2002, vol. 149, p. 18.
Perez, L., Attenborough, K., De Boeck, J., Celis, J.P., Aroca, C., Sánchez, P., López, E., and Sánchez, M.C., Magnetic properties of Co–Ni–Fe alloys electrodeposited under potential and current control conditions, J. Magn. Magn. Mater., 2002, vols. 242–245, p. 163.
Kim, D., Park, D.Y., Yoo, B.Y., Sumodjo, P.T.A., and Myung, N.V., Magnetic properties of nanocrystalline iron group thin film alloys electrodeposited from sulfate and chloride baths, Electrochim. Acta, 2003, vol. 48, p. 819.
Huang, Q. and Podlaha, E.J., Simulation of pulsed electrodeposition for giant magnetoresistance Fe–Co–Ni–Cu/Cu multilayers, J. Electrochem. Soc., 2004, vol. 151, p. 119.
Khomenko, E.V., Shalygina, E.E., Polyakov, S.N., and Checherin, N.G., Electrochemical deposition and properties of ferromagnetic films Co–Fe–Ni with the thickness up to 500 nm, Perspekt. Mater., 2006, vol. 2, p. 66.
Khomenko, E.V., Shalyguina, E.E., and Chechenin, N.G., Magnetic properties of thin Co–Fe–Ni films, J. Magn. Magn. Mater., 2007, vol. 316, p. 451.
Yun, T. and Jiang, W., Effects of additives on magnetic properties of electroplated Co-Ni-Fe films, Master’s Theses, San Jose State University, 2008.
Rohan, J.F., Ahern, B.M., Reynolds, K., Crowley, S., Healy, D.A., Rhen, F.M.F., and Roy, S., Electroless thin film Co–Ni–Fe–B alloys for integrated magnetics on Si, Electrochim. Acta, 2009, vol. 54, p. 1851.
Péter, L., Csik, A., Vad, K., Toth-Kadar, E., Pekker, A., and Molnár, G., On the composition depth profile of electrodeposited Fe–Co–Ni alloys, Electrochim. Acta, 2010, vol. 55, p. 4734.
Sundaram, K., Dhanasekaran, V., and Mahalingam, T., Structural and magnetic properties of high magnetic moment electroplated Co–Ni–Fe thin films, Ionics, 2011, vol. 17, p. 835.
Phua, L.X., Phuoc, N.N., and Ong, C.K., Effect of Ni concentration on microstructure, magnetic and microwave properties of electrodeposited Ni–Co–Fe films, J. Alloys Compd., 2012, vol. 543, p. 1.
Gong, J., Riemer, S., Morrone, A., Venkatasamy, V., Kautzky, M., and Tabakovic, I., Composition gradients and magnetic properties of 5–100 nm thin Co–Ni–Fe films obtained by electrodeposition, J. Electrochem. Soc., 2012, vol. 159, p. 447.
Li, J.-M., et al., Effect of boron/ phosphorus containing additives on electrodeposited Co–Ni–Fe soft magnetic thin films, Trans. Nonferrous Met. Soc. China, 2013, vol. 23, p. 674.
Kockar, H., Demirbas, O., Kuru, H., and Alper, M., Differences observed in properties of ternary Ni–Co–Fe films electrodeposited at low and high pH, J. Mater. Sci.: Mater. Electron., 2013, vol. 24, p. 1961.
Azizi, A., Yourdkhani, A., Cutting, D., and Pesika, N., Tuning the crystal structure and magnetic properties of Co–Ni–Fe–B thin films, Chem. Mater., 2013, vol. 25, p. 2510.
Valko, N.G. and Gurtovoy, W.G., Structure and properties of coatings Co−Ni−Fe, electrolytically besieged by X-ray radiation, Fiz. Tverd. Tela, 2013, vol. 55, p. 2086.
Kayani, Z.N., Riaz, S., and Naseem, Sh., Structural and magnetic properties of Fe–Co–Ni thin films, Indian J. Phys., 2014, vol. 88, p. 17.
Kuru, C.H., Kockar, H., Demirbas, O., and Alper, M., Characterizations of electrodeposited Ni–Co–Fe ternary alloys: Influence of deposition potential, J. Sci. Mater., 2015, vol. 26, p. 4046.
Watanabe, Yo., Otsubo, M., Takahashi, A., and Fukunaga H., Temperature characteristics of a fluxgate current sensor with Fe–Ni–Co ring core</b, IEEE Trans. Magn., 2015, vol. 51, p. 1.
Kourov, N.I., Pushin, V.G., Korolev, A.V., Knyazev, Yu.V., and Ivchenko, M.V., Peculiar features of physical properties of the rapid quenched Al–Cr–Fe–Co–Ni–Cu high-entropy alloy, J. Alloys Compd., 2015, vol. 636, p. 304.
Romankov, S., Park, Y.C., and Shchetinin, I.V., Mechanical intermixing of elements and self-organization of (Fe–Ni) and (Co–Fe–Ni) nanostructured composite layers on a Ti sheet under ball collisions, J. Alloys Compd., 2015, vol. 653, p. 175.
Yanai, T., and all, Electroplated Fe–Co–Ni films prepared from deep-eutectic-solvent-based plating baths, AIP Adv., 2016, vol. 6, p. 917.
Eguchi, K., Azuma, K., Akiyoshi, T., and Fukunaga, H., DC/pulse plating of Fe-Ni-Co films, 2016, Int. Conf. Asian Union Magn. Soc. (ICAUMS).
Yanai, T., Koda, K., Eguchi, K., and Takashima, K., Effect of ammonium chloride in plating baths on soft magnetic properties of electroplated Fe–Ni films, IEEE Trans. Magn., 2017, vol. 99, p. 1.
Li, D. and Podlaha, E., Template-assisted electrodeposition of Fe–Ni–Co nanowires: Effects of electrolyte pH and sodium lauryl sulfate, J. Electrochem. Soc., 2017, vol. 164, p. D843.
Yanai, T., and all, Electroplated Fe–Co–Ni films prepared in ammonium-chloride-based plating baths, AIP Adv., 2018, vol. 8(056127), p. 1.
Romankov, S., Park, Y.C., and Shchetinin, I.V., Structural transformations in (Co–Fe–Ni)/Ti nanocomposite systems during prolonged heating, J. Alloys Compd., 2018, vol. 745, p. 44.
Tabakovic, I. and Venkatasamy, V., Preparation of metastable Co–Fe–Ni alloys with ultra-high magnetic saturation (B s = 2.4–2.59 T) by reverse pulse electrodeposition, J. Magn. Magn. Mater., 2018, vol. 452, p. 306.
Zaharov, Yu.A., Pugachev, V.M., Ovcharenko, V.I., Datiy, K.A., Popova, A.N., and Bogomyakov, A.S., Phase composition and magnetic properties of nanostructured Fe–Co–Ni powders, Phys. Status Solidi B, 2018, vol. 255:170175, p. 1.
Cesiulis, H., Tsyntsaru, N., Podlaha, E.J., Li, D., and Sort, J., Electrodeposition of iron-group alloys into nanostructured oxide membranes: Synthetic challenges and properties, Curr. Nanosci., 2019, vol. 15, p. 84.
Budi, S., Muhab, S., Purwanto, A., Kurniawan, B., and Manaf, A., Effect of the electrodeposition potential on the magnetic properties of FeCoNi films, Mater. Sci.-Pol., 2019, vol. 37, p. 389.
Milyaev, M.A., Bannikova, N.S., Naumova, L.I., Proglyado, V.V., Patrakov, E.I., Kamenskii, I.Yu. and Ustinov, V.V., Magnetoresistance of Co–Ni–Fe/Cu superlattices with different composition of the ferromagnetic alloy, Fiz. Met. Metalloved., 2019, vol. 120, p. 905.
Saraç, U., Kaya, M., and Baykul, M.C., A comparative study on microstructures, magnetic features and morphologies of ternary Fe–Co–Ni alloy thin films electrochemically fabricated at different deposition potentials, J. Supercond. Novel Magn., 2019, vol. 32, p. 917.
Shekhanov, R.F., Electrodeposition of alloys containing iron-group metals from polyligand electrolytes, Doctoral Dissertation, Ivanovo, 2020.
Ledwig, P., Kac, M., Kopia, A., Falkus, J., and Dubiel, B., Microstructure and properties of electrodeposited nanocrystalline Ni–Co–Fe coatings, Mater. Sci., 2021, https://www.researchgate.net/publication/353213741.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by T. Safonova
Rights and permissions
About this article
Cite this article
Tikhonov, R.D., Cheremisinov, A.A. & Tikhonov, M.R. Congruent Electrochemical Deposition of Co–Ni–Fe Films. Russ J Electrochem 58, 1094–1102 (2022). https://doi.org/10.1134/S1023193522120072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193522120072