Abstract
CoNiFe alloy thin films deposited at various cobalt concentrations were galvanostatically electrodeposited on the pre-cleaned copper substrates. The effects of cobalt concentration on the structural, compositional, morphological, and magnetic properties of the films were investigated. X-ray diffraction patterns revealed that the deposited films possess polycrystalline in nature with mixed (fcc–bcc) cubic structure at optimized cobalt concentration. Microstructural properties of the films were calculated from predominant diffraction lines. The surface morphology and surface roughness were characterized using scanning electron microscopy and atomic force microscopy, respectively. EDAX results were revealed that the cobalt content increases as nickel content decreases whereas ferrous content initially increases and then eventually decreases in the CoNiFe alloy. VSM results show a higher value of saturation magnetization (4πM s) above 2 T with coercivity 154 A/m for films deposited in the optimized deposition condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ternary alloys of iron group metals have attracted many researchers due to their wide range of applications in computer read/write heads and microelectromechanical systems [1–3]. Materials with low coercivity and higher value of saturation magnetization have been used in read/write heads. A soft magnetic NiFe alloy has been extensively studied as core material in read/write heads [4]. Recently, CoNiFe alloy thin films have received much attention because of their applications in high-density read/write heads instead of NiFe alloys. CoNiFe alloy thin films with soft magnetic properties having saturation magnetization above 2 T and low coercivity below 160 A/m have been obtained by electroplating method earlier [5, 6]. Several methods of preparing CoNiFe alloy thin films such as electroless deposition [7], pulsed electrodeposition [8, 9], sputtering [10], and electroplating [5, 6, 11, 12] have been reported earlier. Among these methods, electrodeposition appears to be attractive due to its low-cost synthesis, low-temperature processing, arbitrary substrate shapes, and the possibility to control the film thickness and morphology by readily adjusting the electrical parameters as well as the composition of electrolytic solution [13, 14]. Ohashi et al. [15] observed that the electrodeposited CoNiFe alloy thin film obtained from sulfate electrolytic bath could be a promising material for high-density magnetic recording heads. Recently, Co containing some alloys such as CoFe [16] and CoNiFe [17] has been investigated as these materials were found to exhibit larger saturation magnetization values. CoNiFe alloy thin films with face-centered cubic (fcc), body-centered cubic (bcc), and hcp structures with single phase shows variation in saturation magnetization and coercivity values. So it is obvious that the mixed (bcc–fcc) phase of CoNiFe is accompanied by higher value of saturation magnetization with low coercivity will be suitable for core material in read/write head.
In the present work, CoNiFe alloy thin films were electrodeposited on the pre-cleaned copper substrates with various concentration of cobalt sulfate. The structural, morphological, and magnetic properties of the prepared thin films were studied using X-ray diffraction, scanning electron microscopy (SEM), atomic force microscopy (AFM), and vibrating sample magnetometer (VSM), respectively.
Experimental details
The soft magnetic CoNiFe thin alloy films were deposited on the pre-cleaned Cu substrates by galvanostatic electrodeposition from an aqueous electrolytic bath. The electrochemical experiments were performed in a standard three electrode cell without agitation. The electrolytic bath consists of (10–100) mM CoSO4, 20 mM NiSO4, 40 mM FeSO4, 350 mM H3BO3, and 300 mM NH4Cl. The NH4Cl and H3BO3 serving as supporting electrolyte and pH buffer, respectively. All the solutions were prepared by dissolving Analytical Grade Reagents (MERCK Chemicals, Mumbai, India) in de-ionized water. The pH of the electrolytic bath was adjusted to 2.9 ± 0.1 by adding adequate amount of H2SO4 up to the required value using a digital pH meter. The deposition time and current density were fixed for 30 min and 9.5 mA/cm2 to obtain fine quality of CoNiFe alloy thin films. The electrochemical experiments were carried out using a potentiostat/galvanostat (EG&G, Model 362, Princeton Applied Research, USA). The counter and reference electrodes were graphite plate and saturated calomel electrode, respectively.
The film thickness was measured using stylus profilometer (Mitutoyo, SJ 301) and the values of thickness are found to be in the range between 700 and 950 nm. X-ray diffraction data of the obtained samples were recorded using an X-ray diffractometer (X’PERT PRO PANalytical, The Netherlands) employing CuKα radiation with λ = 1.540 Å. Surface morphology of the film composition was analyzed using an energy dispersive X-ray analysis set up attached with a scanning electron microscope (Philips Model XL 30). Magnetic properties of the films were investigated using vibrating sample magnetometer (7404 Lakeshore, USA).
Results and discussion
XRD analysis
Figure 1 depict that the XRD patterns of CoNiFe alloy thin films electrodeposited at various Co2+ concentrations of electrolytic bath (10, 25, 50, 75, and 100 mM). The observed d-spacing values are indexed with standard values [JCPDS file no. 65–5131 and 65–4131]. The presence of diffraction peaks indicates that the deposited films possess polycrystalline in nature with cubic structure. It is observed from Fig. 1 (a) that the CoNiFe alloy thin films obtained at 10 mM Co2+ concentration exhibits only body-centered cubic structure specifically, with (110), (200), (211), and (220) planes. Generally, the common form of nickel (Ni) and cobalt (Co) is face-centered cubic and the common form of ferrous (Fe) is bcc. Co–Ni–Fe electrodeposits, from the solution containing 25 mM of Co2+ concentration (Fig. 1 (b)), show that the intensity of bcc (110), (200), and (211) planes decreased gradually and bcc (220) plane disappeared. Fcc (111) structure plane is also present. This indicates the inclusion of cobalt content in electrodeposited alloy. Intensities of bcc peaks decrease slightly, indicating the reduction of Fe content in the Co–Ni–Fe films. Figure 1 (c) shows that the bcc peak intensities decreased continuously and fcc peak intensities increased for film deposited with 50 mM Co2+ concentration. In this case fcc (200) plane is also emerged. The film electrodeposited at 75 mM Co2+ concentration (Fig. 1 (d)) exhibited the appearance of fcc (220) plane. It is also found that the intensity of fcc planes increases and the intensity of bcc planes decreases. Further, when Co2+ concentration is increased above 75 mM, the orientation of plane changes from bcc (110) to fcc (111) as indicated in Fig. 1 (e). If the content of Co is high, the films exhibit well-oriented fcc phases with slight incorporation of bcc (110) plane. Similar behavior exhibited by CoNiFe alloy thin films has been reported earlier [18].
X-ray diffraction patterns were taken out in order to identify the crystallinity and crystallographic planes of deposited films. From X-ray diffraction data, the interplanar spacing d hkl was calculated using Bragg’s relation [19]
where “λ” is the wavelength of X-rays used, “d” the interplanar spacing, “n” the number of order, and “θ” is the Bragg’s diffraction angle. The factor “d” is related to (hkl) miller indices of the plane and the dimension of unit cell using the following relation,
using Eq. 2 the lattice constant of cubic cell can be calculated.
The variation of lattice parameter with CoSO4 concentration for CoNiFe alloy thin films obtained at different CoSO4 concentrations is shown in Table 1. The obtained lattice parameters are 3.598 and 2.847 Å for fcc—NiFe and bcc—CoFe, respectively. It is observed from the table that the value of lattice parameter increases with the concentration of CoSO4 for both fcc and bcc phases. On the other hand, the lattice constant for fcc (111) plane and bcc (110) plane values are increase when increasing the CoSO4 concentration from 25 to 75 mM and attains its maximum value (3.598 and 2.849 Å) for the 75 mM concentration; subsequently, the values of lattice constant slightly decrease. The reason for the variation in fcc and bcc lattice constants may be due to the volume required per one atom in Fe is about 5.9% larger than Co and about 8.5% larger Ni atoms [20, 21].
The size of the crystallites is calculated using FWHM data and Debye-Scherer formula [22]
where “λ” is the wavelength of CuKα target (λ = 0.1540 nm), “β” is full width at half maximum of the peak in radians, “θ B” is Bragg’s diffraction angle at peak position in degrees. The sizes of the crystallites are found to be in the range between 17 and 23 nm. The variation of intensity in bcc (110) plane is attributed to crystallite size variation. The fcc–bcc mixed phases are formed with fine crystal structure compared to that of single phases of bcc or fcc structures [23]. The crystallite size of the mixed phase (fcc–bcc) has been obtained taking the average value of both the predominate peak. The lowest crystallite size is obtained at optimized cobalt sulfate concentration (75 mM) of the CoNiFe alloy thin film. This lower crystallite size has very good application in soft magnetic properties. The variation in lattice spacing leads to crystallite size variation may be responsible for change in dislocation density values [14]. In our study, well-oriented fcc–bcc mixed phase CoNiFe alloy thin film with lower crystallite size achieved at 75 mM Co2+ concentration. It is on the crystallite size reduction and smoothness.
In order to calculate the strain separation in the films, we have followed the procedure using Williamson-Hall method [24]. The calculation of microstrain was based on the assumption of strain line profiles and the crystallite size with the proper equation connecting these two parameters is given by
where λ is the wavelength of X-ray, θ 0 is the diffraction angle, D is the crystallite size, and e is the microstrain. Table 1 shows all the values of obtained microstructured parameters. Dislocation density is defined as the length of dislocation lines per unit volume of the crystal. The dislocation density is calculated using the relation [25].
The variation of crystallite size and dislocation density with respect to CoSO4 concentration is shown Table 1. It is observed from the table that the sizes of the crystallites are found to decrease while increasing CoSO4 upto 75 mM, afterwards it increase slightly. It is also observed that the dislocation density increases while increasing CoSO4 concentration from 25 to 75 mM and attains its maximum value for films obtained at 75 mM CoSO4 concentration; thereafter, it decreases faintly. The variation in lattice spacing leading to the variation in crystallite size may be responsible for change in dislocation density values. The broad fcc and bcc peak observed in cobalt sulfate concentration increases from 10 to 75 mM. The increase of concentration of Cobaltus ion may pave the way for filling more metallic vacant sites and intertials resulting in a more compact formation which leads to decrease in crystallite size. From Table 1 it is observed that number of crystallites per unit area increases with the increase of cobalt concentration from 10 to 75 mM, further increase of Co concentration and the number of crystallites is found to decrease, which may be attributed to the reduction in crystallite size with increase in cobalt concentration.
Morphological and compositional analyses
The surface morphology of CoNiFe alloy thin films was analyzed using scanning electron microscopy. The surface morphological micrographs of CoNiFe alloy thin films obtained at (50–100) mM of cobalt concentration is shown in Fig. 2. It is observed from Fig. 2a that the grains are in columnar-like structure grown perpendicular on the substrate. The surface is covered with nonuniform distribution with dense grains. It is observed from Fig. 2a that the films deposited at 50 mM Co2+ concentrations exhibit rough surface with different grain sizes and the surface is covered with more number of grains that are packed within 1 μm scale. Figure 2b shows the optimized cobalt concentration of morphological image in which the film surface is found to be smooth, uniform, and free from pinholes. The grains are distributed uniformly over the entire surface of the film. The sizes of the grains are found to be in the range between 0.25 and 0.42 μm. The average size of the grains is found to be 0.34 μm. Figure 2c shows the higher deposition concentration (100 mM) of surface morphology with the appearance of powderly deposited grains with some pin holes. Figure 2c shows rough surface with larger grains when compared to films obtained at 75 mM Co2+ concentration. This is due to the evolution of hydrogen with the increase in cobalt concentration during the process of deposition. Jafarian et al. [26] have synthesized CoNiFe alloy thin films with high cobalt content, the morphology of that film have been observed hemispherical crystallites distributed on the substrate. The films obtained at 75 mM Co2+ concentration have very small size and uniformly distributed grains when compared to films obtained at other Co2+ concentrations.
The composition of the films was analyzed using energy-dispersive analysis by X-rays. The composition analysis was carried out in order to determine the relationship between Co, Ni, and Fe content and the influence of Co concentration. The variation of Co, Ni, and Fe content with CoSO4 concentrations is shown in Fig. 3. It is observed that the content of Co increases from 50 to 65 at.% while increasing CoSO4 concentration from 10 to 100 mM. It is also observed that the content of Ni decreases from 20 to 10 at.% while increasing CoSO4 concentration upto 75 mM, afterwards it increases faintly whereas the behavior of Fe is found to be oscillating. The content of Fe increases from 28 to 30 at.%, while increasing CoSO4 concentration from 10 to 75 mM; subsequently, it decreases slightly. The film prepared at 10 mM CoSO4 concentration having Co, Ni, and Fe content values 50, 22, and 28 at.% exhibits only fcc phase. The film prepared at optimize CoSO4 concentration containing Co, Ni, and Fe composition values are 60, 10, and 30 at.% exhibits mixed phases with low crystallite size and grains are finely dispersed morphological properties. The AFM (2D and 3D view) micrographs of CoNiFe alloy thin film obtained at 75 mM Co2+ concentration is shown in Fig. 4. It is noted from Fig. 4 that the surface is found to exhibit cone-shaped grains. The grains are distributed evenly over the entire surface of the films. The R a value of the films obtained in this concentration is found to be 13.49 nm. The roughness value obtained in this work is found to be lower than CoNiFe thin film reported earlier [27].
Magnetic properties
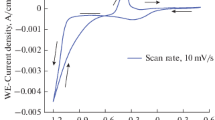
The crystalline natures of the material determine the magnetic properties of the materials. The saturation magnetization and coercivity are important parameters that determine the magnetic properties of soft magnetic materials which are found in numerous applications especially in magnetic recording and reading technology. Hence, we have planned to examine the saturation magnetization and coercivity of deposited CoNiFe alloy thin films with different concentration of cobalt. The magnetic hysteresis loop of CoNiFe alloy thin films prepared at optimized CoSO4 concentration of 75 mM is as shown in Fig. 5.
Figure 6 shows the variation of saturation magnetization and coercivity for CoNiFe alloy thin films prepared at various CoSO4 concentrations. Higher saturation magnetization (4πM s) 2.08 T and the coercivity of 154 A/m were observed for the film prepared at optimized Co concentration of 75 mM as shown in Fig. 6. It is observed that the value of saturation magnetization increases from 1.88 to 2.08 T while increasing CoSO4 concentration and it starts decreasing with further increase of Co concentration. The value of coercivity (H c) decreases from 635 to 154 A/m when increasing CoSo4 concentration upto 75 mM; thereafter, it increases slightly. The films prepared at optimized CoSO4 concentration exhibits a higher value of saturation magnetization with low value of coercivity. This is due to the fact that the crystallite size attains a minimum value of 17 nm exhibiting mixed (fcc–bcc) phases of CoNiFe alloy. The exotic magnetic behavior arising with the minimum obtained crystallite size for the optimized CoNiFe alloys with the ratio (Co 60 at.%, Ni 10 at.%, and Fe 30 at.%) is due to the origin of the nanoregime for this film. We are able to achieve the best soft magnetic property which is the outcome of the obtained hysteresis loop from the above appropriate composition ratio of these alloys. The optimized CoNiFe alloy film having smaller crystallite size, high magnetic saturation, and low coercivity prepared by our method, which we believe, is suitable for the application of core material in read/write head. Similar behavior for CoNiFe alloy thin films have been reported earlier [27]. The CoNiFe alloy thin films with higher Co-content exhibiting saturation magnetization value greater than 2 T and low coercivity have been reported without any additives [10]. CoNiFe alloy thin films with coercivity value less than 160 A/m must be utilized as core material in read/write head in computer applications [17]. But we report the value of the coercivity as 154 A/m from our experiment which we believe that our method is the best method of preparing these types of magnetic materials with low cost and high efficiency.
Conclusions
In conclusion, cathodic deposition of CoNiFe alloy thin films on the pre-cleaned Cu substrate has been carried out by galvanostatically using electrodeposition technique. The effect of CoSO4 concentration on structural, morphological, compositional, and magnetic properties has been investigated. The deposited film exhibited single bcc phase (below 50% Co content) and mixed (bcc–fcc) phases (above 50% Co content) for films prepared at various CoSO4 concentrations. The films obtained under optimized deposition conditions have low crystallite size value nearly 17 nm with mixed (bcc–fcc) phases exhibiting higher saturation magnetization and low value of coercivity. Surface morphology reveals smooth, uniform surface covered with evenly distributed grains. The grains in the film are evenly distributed with densely grown on the substrate surface. The change in the structural phases from single phase (fcc) to mixed phases (bcc–fcc) significantly influences the magnetic properties. The M s and H c are obtained as 2.08 T and 154 A/m at the optimized condition of the CoNiFe alloy thin films. The soft magnetic property is observed at low pH and low current density. It is concluded from VSM studies that CoNiFe alloy thin films have high saturation magnetization and low coercivity values and also associated with high fcc–bcc mixture structure with high cobalt content. Hence, the results obtained in the present work will construct CoNiFe alloy thin films as core material in high magnetic recording head for practical purpose.
References
Hong J, Furukawa A, Sunn N, Wang SX (1999) IEEE Trans Magn 35:2502–2504
Kim D, Park DY, Yoo BY, Sumodjo PTA, Myung NV (2003) Electrochim Acta 48:819–830
Kohmoto O (1991) IEEE Trans Magn 27:3640–3647
Chiu A, Croll I, Heim DE, Jones RE Jr, Kasiraj P, Kiaassen KB, Mec CD, Simmons RG (1996) IBM J Res Dev 40:283–300
Osaka T, Takai M, Hayashi K, Ohashi K, Yasue Y, Saito M, Yamada K (1998) Nature 392:796–798
Osaka T, Takai M, Hayashi K, Sogawa Y, Ohashi K, Yasue Y, Saito M, Yamada K (1998) IEEE Trans Magn 34:1432–1434
Osaka T, Yokoshima T, Corros (2004) Eng Sci Tech 39:38–44
Eftekhari A (2004) Phil Mag Lett 84:587–592
Brankovic SR, Vasiljevic N, Klemmer TJ, Johns EC (2005) J Electrochem Soc 152:C196–C202
Kim YM, Choi D, Kim SR, Kim KH, Kim J, Han SH, Kim HJ (2001) J Magn Magn Mater 226:1507–1509
LI J, Zhang Zh, Yin J, Yu G, Cai Ch, Zhang J, Nonferr T (2006) Metal Soc 16:659–665
Liu X, Zangari G, Shen L (2000) J Appl Phys 87:5410–5412
Kyung Seek Lew, Raja M, Thanikaikarasan S, Taekyu Kim, Yong Deak Kim, Mahalingam T (2008) Mater Chem Phys 112:249–253
Thanikaikarasan S, Mahalingam T, Sundaram K, Kathalingam A, Kim YD, Kim T (2009) Vacuum 83:1066–1072
Ohashi T, Yasue Y, Saito M, Yamada K, Osaka T, Takai M, Hayash K (1998) IEEE Trans Magn 34:1462–1464
Lallemand F, Ricq L, Berc P, Pagetti J (2002) Electrochim Acta 47:4149
Tabokovic I, Inturi V, Riemer S (2002) J Electrochem Soc 149:C18–C22
Shinoura O, Kamijima A, Narumiya Y (1994) J Magn Soc Jpn 18:277
Cullity BD (1978) Elements of X-ray diffraction. Addison-Wesley, Boston, 555
Chechenin NG, Khomenko EV, Vainchtein DI, de Hosson JThM (2008) J Appl Phys 103:07E738–3
Nam HS, Yokoshima T, Nakanishi T, Osaka T, Yamazaki Y, Lee DN (2001) Thin Solid Films 384:288–293
Mahalingam T, Dhanasekaran V, Ravi G, Lee S, Chu JP, Lim Han-jo (2010) J Optoelectron Adv Mater 12:1327–1332
Osaka T (2000) Electrochim Acta 45:3311–3321
Williamson GK, Smallman RE (1956) Philos Mag 1:34–36
Velumani S, Narayandass SaK, Mangalaraj D (1998) Semicond Sci Technol 13:1016–1024
Jafarian M, Azizi O, Gobal F, Mahjani MG (2007) Int J Hydrogen Energy 32:1686–1693
Liu X, Evans P, Zangari G (2000) IEEE Trans Magn 36:3479–3481
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundaram, K., Dhanasekaran, V. & Mahalingam, T. Structural and magnetic properties of high magnetic moment electroplated CoNiFe thin films. Ionics 17, 835–842 (2011). https://doi.org/10.1007/s11581-011-0580-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-011-0580-0