Abstract
Nowadays, industrial waste from feedstock contains a lot of organic acids, which are being discharged into the water streams causing environment load with wastage of resources. Electrodialysis performs an important role in wastewater treatment. In this study, we have prepared different anion exchange membranes, by casting solution of poly(vinylchloride-co-vinylacetate) (PVC–VA) with uniformly dispersed micronized anion exchange resin (polystyrene–divinylbenzene–trimethyl ammonium chloride) (PS–DVB–TAC). These membranes were also processed for the deposition of polyaniline at different time intervals by in situ polymerization. The cation exchange membrane (CEM) was also produced by casting a solution, containing (PVC–VA) with micronized cation exchange resin [sulfonated poly(styrene-co-divinylbenzene)] (SPS–DVB). The separation of the model solution of lactic acid was performed by using anion exchange membrane (with coated/without coated PANI) and cation exchange membranes (CEM) in a four-compartment electrodialyzer at same voltage. These different modified forms of anion exchange membranes were characterized by FTIR/ATR for identification of functionalities within the polymer chain, SEM analysis for surface morphology, TGA for thermal stability and a four-probe method for electrical conductivity of membranes. While, % age of water uptake and ion exchange capacity were also determined. The results of electrodialysis showed that the 14.78% of the lactate ions moved through anion exchange membrane (without a coating of PANI) to the product compartment within 30 min of the experiment at (30 V). While, the membranes with polyaniline’s coating time 2, 12, and 24 h have separation efficiency of lactic acid 21.65, 27.46, and 33.36% respectively at the same voltage. The results clearly showed that higher PANI coating has a positive trend in the separation of lactic acid. This separation technique has a great potential in the separation of organic acids and amino acids from a feedstock of microbial fermentation at an industrial scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Recently, composite membranes comprising intrinsically conductive polymers (ICPs) have been prepared [1, 2]. These composite membranes have applications in different fields of membrane processes such as electrodialysis [3–5], gas separation [6], pervaporation, and nanofiltration. Various methods have been used to prepare these composite membranes including polymer blending [7], in situ polymerization [8], grafting, and electrochemical polymerization. Among various ICPs, PANI is one of the largest studied ICP [9].
PANI, an intrinsically conducting polymer, has some unique characteristics such as relatively low cost, easy synthesis, large intrinsic electronic conductivity, simple tunable chemistry with the aid of acid and base as well as having environmental and thermal stability [10–14].
Many investigations have been focused on the modification of solid surfaces with a layer of PANI that consequently serves as the active constituent, while the resin support provides the required mechanical features [5, 15].
Various approaches to produce membranes by using PANI have already been reported including drop casting, dip coating, fractional thermal vacuum deposition and electrochemical growth by voltammetric, galvanostatic or potentiostatic routes [16].
Spin coating is the most common technique used to produce thin PANI films [17]. The major limitation to implement this approach is the availability of very few solvents to completely dissolve PANI for subsequent spin coating. Moreover, the spin-coated PANI films have less adhesion to the supporting surface. Therefore, a scalable deposition method remains a challenge for morphological homogeneity and control thickness of film on the solid-supported substrate. In situ polymerization of aniline during deposition has a potential to become a continuous, scalable and simple method to produce smooth thin PANI film on supported surfaces. Sapurina with coworkers have reported that the adsorption of aniline’s oligomers on the upper surface of the supported substrate plays a primary role in PANI film formation [18].
Research studies showed that the deposition rate, electronic and morphological properties of thin films of PANI are greatly dependent on the nature of the substrate surface, whether hydrophobic or hydrophilic. Hydrophobic substrates are smoother in PANI deposition than hydrophilic substrates [19].
In biochemical and chemical industries ion exchange membranes (IEMs) play an essential role, as these are more advanced separation membranes [10]. Electrodialysis has attracted the attention to separate and purify the organic acids and amino acids from feedstock because of its environmentally benign nature [20, 21]. In present work, we studied the separation efficiency of PANI coated composite membrane for the separation of lactic acid through electrodialysis that has the most important potential applications in food, chemical and pharmaceutical industries [22–24].
EXPERIMENTAL
Chemicals
Polystyrene-divinylbenzene-trimethyl ammonium chloride (PS–DVB–TAC) anion exchange resin (Mecolite PA 101) and sulfonated polystyrene divinylbenzene (SPS–DVB) (mecolite PC003 ID) was obtained from the local market and used after crushing (400 mesh). Poly(vinylchloride-co-vinylacetate) (PVC–VA) was purchased from the commercial supplier [Akbari store] while, analytical grade Lactic acid (88%) was obtained from [Sigma-Adrich]. Aniline, Ferric chloride (FeCl3), methyl ethyl ketone (MEK), phenolphthalein was purchased from Merck®, USA. Hydrochloric acid and sodium hydroxide were purchased from Sigma-Aldrich. Because all reagents were of analytical grades so they were used as received. For the preparation of all solutions in the whole experimental work deionized water was used.
Equipments
Scanning electron microscopy (SEM) micrographs were recorded using JEOL JSM-6480LV at various magnifications. Particle size analyzer, Litesizer-500 (Anton Paar GmbH ) with a measuring range of 0.3 nm–10 μm, was used to determine the particle size of the anion exchange resin. The FTIR-ATR analysis was performed by using JASCO FTIR 4100 spectrophotometer in attenuated total reflectance (ATR) mode in the range of 650–4000 cm–1. The electrical conductivity of the membranes was measured by using (Keithley, 6220-Precision current source, 2182-Nanometer) four-probe method. DC power supply (Adiget PS 3030DD) was used as a potential source. The thermogravimetric analysis of membranes was conducted under a nitrogen atmosphere using Schimadzu TGA-50 analyzer. The heating rate for the membranes was 20°C/min.
Procedure to Synthesize Anion Exchange Membrane
(PS–DVB–TAC) anion exchange resin was crushed in pestle and mortar and subsequently soaked in distilled water for more than 24 h. Then it was ground again to a very fine size to be sieved through 300–400 mesh after drying for 6 h at 50°C. (PVC–VA) (2.0 g) was added in MEK solvent (16 g) and stirred for 4 h. When it became a clear solution then (PS–DVB–TAC) anion resin (2.0 g) was added in the solution of (PVC–VA), and then stirred for another 4 h until the resin was uniformly distributed. Then resin dispersed solution was cast on the glass plate by casting knife, the solvent was allowed to evaporate for 4 h at 25°C and the formed membrane was removed from the casting plate.
Aniline (0.4 M) and FeCl3 (0.3 M) solutions were prepared in 0.4 M aqueous HCl separately. Then [(PVC–VA) + (PS–DVB–TAC))] composite membrane was coated with PANI by in situ polymerization. For this purpose, [(PVC–VA) + (PS–DVB–TAC)] composite membrane was dipped in 0.4 M anilinium chloride solution and then was slowly added with 0.3 M FeCl3 solution. This deposition of PANI was carried out for 2, 12, and 24 h durations with three different membranes respectively and PANI was coated on the membrane. After the deposition, the membranes were removed and washed with 1 M HCl solution to remove the oligomers and then again washed with deionized water. Similarly, the cation exchange membranes were also self-prepared by using the cation exchange resin (SPS–DVB) and (PVC–VA) in the same ratio as in the synthesis of anion exchange membrane.
The schematic route for the synthesis of polyaniline deposited anion exchange membranes has been shown below in Fig. 1.
Water Uptake
For the % age measurement of water uptake, Membranes of almost 2 cm2 were soaked up in deionized water for 24 h at room temperature for equilibration. Membranes were weighed after the removal of excessive water by the absorbent paper and then dried in an oven for four hrs at 60°C, and then weighed again. By taking the difference of mass between wet (Wwet) and dry (Wdry) membranes the % age of water uptake was calculated by using the following formula [25–27].
Ion Exchange Ccapacity
2 cm2 specimen of anion exchange membrane without PANI coating [(PVC–VA) + (PS–DVB–TAC)] and PANI-modified membranes were immersed in 30 mL of 0.1 M NaOH solution for 24 h, separately. The chloride ions attached with the quaternary ammonium part of resin and with quinoid part of polyaniline were replaced by OH– ions. The depletion of the hydroxyl ions was determined by titrating with 0.1 M HCl solution using phenolphthalein as an indicator. Before drying, the membranes were washed by rinsing with deionized water. The IEC was determined as milliequivalents (meq/g) of OH– per gram of dry membrane by using the following formula [26]:
Electrical Conductivity
The electrical conductivity of nonfabricated and the polyaniline’s fabricated membranes was measured by the four-probe method. In this method, a 3 cm spherical sample of the membrane was prepared by punching with the help of puncher of internal diameter 3 cm and then its conductivity was measured by using the four-probe, each probe being separated by 0.2 cm because its thickness (130–140 μm) is very small as compared to the probe spacing, and its value is calculated by using the following formula [31]:
Here V is the voltage, I is the value of current, obtained from the conductivity data. While, C is correction factor, its value was calculated by dividing the diameter (d) of the membrane’s sample by probe spacing (s). Then conductivity was calculated by taking the inverse of resistivity, using the following formula.
Electrodialysis
The process of electrodialysis was carried out in a four-compartment electrodialyzer cell as shown in Fig. 2. Titanium plates were used as an anode and cathode. The exposed area of these electrodes was 12.56 cm2. Self-prepared anion and cation exchange membranes were used for the separation of lactic acid. The exposed area of the membranes was (12.56 cm2). NaOH solution (0.1 N) was used in anodic and cathodic compartments. The chamber beside the cathodic compartment was tagged as the feed compartment and the compartment alongside the anodic compartment was tagged as the product chamber. In both of these chambers, 1% lactic acid solution was placed.
Before electrodialysis, membranes were placed in their corresponding solutions for 24 h. All solutions were prepared in the deionized water. At room temperature, all experiments were conducted for 30 min by using the DC potential source at standard voltage (30 V) for 30 min duration and monitor the separation of lactic acid from different forms of polyaniline coated anion exchange membrane by acid–base titration using phenolphthalein as an indicator [28].
RESULTS AND DISCUSSION
Particle Size Determination
The particle size of the anion exchange resin is within the range of 1.976 to 2.964 µm as shown in Fig. 3. The most frequently occurring particle size is 2.4 to 2.5 µm which is a quite narrow range and a good indicator of uniform distribution. Particle size distribution is an important factor because it affects the phase morphology of membranes. Finer the resin particles are, the more uniform and homogenous is the phase morphology. As the resin particles become coarser, phase morphology will be more irregular and non-uniform. The poly(vinylchloride-co-vinylacetate) (PVC–VA) is a film-forming material, while the particles of anionic exchange resins are infusible and incapable to form a film. As the particle size of resin become coarser, it will affect the continuous film formation of the resulting membranes. So, in this research very fine size of resin (PS–DVB–TAC) is used to form a homogenous phase of anion exchange membrane as evident from SEM images in Fig. 4.
SEM Analysis
SEM images of the pure polymeric membranes and its modified version were recorded which are shown in Fig. 4. Figure 4a is a SEM image of the pristine membrane of (PVC–VA) showing the smooth surface. Figure 4b is presenting the rough surface texture of membrane formed by compositing (PVC–VA) with anion exchange resin (PS–DVB–TAC) of particle size 1.976 to 2.964 micron. Figure 4c is displaying the deposition of PANI on the surface of the composited membrane after 2 h polymerization’s time of aniline. Figure 4d is showing the dense character of polyaniline deposited during 12 h of polymerization on the composite membrane. Figure 4e represents even more dense deposition of polyaniline during 24 h of polymerization on composite membrane.
We expect that there is always a strong interaction between the benzenoid or quinoid part of polyaniline i.e. (N–H or –N=) and Cl or C=O moieties in the (PVC–VA). A similar interaction is expected between the quaternary ammonium [C4(N+)] part of the anion exchange resin and the functional moieties in the (PVC–VA). This might have resulted in the covering of [(PVC–VA) + (PS–DVB–TAC)] with PANI. The higher magnification of the (PVC–VA) shows that the matrix membrane is porous as SEM image is inset in Fig. 4a.
Water Uptake Percentage
(PVC–VA) membrane is hydrophobic so it does not absorb water. The [(PVC–VA) + (PS–DVB–TAC)] membrane absorbed (10.67%) amount of water due to the presence of quaternary ammonium functionalities of resin. In [(PVC–VA) + (PS–DVB–TAC)] + PANI 2 h membrane water uptake increased (16.56%) significantly due to the combined effect of benzenoid, quinoid and quaternary ammonium moieties. But in [(PVC–VA) + (PS–DVB–TAC)] + PANI 12 h membrane water uptake (23.72%) increased effectively with increasing the time of PANI coating. Moreover, in [PVC–VA) + (PS–DVB–TAC)] + PANI 24 h membrane water uptake is (29.33%) which showed that by further increasing the time of PANI coating, thickness of PANI on the composited membrane is increased.
The results of % age of water uptake given in Table 1, showed the increasing trend of water uptake with increasing the deposition time of PANI coating.
Ion Exchange Capacity
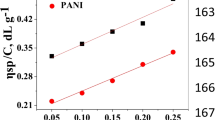
Table 2 shows the values of ion exchange capacities of [(PVC–VA) + (PS–DVB–TAC)] composite and PANI modified membranes. The value of ion exchange capacity of [(PVC–VA) + (PS–DVB–TAC)] is (1.268) which is due to the presence of exchangeable chloride moieties on the Trimethyl quaternary ammonium. The trend for ion exchange capacity is similar as the % age of water uptake. The value of IEC of PANI coated membrane for two hrs, increased (1.417) due to the synergic effect of exchangeable chloride associated with quinoid (–N=) and quaternary ammonium functionalities. This can also be observed in the increased value of water uptake for the PANI modified membrane for 2 h.
The value of ion exchange capacity further increased (1.724) for the modified membrane with PANI coating for 12 h. This is probably due to thick coating of PANI with exchangeable chloride of quaternary ammonium resin. Finally, the value of IEC of PANI coated membrane for 24 h is (1.976) increased again. This means, by densing the coating of PANI on the composite membrane the amount of exchangeable chlorides is increased.
Structure Elucidation
FTIR analysis was used to investigate the structure of ion exchange membranes as shown in Fig. 5 and absorption signals have been given in Table 3. The peak appears at 689 cm–1 shows the existence of C-l, at 1737 cm–1 attributed to C=O stretch and at 1232 cm–1 corresponded to C–O stretch. It is observed that with increasing the time of deposition of Polyaniline, the height of the peak of these groups decreases as well. The peak of quaternary amine (CN+) appears at 1367 cm–1 and C–H stretch band is at 2913 cm–1. While, the peak appears at 3234 cm–1 is due to N–H bond and the broadening of this peak shows hydrogen bonding with C=O group.
Thermogravimetric Analysis
Figure 6 shows the TGA curves of (PVC–VA), [(PVC–VA) + (PS–DVB–TAC)] and PANI-modified [(PVC–VA) + (PS–DVB–TAC)] cast films. In (PVC–VA), the weight-loss between 60–270°C, is corresponding to the loss of moisture, residual MEK (solvent) and additional volatile components. The second weight loss between 270–360°C attributed to dehydrochlorination and deacetification of (PVC–VA) that results in the formation of polyene which is further degraded between 400–580°C into lower molecular weight molecules [29, 30]. Above this temperature carbonaceous polymer residues and ash are formed as shown by the TGA profile.
In [(PVC–VA) + (PS–DVB–TAC)], membrane weight loss compared to PVC–VA) membrane starts at early temperature region (30–270°C), which shows the removal of water, trapped in (PS–DVB–TAC) resin. Moreover, in addition to dehydrochlorination, deacetification the deamination also occurred because of the presence of quaternary ammonium moiety in the anion exchange resin. The decreasing weight loss in the second transition between 270–360°C can be described in terms of the lower extent of dehydrochlorination and deacetification and this is due to smaller (PVC–VA) content in [(PVC–VA) + (PS–DVB–TAC)] composite membrane. Similarly, between 360–540°C, the decreased weight loss as compared to (PVC–VA) membrane indicating the fusion and subsequent charring of anion exchange resin with the removal of the gaseous products.
In the case of [(PVC–VA) + (PS–DVB–TAC)] composite membranes, coated with PANI for 2, 12, and 24 h, higher weight losses occurred between 30–270°C as compared to [(PVC–VA) + (PS–DVB–TAC)] composite membrane. Here, PANI is doped with HCl and it is released from PANI along with moisture. In the temperature range between 270–360°C, a decreasing trend of weight loss is shown as compared to [(PVC–VA) + (PS–DVB–TAC)] representing that, increasing the PANI coating, increased the thermal stability of membrane.
The decreased weight loss of PANI-modified [(PVC–VA) + (PS–DVB–TAC)] composite membranes as compared to [(PVC–VA) + (PS–DVB–TAC)] membrane in the range 450–560°C indicates that deposition of PANI, decreased dehydrochlorination, deacetification and deamination of [(PVC–VA) + (PS–DVB–TAC)] and enhanced the stability of PANI coated membranes.
Measurement of Electrical Conductivity
The results given in Table 4 indicate that the electrical conductivity of the membranes is ranged from 0.009 × 10–7 to 3.4 × 10–7. As the coating time of polyaniline was increased, the conductivity of polyaniline composite membranes was also enhanced.
Electrodialysis
After electrodialysis, the concentration of lactic acid in the feed compartment was determined by titrating against 0.01 N NaOH using phenolphthalein indicator. Before electrodialysis operation, a blank reading for 1% lactic acid was taken. The experimental separation results of the lactic acid are summarized in Table 5.
Results given in Table 5 show that anion exchange membrane without PANI coating is capable of separating the lactic acid upto 14.78%. While, the anion exchange membranes with 2 and 12 h PANI coating has 21.65 and 27.46% separation efficiency respectively. Moreover, the separation of lactic acid with 24 h PANI coating is 33.36%. It was observed during the electrodialysis process that energy consumption was varied from 1.5 to 0.8 kW h/kg and current efficiency (η) was varied from 60 to 80% with an increasing trend of PANI coating. The results of fabricated membranes show that with increasing PANI coating, separation percentage of lactic acid is increased.
CONCLUSIONS
Heterogeneous anion exchange membranes were synthesized by coating polymeric anion exchange membrane of [(PVC–VA) + (PS–DVB–TAC)] with PANI. The PANI was coated on the anion exchange membrane via in situ polymerization of aniline at different polymerization times. SEM images of the PANI coated membranes showed that the thickness of the polyaniline coating was dependent on the time duration. Moreover, coating of PANI is denser at longer polymerization time than the shorter polymerization time. The verification of specific interaction between PANI and [(PVC–VA) + (PS–DVB–TAC)] membrane is also confirmed by FTIR analysis. The ion exchange capacity and water uptake of the fabricated membranes are positively affected by the PANI coating, showing an increasing trend in performance with more deposition time of PANI. The data of electrical conductivity shows that with increasing PANI coating the electrical conductivity is also increased. The experimental results showed that the acid separation capability of the membranes increases as PANI coating thickness increased in the membranes by employing the DC potential source, which can be used as a better solution for the industry of feedstock to separate organic acids from their waste.
REFERENCES
Qaiser, A.A., Hyland, M.M., and Patterson, D.A., Control of polyaniline deposition on microporous cellulose ester membranes by in situ chemical polymerization, J. Phys. Chem. B, 2009, vol. 113, p. 14986.
Rohani, R., Yusoff, I.I., and Efdi, F.A.M., Polyaniline composite membranes synthesis in presence of various acid dopants for pressure filtration, J. Kejuruteraan, 2017, vol. 29, p. 121.
Shkirskaya, S.A., Senchikhin, I.N., Kononenko, N.A., and Roldugin, V.I., Effect of polyaniline on the stability of electrotransport characteristics and thermochemical properties of sulfocationite membranes with different polymer matrices, Russ. J. Electrochem., 2017, vol. 53, p. 78.
Loza, N.V., Loza, S.A., Kononenko, N.A., and Magalyanov, A.V., Ion transport in sulfuric acid solution through anisotropic composites based on heterogeneous membranes and polyaniline, Pet. Chem., 2015, vol. 55, p. 724.
Montes-Rojas, A., Rentería, J.A.Q., and Chávez, N.B.J., Influence of anion hydration status on selective properties of a commercial anion exchange membrane electrochemically impregnated with polyaniline deposits, RSC Adv., 2017, vol. 7, p. 25208.
Sairam, M., Nataraj, S.K., and Aminabhavi, T.M., Polyaniline membranes for separation and purification of gases, liquids, and electrolyte solutions, Sep. Purif. Rev., 2006, vol. 35, p. 249.
Amado, F.D.R., Rodrigues, L.F., and Rodrigues, M.A.S., Development of polyurethane/polyaniline membranes for zinc recovery through electrodialysis, Desalination, 2005, vol. 186, p. 199.
Nagarale, R.K., Gohil, G.S., and Shahi, V.K., Preparation and electrochemical characterization of cation- and anion-exchange/polyaniline composite membranes, J. Colloid. Interface Sci., 2004, vol. 277, p. 162.
Stejskal, J., Sapurina, I., and Trchová, M., Polyaniline nanostructures and the role of aniline oligomers in their formation, Prog. Polym. Sci., 2010, vol. 35, p. 1420.
Hosseini, S.M., Jeddi, F., and Nemati, M., Fabrication of mixed matrix heterogeneous ion exchange membrane by multiwalled carbon nanotubes: electrochemical characterization and transport properties of mono and bivalent cations, Desalination, 2014, vol. 341, p. 107.
Ćirić-Marjanović, G., Recent advances in polyaniline research: polymerization mechanisms, structural aspects, properties and applications, Synth. Met., 2013, vol. 177, p. 1.
Zhao, S., Wang, Z., and Wei, X., Performance improvement of polysulfone ultrafiltration membrane using PANiEB as both pore forming agent and hydrophilic modifier, J. Membr. Sci., 2011, vol. 385, p. 251.
Qaiser, A.A., Hyland, M.M., and Patterson, D.A., Surface and charge transport characterization of polyaniline-cellulose acetate composite membranes, J. Phys. Chem. B, 2011, vol. 115, p. 1652.
Farrokhzad, H., Moghbeli, M.R., Van Gerven, T., and Van der Bruggen, B., Surface modification of composite ion exchange membranes by polyaniline, React. Funct. Polym., 2015, vol. 86, p. 161.
Hosseini, S.M., Jashni, E., Habibi, M., and Van der Bruggen, B., Fabrication of novel electrodialysis heterogeneous ion exchange membranes by incorporating PANI/GO functionalized composite nanoplates, Ionics, 2018, vol. 24, p. 1789.
Blinova, N.V., Stejskal, J., Frechet, J.M.J., and Svec, F., Effect of reaction conditions on film morphology of polyaniline composite membranes for gas separation, J. Polym. Sci. A: Polym. Chem., 2012, vol. 50, p. 3077.
Chinn, D. and Janata, J., Spin-cast thin films of polyaniline, Thin Solid Films, 1994, vol. 252, p. 145.
Sapurina, I., Riede, A., and Stejskal, J., In-situ polymerized polyaniline films: 3. Film formation, Synth. Met., 2001, vol. 123, p. 503.
Wang, P.C., Huang, Z., and MacDiarmid, A.G., Critical dependency of the conductivity of polypyrrole and polyaniline films on the hydrophobicity/hydrophilicity of the substrate surface, Synth. Met., 1999, vol. 101, p. 852.
Xu, T. and Huang, C., Electrodialysis-based separation technologies: a critical review, AIChE J., 2008, vol. 54, p. 3147.
Eliseeva, T.V., Shaposhnik, V.A., Krisilova, E.V., and Bukhovets, A.E., Transport of basic amino acids through the ion-exchange membranes and their recovery by electrodialysis, Desalination, 2009, vol. 241, p. 86.
Doležel, M. and Machuča, L., Production of lactic acid by ion substitution in 3-compartment electrodialysis and comparing of stack arrangements, Period. Polytech.-Chem., 2015, vol. 59, p. 247.
Lech, M. and Trusek, A., Batch electrodialysis of lactic acid obtained from LAB fermentation, Pol. J. Chem. Tech., 2018, vol. 20, p. 81.
Hábová, V., Melzoch, K., Rychtera, M., and Sekavová, B., Electrodialysis as a useful technique for lactic acid separation from a model solution and a fermentation broth, Desalination, 2004, vol. 162, p. 361.
Hassanvand, A., Wei, K., Talebi, S., Chen, G.Q., and Kentish, S.E., The role of ion exchange membranes in membrane capacitive deionisation, Membranes, 2017, vol. 7, p. 54.
Ezzeldin, H.A., Apblett, A., and Foutch, G.L., Synthesis and properties of anion exchangers derived from chloromethyl styrene codivinylbenzene and their use in water treatment, Int. J. Polym. Sci., 2010, vol. 2010, p. 1.
Sharma, S., Dinda, M., Sharma, C.R., and Ghosh, P.K., A safer route for preparation of anion exchange membrane from inter-polymer film and performance evaluation in electrodialytic application, J. Membr. Sci., 2014, vol. 459, p. 122.
Guo, R.-Q., Wang, B.-B., Jia, Y.-X., and Wang, M., Development of acid block anion exchange membrane by structure design and its possible application in waste acid recovery, Sep. Purif. Technol., 2017, vol. 186, p. 188.
Malik, M.S., Qaiser, A.A., and Arif, M.A., Structural and electrochemical studies of heterogeneous ion exchange membranes based on polyaniline-coated cation exchange resin particles, RSC Adv., 2016, vol. 6, p. 115046.
Galíková, A., Šubrt, J., Bastl, Z., and Kupčík, J., Thermal degradation of poly(vinyl chloride-co-vinyl acetate) and its laser-derived analogue, Thermochim. Acta, 2006, vol. 447, p. 75.
Smits, F.M., Measurement of sheet resistivities with the four-point probe, Bell Syst. Tech. J., 1958, vol. 37, p. 711.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Khurram, Qaiser, A.A., Ghaffar, A. et al. Development of Polyaniline Based Anion Exchange Membrane for the Separation of Lactic Acid via Electrodialysis. Russ J Electrochem 56, 587–595 (2020). https://doi.org/10.1134/S1023193520060099
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193520060099