Abstract
Streptomyces rimosus ATCC 10970 contains 14 genes annotated as aminoglycoside phosphotransferases in its genome: aphSR1–aphSR14. We have previously shown that the aphVIII (aphSR5) and aph(3'')-Id (aphSR3) genes, when cloning in E. coli, cause resistance to kanamycin, neomycin, paromomycin, and streptomycin. It was found for Aph(3')-VIII that antibiotic resistance increased after phosphorylation at the Ser146 motif in the active site of the enzyme by serine-threonine protein kinases (STPKs). The aphSR2 gene, when cloning in E. coli, causes resistance to neomycin and hygromycin. In this work, in order to assess the possibility of influence of STPK genes on increasing resistance to aminoglycoside antibiotics, we performed a combined cloning into E. coli at pET32a of the aphSR2 gene and the STPK genes (pkSR1 and pkSR2) localized in one cluster of the S. rimosus ATCC 10970 genome. We detected that, in the construction E. coli/aphSR2/pkSR1, there is a 2-fold increase in resistance to neomycin. The presented data are the second example of the STPK effect on the modulation of the level of resistance to aminoglycoside antibiotics in bacteria of the genus Streptomyces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The ability of bacteria to acquire antibiotic resistance has been known since the discovery of antimicrobial agents [1]. The concept of “resistance,” which stands for a pool of resistance genes specific to a particular bacterial community, has been established in recent decades. The Antibiotic Resistance Genes Database (ARDB) currently numbers 23 137 resistance genes. The downregulation of resistance genes makes it possible to use the existing antibiotics in fighting against antibiotic-resistant bacteria [2].
Bacterial resistance to aminoglycoside antibiotics was first discovered in 1952. A hypothesis for explaining the origin of resistance genes was put forward in the early 1970s, in particular, of aminoglycoside transferases from soil-dwelling antibiotic-producing bacteria [3]. In 1983, aminoglycoside-3'-phosphotransferase genes were identified and sequenced in plasmids and mobile elements in clinical strains of gram-negative and gram-positive bacteria [4].
It is known that antibiotic resistance genes could have originated from bacterial antibiotic producers that belong to the Streptomyces genus. The genome annotation revealed four to 14 aminoglycoside phosphotransferase genes in Actinobacteria. However, the functions of genes annotated as aph in the sequenced genomes (https://www.ncbi.nlm.nih.gov/genome/) are still to be elucidated [5].
When sequencing the genome of the strain Streptomyces rimosus subsp. rimosus АТСС 10970 (oxytetracycline producer) [6], 14 aph genes were annotated, which we designated as aphSR1–aphSR14. We conducted a comparative and phylogenetic analysis of amino acid sequences for the products of 14 aph genes with previously known aph genes from clinical isolates and aminoglycoside antibiotic-producing strains from seven subfamilies. It was revealed that AphSR5 (AphVIII) belongs to the Aph(3') subfamily, AphSR3 belongs to the Aph(3'') subfamily, and AphSR2 is located on the same branch with Aph(7'')-Ia.
We previously identified and characterized two aminoglycoside phosphotransferases in S. rimosus АТСС 10970 with a high-level aminoglycoside resistance. AphSR5 (Aph(3')-VIII) leads to resistance to kanamycin, neomycin, and paromomycin; an important feature of AphVIII from S. rimosus is the dependence of its enzymatic activity on the degree of phosphorylation by serine-threonine proteinkinases; the 3D structure of AphVIII (code PDB 4H05) was obtained [7–10]. Cloning of the aphSR3 gene (aph(3'')-Id) in E. coli revealed that this gene causes resistance to streptomycin [11].
The object of this study is AphSR2 aminoglycoside phosphotransferase. According to the phylogenetic analysis, AphSR2 is located on the same branch with Aph(7'')-Ia, but the tree node was not well supported (the bootstrap value was <60%); it was however possible to classify them with the Aph(7'') subfamily. At the same time, the comparative analysis of AphSR2 using the SAS software program (https://www.ebi.ac.uk/ thornton-srv/databases/sas/) with the known 3D structures showed similarity between 3D structures of AphSR2 and Rv3168 transferase (PDB ID—3ATS) of the strain Mycobacterium tuberculosis H37Rv (31.2% identity).
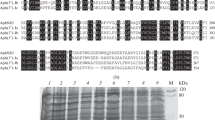
An analysis of amino acid sequence alignment conducted in the Clustal Omega software program (https://www.ebi.ac.uk/Tools/msa/clustalo/) showed that the sequence of AphSR2 shares a significantly greater number of common conserved amino acid residues with the sequence of Rv3168 than with aminoglycoside phosphotransferase Aph(7'')-Ia (Fig. 1a).
Characterization of the aphSR2 and pkSR1, pkSR2 gene cluster from the strain S. rimosus АТСС 10970 and the encoded proteins. (a) Comparison of the amino acid sequence of AphSR2 with sequences of APH(7'')-Ia and Rv3168 (residues conserved between all three sequences are highlighted in black; between two, in gray); (b) transcription organization of the aphSR2 and pkSR1, pkSR2 gene cluster of the strain S. rimosus АТСС 10970 (hp—hypothetical protein, citB—putative two-component system response transcriptional regulator, pdha—pyruvate dehydrogenase E1); (c) electrophoresis of soluble fraction of proteins from the strain E. coli BL21(DE3), containing plasmids: (1) pET32a, (2) pET32a:aphSR2; (3) pET32a:pkSR1, (4) pET32a:aphSR2 + pkSR1, (5) pET32a:pkSR2, (6) pET32a:aphSR2 + pkSR2. М—molecular weight protein marker SM0441 (Fermentas, Lithuania).
Using the NCBI and UniProt databases (http://www.uniprot.org/), it was found that the aphSR2 gene is located in the same cluster with the genes of two serine-threonine protein kinases (STPKs), which we designated as follows (according to the numbers of the gene loci): SRIM_07563—pkSR1, SRIM_07568—pkSR2. The transcription of the pkSR1 gene occurs in the same direction as that of the aphSR2 gene (Fig. 1b).
We previously classified STPKs of gram-positive bacteria on the basis of an analysis of the signature of nine variable amino acid residues, the side chains of which are exposed to the adenine binding region. According to the results of this classification, all STPKs were divided into 20 groups. According to the proposed classification of STPKs, PkSR1 belongs to the group IIa, and PkSR2 belongs to group IIb [12, 13]. The orthologs for these STPKs are kinases from Streptomyces coelicolor Pk13 (BLAST identity 89%) and Pk12 (BLAST identity 93%), respectively [14]. The discussed kinases from S. coelicolor are located on the chromosome in the same way as kinases from S. rimosus—adjacent to each other, but facing in the opposite directions. None of the 11 described and well-studied STPKs from M. tuberculosis are orthologs of the considered STPKs of S. rimosus.
In this work, we have studied experimentally the effect of the pkSR1 and pkSR2 STPK genes on the resistance of E. coli BL21(DE3), which contains the aphSR2 gene, to aminoglycoside antibiotics by combined cloning of these genes in the pET32a expression vector.
The PkSR1 protein consists of 573 amino acid residues; the domain structure of the protein consists of the catalytic domain (19–288 a.a.) and the PASTA domain (503–569 a.a.). The PkSR2 protein consists of 516 amino acid residues; the domain structure of the PkSR2 protein comprises the catalytic domain (5–265 a.a.).
At the first stage, the catalytic domains of protein kinases were cloned in E. coli. The pkSR1 and pkSR2 genes were amplified from the genomic DNA of the S. rimosus strain using PCR (РСK-100 kit manufactured by Dialat Ltd. with the PTC-0150 instrument (MJ Research, Inc.)) using oligonucleotides PkSR1-N (5'-tcgcggatcccgctaccagctccgtgatct-3') and PkSR1-C (5'-tcgcggatcccgctaccggctcacgcgccg-3') for the pkSR1 gene and oligonucleotides PkSR2-N (5'-tcgcggatcccgctaccggctcacgcgccg-3') and PkSR2-C (5'-ccgcaagcttgcgcatctcctccgcggtctg-3') for the pkSR2 gene. The fragments were cloned at the restriction sites of BamHI and HindIII endonucleases into the pET28a expression vector (selection marker Km). Then, the resulting hybrid plasmid pET28a:pkSR1 and 28a:pkSR2 was treated with XbaI and XhoI restriction endonucleases and inserted at the specified restriction sites into the pET32a expression vector (selection marker ampicillin). The pET32aM:pkSR1 and pET32aM:pkSR2By hybrid plasmids were obtained by cloning.
The aphSR2 gene was then cloned into pET32aM:pkSR1 and pET32aM:pkSR2 plasmids at the XbaI endonuclease restriction site. As a control, the aphSR2 gene was cloned into the pET32a plasmid at the XbaI endonuclease restriction site. The aphSR2 gene was amplified from the pET16b:aphSR2 plasmid DNA using the oligonucleotides T7prom (5'-ttaatacgactcactatagg-3') and AphSR2C-XbaI (5'-agcctcta-gatcactccgtgaaggccgcc-3'). Screening of clones was performed with PCR using the oligonucleotides T7prom and AphC-XbaI, which made it possible to select clones with the desired orientation. The plasmids were designated as pET32aM:aphSR2/pkSR1, pET32aM:aphSR2/pkSR2 and pET32a:aphSR2.
To study the expression of the aphSR2, pkSR1, and pkSR2 genes in E. coli, the hybrid plasmids were transformed to competent E. coli cells of the strain BL21(DE3) (F–, dcm, ompT, hsdS(\({\text{r}}_{{\text{B}}}^{-}{\text{m}}_{{\text{B}}}^{-}\)), gal λ (DE3)) (Novagen) and grown in liquid LB medium at 37°C to an optical density of 0.6 (~2 h), and the expression was then induced by adding isopropyl β‑thiogalactopyranoside (IPTG) to a final concentration of 1.3 mM. Next, the cells were cultured at 28°C for 18 h, precipitated by centrifugation (5000 rpm, 10 min, 4°C), and suspended in the Sample buffer and analyzed by 12.5% SDS-PAGE electrophoresis according to the Laemmli protocol. The protein fractions of E. coli strains containing the pET32a plasmid without the insertion were used as controls. The electropherogram analysis revealed the expression of proteins with molecular weight of 33, 32, and 41 kDa, which corresponds to the estimated molecular weights of the catalytic domains of the PkSR1 and PkSR2 protein kinases and the AphSR2 protein molecular weight (Fig. 1c).
At the next stage, the spectrum of resistance to aminoglycoside antibiotics was tested using standard disks of E. coli BL21(DE3) strains containing all recombinant plasmids. The spectrum of resistance was tested using paper disks with aminoglycoside antibiotics: kanamycin (30 μg/disk), neomycin (30 μg/disk), amikacin (30 μg/disk), streptomycin (10 μg/disk), gentamycin (10 μg/disk), tobramycin (10 μg/disk), sisomycin (10 μg/disk), netilmycin (10 μg/disk), isepamycin (30 μg/disk), and hygromycin (100 μg/disk). The results were evaluated after incubation for 16–18 h at 37°C.
The investigation of the growth inhibition zone surrounding the antibiotic disks showed that the aphSR2 gene causes resistance to neomycin and hygromycin in E. coli BL21(DE3) (Table 1). The combination of the aphSR2 and pkSR1 genes in one vector increased the level of E. coli resistance to neomycin, and the combination of the aphSR2 and pkSR2 genes did not change the resistance. The combination of the aphSR2 and STPK (pkSR1 or pkSR2) genes had no effect on hygromycin resistance. The effect of STPK genes on the resistance of E. coli BL21(DE3) was investigated as a control; these genes had no effect on resistance to neomycin, but increased susceptibility to hygromycin. The results suggest that the aphSR2 gene may be a candidate hygromycin resistance gene from S. rimosus. According to the published data, aph(7'')-Ia causes resistance to hygromycin B [15] and rv3168 causes resistance to kanamycin [16]. In contrast to rv3168 and aph(7'')-Ia, aphSR2 influenced the resistance of E. coli cells to neomycin and hygromycin.
Next, the level of resistance to neomycin and hygromycin was tested using the analysis of the minimum-inhibitory concentrations (MIC). For the analysis, we used clones of the E. coli BL21(DE3) transformants containing recombinant plasmids pET32a, pET32a:aphSR2, and pET32aM:aphSR2/pkSR1. Individual colonies were collected and transferred to tubes containing 2 mL of LB broth; an overnight culture was grown to an optical density of OD625 = 0.3 and then diluted with LB medium to obtain the final density of 105–106 CFU/mL. Cell culture (100 µL) (IPTG, 100 μM) was added to each of the series of tubes containing twofold dilutions of neomycin in 2 mL of LB medium in order to induce the expression of the aphSR2 and pkSR1 genes. After incubation of the cultures at room temperature (~25°C) and 250 rpm for 18 h, the MIC values were estimated as the lowest concentration of neomycin or hygromycin which resulted in the complete growth inhibition (assessed by spectrophotometry at OD625).
In experiments on MIC estimation using a method of serial dilution, the concentration of neomycin was 8 µg/mL for the control strain E. coli BL21(DE3); upregulation of the recombinant protein expression increased MIC to 16 µg/mL; combined pkSR1 and aphSR2 gene expression caused an increase in MIC to 32 μg/mL. Thus, it was found that, upon combined cloning in E. coli, aphSR2 causes resistance to neomycin, showing the modulation of the antibiotic resistance level by pkSR1. The MIC of hygromycin for the control strain of E. coli BL21(DE3) was 100 µg/mL, indicating that the strain of E. coli is resistant to hygromycin, which complicates the MIC measurement [17].
Thus, AphSR2 is the second aminoglycoside phosphotransferase of Streptomyces, in particular, S. rimosus, for which the data show that the level of resistance increases under the influence of STPKs, being a cumulative result of their combined expression in E. coli. The results confirm the hypothesis on the role of signal-transmitting systems of soil-dwelling bacteria in the modulation of expression of antibiotic resistance genes in Actinobacteria [18].
The tasks for subsequent work include the isolation of recombinant proteins AphSR2, PkSR1, and PkSR2 and an investigation in vitro of the level and sites of phosphorylation in the studied aminoglycoside phosphotransferase and the effect of phosphorylation on enzymatic activity.
REFERENCES
Davies, J., Antibiotic resistance and the golden age of microbiology, Ups. J. Med. Sci., 2014, vol. 119, no. 2, pp. 65–67. https://doi.org/10.3109/03009734.2014.898718
Gerard, D. and Wright, G.D., The antibiotic resistome: the nexus of chemical and genetic diversity, Nat. Rev. Microbiol., 2007, vol. 5, pp. 175–186. https://doi.org/10.1038/nrmicro1614
Davies, J., Bacterial resistance to aminoglycoside antibiotics, J. Infect. Dis., 1971, vol. 124, suppl., pp. S7–S10.
Wright, G.D., Molecular mechanisms of antibiotic resistance, Chem. Commun. (Cambridge), 2011, vol. 47, no. 14, pp. 4055–4061. https://doi.org/10.1039/c0cc05111j
Anderson, A.S., Clark, D.J., Gibbons, P.H., et al., The detection of diverse aminoglycoside phosphotransferases within natural populations of actinomycetes, J. Ind. Microbiol. Biotechnol., 2002, vol. 29, no. 2, pp. 60–69.
Pethick, F.E., MacFadyen, A.C., Tang, Z., et al., Draft genome sequence of the oxytetracycline-producing bacterium Streptomyces rimosus ATCC 10970, Genome Announc., 2013, vol. 1, no. 2. e00063-13. https://doi.org/10.1128/genomeA.00063-13
Sizova, I.A., Khegemann, P., Furmann, M., et al., Streptomyces rimosus aminoglycoside 3'-phosphotransferase VIII: comparisons with aminoglycoside 3'-phosphotransferases of aminoglycoside-producing strains and with eukaryotic protein kinases, Mol. Biol. (Moscow), 2002, vol. 36, no. 1, pp. 18–25. https://doi.org/10.1023/A:1014282003679
Elizarov, S.M., Sergienko, O.V., Sizova, I.A., et al., Dependence of aminoglycoside 3′-phosphotransferase VIII activity on serine/threonine protein kinases in Streptomyces rimosus,Mol. Biol. (Moscow), 2005, vol. 39, no. 2, pp. 226–233. https://doi.org/10.1007/s11008-005-0033-9
Elizarov, S.M., Alekseeva, M.G., Novikov, F.N., et al., Identification of phosphorylation sites in aminoglycoside phosphotransferase VIII from Streptomyces rimosus,Biochemistry (Moscow), 2012, vol. 77, no. 11, pp. 1258–1265. https://doi.org/10.1134/S0006297912110041
Boyko, K.M., Gorbacheva, M.A., Korzhenevskiy, D.A., et al., Structural characterization of the novel aminoglycoside phosphotransferase AphVIII from Streptomyces rimosus with enzymatic activity modulated by phosphorylation, Biochem. Biophys. Res. Commun., 2016, vol. 477, no. 4, pp. 595–601. https://doi.org/10.1016/j.bbrc.2016.06.097
Alekseeva, M.G., Rudakova, N.N., Zakharevich, N.V., et al., New gene of aminoglycoside phosphotransferase aph(3'')-Id from Streptomyces rimosus ATCC10970, encoding streptomycin resistance, Russ. J. Genet., 2018, vol. 54, no. 10, pp. 1254—1258. https://doi.org/10.1134/S1022795418100034
Zakharevich, N.V., Osolodkin, D.I., Artamonova, I.I., et al., Signatures of the ATP-binding pocket as a basis for structural classification of the serine/threonine protein kinases of gram-positive bacteria, Proteins, 2012, vol. 80, no. 5, pp. 1363–1376. https://doi.org/10.1002/prot.24032
Zakharevich, N.V. and Danilenko, V.N., Serine—threonine bacterial protein kinases are a potential target for regulating the composition of human microbiota, Vestn. Ross. Gos. Med. Univ., 2017, no. 2, pp. 20–29.
Petrícková, K. and Petrícek, M., Eukaryotic-type protein kinases in Streptomyces coelicolor: variations on a common theme, Microbiology, 2003, vol. 149, no. 7, pp. 1609–1621. https://doi.org/10.1099/mic.0.26275-0
Berthold, P., Schmitt, R., and Mages, W., An engineered Streptomyces hygroscopicus aph 7'' gene mediates dominant resistance against hygromycin B in Chlamydomonas reinhardtii,Protist, 2002, vol. 153, no. 4, pp. 401–412. https://doi.org/10.1078/14344610260450136
Kim, S., Nguyen, C.M., Yeo, S.J., et al., Cloning, expression, purification, crystallization and X-ray crystallographic analysis of Rv3168 from Mycobacterium tuberculosis H37Rv, Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun., 2011, vol. 67, no. 5, pp. 627–629. https://doi.org/10.1107/S1744309111010487
Rao, R.N., Allen, N.E., and Hobbs, J.N., Genetic and enzymatic basis of hygromycin B resistance in Escherichia coli,Antimicrob. Agents Chemother., 1983, vol. 24, no. 5, pp. 689–695.
Danilenko, V.N., Mironov, V.A., and Elizarov, S.M., Calcium as a regulator of intracellular processes in actinomycetes: a review, Appl. Biochem. Microbiol., 2005, vol. 41, no. 4, pp. 319–329.
Funding
This study was partially supported by the Russian Foundation for Basic Research (project no. 17-04-01106 of April 6, 2017) and by the State Task “Genetic Technology in Biology, Medicine, Agriculture, and Environmental Management” (no. 0112-2019-0002 of 2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Novikova
Rights and permissions
About this article
Cite this article
Rudakova, N.N., Alekseeva, M.G., Zakharevich, N.V. et al. Aminoglycoside Phosphotransferase AphSR2 from Streptomyces rimosus ATCC 10970: Dependence of Antibiotic Resistance on Serine-Threonine Protein Kinases PkSR1 and PkSR2. Russ J Genet 56, 112–117 (2020). https://doi.org/10.1134/S1022795420010093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795420010093