Abstract
Here, two field-grown grape species including Vitis amurensis Rupr. ‘Shuangfeng’ and V. riparia × V. labrusca ‘Beta’ showed different survival capabilities after overwintering. Their shoots’ cold hardiness and associated physiological changes were analyzed during seasonal cold acclimation and deaaclimation to illuminate response mechanisms on low temperature. The fluctuations of cold hardiness were synchronized in the two species, but ‘Shuangfeng’ had a higher tolerance than ‘Beta’ to cold stress, which was consistent with the field performance. Leaves and shoots of ‘Shuangfeng’ reddened, responds quickly to the natural low temperature, while those of ‘Beta’ remained green during the same period. Perhaps owing to variations in the carbohydrate accumulations in different colored leaves, so the freezing tolerance of ‘Shuangfeng’ correlated with total soluble sugars in the phloem, while the starch in the xylem and phloem determined the ‘Beta’s tolerance. The timing and types of the accumulated carbohydrates may be responsible for the difference in the water status, energy accumulation and cold hardiness between the two grape species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Geographical distributions of horticultural crops and their cultivation in temperate and boreal zones are highly dependent on their freezing tolerance [1, 2]. Most grape cultivars need to be buried in the soil when the temperature is below –10°C in boreal zones to avoid freezing injury in the winter [3]. Thus, research on the cold resistance of grape cultivars during overwintering is slow. However, Vitis amurensis is a cold-resistant grape species, which can endure extreme low temperatures during overwintering, without being buried in the soil to prevent cold injury [4]. Consequently, it is a good material to study the mechanisms of cold hardiness formation during overwintering. The levels of freezing tolerance and associated adaptations vary among grape species, cultivars and ecotypes, demonstrating the genetic variability in cold hardiness and the genetic adaptation to the local climate [5].
Freezing tolerance can be induced by the seasonal cold acclimation, which involves physiological and biochemical changes that are driven by temperature and photoperiod, and these changes enable woody perennials to survive under severe winter conditions [6–8]. Deacclimation, the reverse process of cold acclimation, occurs in the spring and is mainly driven by warm temperatures in the field [9, 10]. In the fall, starch produced during the summer is hydrolyzed into soluble carbohydrates that accumulate in tissues. In the spring, starch is resynthesized and then mobilized during bud break [11]. The appropriate timing and rates of cold acclimation and deacclimation are important for the growth and survival of woody plants and they may determine the cold hardening of species [12].
Changes occur in soluble carbohydrates during cold acclimation and deacclimation in woody plants. Sugar metabolism and carbon partitioning are two effective ways to sustain the balance required for growth and energy supply in cold acclimation [13]. In many woody plants, carbohydrates produced mainly by starch degradation, and cold hardiness is closely correlated with the soluble carbohydrate concentration [4, 14, 15], because soluble carbohydrates play roles in ROS scavenging, protecting cell structures from freeze-induced dehydration and in depressing the freezing point under deep supercooling [16, 17].
To date, most studies in grapes have focused on associations between cold hardiness and key physiological parameters, such as tissue water content, related compounds and antioxidant enzymes [4, 18–21], but very little information is available on changes in the varieties of carbohydrate during cold acclimation and deacclimation. Particularly, the different low-temperature response mechanisms that act in the different tissues of grape shoots under cold stress remain elusive. However, understanding these mechanisms is vital, given the theoretical and commercial importance of the improvement and breeding of cold -resistant grapes cultivars in the field.
The objectives of the present study were to (1) estimate seasonal changes in cold hardiness in the shoots of two grape species without burying them in the soil for the winter and to (2) determine differences in carbohydrate levels during autumn acclimation and spring deacclimation in the phloem and xylem of two grape species (V. amurensis ‘Shuangfeng’ and V. riparia × V. labrusca ‘Beta’). Particularly, an attempt was made to correlate alterations in primary physiological factors in the phloem and xylem with seasonal changes and differences in shoot freezing tolerance in the two species. Additionally, possible reasons for the different temperature responses of the two species are discussed.
MATERIALS AND METHODS
Plant materials and samples. Shoots were sampled from the National Field Gene Bank for Amur Grapevine located in Zuojia Town, Jilin Province, China (44°04′ N and 126°05′ E, 190 m above sea level). The soil at this site is dark brown forest soil, and its meteorological data are recorded by a meteorological monitoring system (model: HOBO, Onset Equipment, United States). Each germplasm in the nursery is a self-root seedling on an espalier. The row spacing at the nursery is 1.0 × 2.5 m, and the column spacing is 6 m. A normal management model was adopted for the nutrient and water supplies.
In generally, the shoots of grape ‘Shuangfeng’ can naturally overwinter, but grape ‘Beta’ needs to be buried in soil before November in Zuojia area. In order to explore the mechanism on the difference in cold tolerance, the 1-year-old shoots of V. amurensis ‘Shuangfeng’ and V. riparia × V. labrusca ‘Beta’ (without burying in winter) as test materials, which were taken on August 18, September 1, September 15, September 29, October 18, November 18 and December 18 of 2018, January 18 and April 18 of 2019. All the sampled shoots were survival, healthy, mature and 0.6–0.8 cm thick, and they were collected at a height of 1.0–1.8 m on the south side of the espalier.
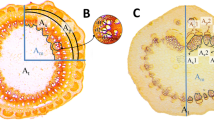
The cold hardiness and water contents of test shoots were determined immediately as sampled from the field. The rest of test shoot epidermises were discard, and the phloem and xylem of shoots in two grape species were independently separated using a sharp surgical blade. Accurate sampling of phloem and xylem based on the cross-sectional anatomical structure of shoots (Fig. 1), then were ground in liquid nitrogen and stored as powders at –80°C for the carbohydrate analysis. Each experiment was repeated three times with three independent biological replicates. Three shoots from one plant of each grape species represented one independent biological replicate.
Freeze–thaw treatment. Shoots were freeze–thaw treated in accordance with the method of Zhao et al. [4]. The freeze–thaw treatment of shoots was carried out using a high–low temperature alternating test chamber (model: GDJW-225, Beijing Yashilin Test Equipment). Ten freezing temperatures were used: 4°C as control, –5, –10, –15, –20, –25, –30, ‒35, ‒40, –45 and –50°C. The freezing treatment lasted 12 h, and the recovery period was 4 h at room temperature for thawing. Both the cooling and heating rates were set at 4°C/h.
Evaluation of cold hardiness. After the freeze–thaw treatment, the cold hardiness of shoots from two grape species were evaluated using the 2,3,5-triphenyltetrazolium chloride (TTC) staining method as described by Zhao et al. [22]. In total, 10 5-mm thick sections were cut from shoot internodes and placed in 10 mL 0.5% TTC in the dark in a 30°C incubator for 4 days. Then, the samples were dissected to obtain longitudinal sections, which were projected onto a LA2400 scanner having image analysis software (WinRHIZOTM; Regent Instruments, Canada), and the areas stained by TTC on the longitudinal sections were measured. The level of cold injury for each shoot was recorded as the area of staining in the whole longitudinal section. The LT50 value was calculated using a logistic equation of staining indices, which is an inverted sigmoid curve appropriate for analyzing data on plant responses to temperature stress.
Measurement of water contents. Total water (%) in shoots was determined as (FW–DW) × 100%, where FW represents the fresh weight, DW represents the dry weight after oven-drying at 90°C for 16 h until a constant weight. Free water (%) was determined using a digital display saccharimeter in accordance with the method of Chen et al. [18]. Bound water (%) = Total water (%) – Free water (%).
Determination of carbohydrate contents. Carbohydrate extractions and analyses were performed in manners similar to those outlined by Xiong et al. [23], with some modifications. The lyophilized sample powder (200 mg) was placed into a 2 mL tube containing 1 mL ice-cooled 80% (v/v) ethanol and homogenized by ultrasound in an ice bath for 15 min. After centrifugation at 8.000 g for 10 min at 4°C, the supernatants were dried to remove the ethanol, dissolved in water and cleaned by passing through a 0.45-μm nylon syringe filter and a Sep-Pak C18 cartridge.
The supernatants were immediately used to analyze the soluble sugars, including sucrose, fructose, glucose and trehalose, by injecting the samples into a high-performance liquid chromatography system (Ultimate 3000, Thermo Scientific, United States) equipped with a Sugar-Pak I column (6.5 × 300 mm, 10 μm) maintained at 80°C and a Shoedex refractive index detector. The starch content was determined using a kit (no. 207748).
Statistics. All the statistical analyses were processed using a SAS 9.0 system (SAS Institute, United States) for Windows. Means separations were performed using Tukey’s honestly significant difference at the 0.05 level. The Pearson’s correlation coefficients and gray relational degrees were used to assess the associations among physiological parameters. The graphs were constructed using SigmaPlot 10.0 (Systat Software, United States).
RESULTS
Seasonal Changes in Air Temperature and Cold Hardiness
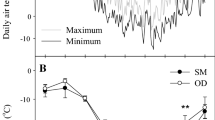
At the end of September, ‘Shuangfeng’ leaves reddened, while most leaves of ‘Beta’ remained green (Fig. 2). The cold hardiness levels of both species were analyzed along with the changes in the air temperature. Daily air temperatures at the experimental site decreased consistently from August and reached a minimum temperature (–33.0°C) at the end of January (Fig. 3a). Daily minimum temperatures were below 0°C for 172 days. The change in winter air temperature was not significantly different from the usual pattern. Then, the daily temperatures continued to increase from the beginning of February to April 2019.
Changes in environmental conditions at the experimental site of Zuojia Town, Jilin Province from August 2018 to April 2019. (a) Changes in the maximum (1) and minimum (2) daily air temperatures; (b) Seasonal changes in cold hardiness estimated using temperatures at which 50% of shoots (LT50) of Vitis amurensis ‘Shuangfeng’ (1) and Vitis riparia × Vitis labrusca ‘Beta’ (2) were injured as assessed by the TTC staining area. Data are means ± standard errors of three replicates.Different lowercase letters indicate significant differences in cold tolerance between the two species at the same sampling time (P ≤ 0.05). The same below.
Changes in cold hardiness from August 2018 to April 2019 were assessed using changes in temperature causing 50% injury (LT50) to shoots of ‘Shuangfeng’ and ‘Beta’ (Fig. 3b). During the cold acclimation, accompanying the seasonal drop in air temperature (August 2018 to January 2019), LT50 values for the two grape species decreased rapidly. That of ‘Shuangfeng’ dropped from –13.47 to –39.27°C, while the value of ‘Beta’ from –6.23 to –38.25°C. The cold hardiness of ‘Shuangfeng’ was significantly stronger than that of ‘Beta’ during the cold acclimation, except in December and January when the cold hardiness levels of both species were similar. Thereafter, the cold hardiness of both species decreased dramatically during deacclimation (from February to April 2018), and the LT50 values of ‘Shuangfeng’ and ‘Beta’ in April increased compared with in January by 15.74 and 13.53°C, respectively. The cold hardiness levels of the two species were not significantly different during this period (Fig. 3b). However, the bud burst rate of ‘Shuangfeng’ (95.00%) shoots was significant higher than that of ‘Beta’ shoots during natural overwintering in the field (30.12%).
Seasonal Changes in Water Content
The total water content of ‘Beta’ was higher than ‘Shuangfeng’ from August to November, but this was reversed from December to April, when the total water content of ‘Shuangfeng’ was higher than that of ‘Beta’ (Fig. 4a). The free water content was significantly different in the two species until January, when the free water content of ‘Shuangfeng’ was higher than that of ‘Beta’ (Fig. 4b). However, the free water contents of the two species decreased greatly during acclimation and then increased during deacclimation. The free water content of ‘Shuangfeng’ decreased from 55.11% at the beginning of this experiment to 13.85% in December, and then increased to 29.18% in April along with the rise in air temperature. The free water content of ‘Beta’ decreased from 70.76 to 19.15% and then increased to 24.37% (Fig. 4b). The change trend of the bound water contents in the two species during cold acclimation and deacclimation were similar to the ratios of bound water to free water, which gradually increased until December and then decreased slightly (Figs. 4c and 4d). During the whole experiment, the bound water content and the ratio of bound water to free water in ‘Shuangfeng’ were greater than in ‘Beta’ (Figs. 4c and 4d).
Changes in physiological parameters in the shoots of Vitis amurensis ‘Shuangfeng’ (1) and Vitis riparia × Vitis labrusca ‘Beta’ (2) from the end of August 2018 to the end of April the following year. (a) total water contents; (b) free water contents; (c) bound water contents; (d) the ratio of bound water to free water.
Seasonal Changes in Carbohydrate Contents
At the beginning of experiment, the total soluble sugar content of ‘Shuangfeng’ and ‘Beta’ in the phloem were 5.29 g and 6.74 g/g FW, respectively, and the contents in the xylem were 4.52 and 3.13 g/g FW, respectively. Accompanying cold acclimation, the total soluble sugar contents in the phloem and xylem of the two grape species increased gradually (Fig. 5a). The maximum total soluble sugar contents in the phloem and xylem were reached in January (‘Shuangfeng’ was 17.52 g/g FW and ‘Beta’ was 11.18 g/g FW) and December (‘Shuangfeng’ was 14.57 g/g FW and ‘Beta’ was 12.03 g/g FW), respectively. Thereafter, the total soluble sugar contents decreased significantly during deacclimation (Fig. 5a). In April 2019, the total soluble sugar contents in the phloem of ‘Shuangfeng’ and ‘Beta’ were 6.92 and 6.08 g/g FW, respectively, and the contents in the xylem were 4.95 and 2.96 g/g FW, respectively, similar to August 2018. The trends in starch accumulation in the phloem and xylem of the two grape species during cold acclimation and deacclimation were coincided with those of the total soluble sugar (Fig. 5b). However, in general, the total soluble sugar and starch contents in the phloem of the two species were higher than in the xylem from October 2018 to April 2019 (Figs. 5a and 5b).
In this study, the soluble sugars, including sucrose, fructose, glucose and trehalose, of the two species were measured from August 2018 to April 2019. The soluble sugar contents varied to different extents depending on the sugar and cultivar (Figs. 6a–6d). Trehalose was present at a low level, while fructose was the most abundant sugar in the shoots (Figs. 5b and 5d). A significant seasonal pattern was observed for fructose in the phloem and xylem of both species, and its contents in the phloem and xylem of ‘Shuangfeng’ were higher than in ‘Beta’ from October 2018 to January 2019 (Fig. 6b). The sucrose contents of the two species in the phloem were higher than in the xylem during cold acclimation and deacclimation, but no significant seasonal pattern was observed for sucrose (Fig. 6a). At the beginning of the experiment, the glucose level temporarily increased in both species, and the glucose contents in the phloem and xylem of both species showed seasonal changes after October (Fig. 6c).
Correlation Grey Relation Analyses between Cold Hardiness and Physiological Factors
The grey relational analysis method was used to explore the correlations between cold hardiness (represented by LT50 values) and physiological factors to determine the primary factors affecting the cold hardiness of two grape species in response to seasonal low temperature. The ranks according to the gray relational degree represented the primary and secondary correlations of physiological factors with LT50 values between the two species. Total soluble sugar in the phloem, as the most crucial factor affecting the cold hardiness of ‘Shuangfeng’, ranked first, followed by bound water and starch in the xylem. Their gray relational degrees were 0.934, 0.923 and 0.904, respectively (Table 1). In addition, seasonal changes in LT50 values for ‘Shuangfeng’ were significantly negatively correlated with changes in total soluble sugar in the phloem and bound water (r = –0.869 and –0.816, respectively, at p ≤ 0.01; Table 1). While the content of total soluble sugar in the phloem of ‘Shuangfeng’ was positively associated with bound water, ratio of bound water to free water, total soluble sugars in xylem, sucrose in phloem, and the content of glucose, fructose, starch in phloem and xylem (Table S1).
The cold hardiness of ‘Beta’ was correlated with bound water, starch in the xylem and starch in the phloem, with grey relational degrees of 0.921, 0.917 and 0.911, respectively, and they were also significantly negatively correlated with LT50 values of ‘Beta’ (r = –0.868, –0.834 and –0.816, respectively, at P ≤ 0.01; Table 1), which was consistent with the results of the grey relational analysis. And the content of starch in the xylem of ‘Beta’ was positively associated with bound water, ratio of bound water to free water, total soluble sugars in xylem, and the content of sucrose, glucose, fructose in phloem and xylem (Table S2).
DISCUSSION
Normally, the shoots of ‘Beta’ need to be buried in late October, but those of ‘Shuangfeng’ can overwinter naturally in the nursery. A significant survival difference was observed between field-grown ‘Shuangfeng’ and ‘Beta’ without burying, corresponding to their bud burst rates were 95.00 and 30.12% after overwintering in April of following year. The low semi-lethal temperature (LT50) values represent the cold-tolerant levels of the plants [24]. There were significant increases in the cold tolerance levels of both species during the cold acclimation basing on their LT50 values.
Cold acclimation in certain trees species is mainly regulated by low temperatures over a period of time. However, different stages of acclimation to low temperature in our experimental periods might proceed depending on the species [25, 26]. The cold acclimation of ‘Shuangfeng’ proceeds in three stages, while ‘Beta’ has four stages. During the first stage, plants achieve tolerance to short frosts of 0 to –3°C in late September. In the second stage of cold acclimation, which starts in October, has minimum temperatures of –10 to 0°C. A third phase occurs in November in response to temperatures from –20 to –10°C. During this phase, ‘Shuangfeng’ reached its strongest cold hardiness. The fourth phase involves December and January, with the acclimation to cold temperatures from –30 to –20°C. The surviving ‘Beta’ proceeded to this phase of acclimation and its LT50 value continued to decrease to its lowest value.
Interestingly, the leave and shoots of ‘Shuangfeng’ reddened gradually and underwent accelerated aging during the first stage, while most leave of ‘Beta’ plants were still green during this period. This may result from the variation in soluble carbohydrate accumulation with different colored leaves [27]. In this study, compare the change of soluble sugar and starch in phloem between two grape species, the soluble sugar content of ‘Shuangfeng’ was significantly lower than ‘Beta’ in August, then subsequently became higher than ‘Beta’ in September and October. But the differences in starch content of two grapes were opposite to the soluble sugar in phloem before October. It suggested that the starch degradation increased the accumulation of soluble sugar accompanying the decrease of air temperature, leading to the leave and shoots of ‘Shuangfeng’ reddened earlier and enhanced its tolerance to low temperature. Meanwhile, the sugar soluble contents of ‘Shuangfeng’ were always higher than ‘Beta’ from October to January. For ‘Beta’ grape, the starch was served as an energy storage to help plants resist low winter temperatures. In woody plants, the majority of studies revealed the crucial roles of soluble sugar as compatible solutes in cold hardiness [19, 22]. So this might be the reason that the cold tolerance and survival rate overwinter of ‘Shuangfeng’ shoots were stronger than ‘Beta’.
In generally, hexoses such as glucose and fructose, are frequently correlated with freezing tolerance in herbaceous plants [28, 29], whereas they are not correlated with the cold acclimation of woody plants. However, our results suggested that glucose and fructose participate in the increasing cold hardiness of the two grape species. In addition, the sucrose content made a relatively small contribution to freezing tolerance. Sucrose may be broken down into glucose and fructose, thereby doubling the osmotic solute concentration, which increases the bound water content during cold acclimation, allowing the more effectively resistance to cold stress [30, 31]. Furthermore, the protective functions of glucose and fructose may be attributed to their abilities to interact with extracellular ice to moderate the rate of ice growth at low temperatures.
Compared to January, the change in water content is not significant of two grape species in April. But their LT50 value has obviously decreased accompany the deacclimation. Meanwhile, the contents of total soluble sugar, fructose, glucose and starch in phloem and xylem of two grape species relating to tolerance showed drop sharply. So it is becoming increasingly clear that compatible solutes play a key role in mediating the cold tolerance during the cold acclimation and deacclimation.
REFERENCES
Turk, H. and Genisel, M., Melatonin-related mitochondrial respiration responses are associated with growth promotion and cold tolerance in plants, Cryobiology, 2020, vol. 92, p. 76. https://doi.org/10.1016/j.cryobiol.2019.11.006
Short, S., Díaz, R., Quiñones, J., Beltrán, J., Farías, J.G., Graether, S.P., and Bravo, L.A., Effect of in vitro cold acclimation of Deschampsia antarctica on the accumulation of proteins with antifreeze activity, J. Exp. Bot., 2020, vol. 71, p. 2933. https://doi.org/10.1093/jxb/eraa071
Fennell, A., Freezing tolerance and injury in grapevines, J. Crop Improv., 2004, vol. 10, p. 201. https://doi.org/10.1300/J411v10n01_09
Zhao, Y., Wang, Z.X., Yang,Y.M., Liu, H. S., Shi, G. L., and Ai, J., Analysis of the cold tolerance and physiological response differences of amur grape (Vitis amurensis) germplasms during overwintering, Sci. Hortic., 2020, vol. 259, p. 108760. https://doi.org/10.1016/j.scienta.2019.108760
Xu, W.R., Li, R.M., Zhang, N.B., Ma, F.L., Jiao, Y.T., and Wang, Z.P., Transcriptome profiling of Vitis amurensis, an extremely cold-tolerant Chinese wild Vitis species, reveals candidate genes and events that potentially connected to cold stress, Plant Mol. Biol., 2014, vol. 86, p. 527. https://doi.org/10.1007/s11103-014-0245-2
Palva, E.T., Welling, A., Tähtiharju, S., Tamminen, I., and Heino, P., Cold acclimation and development of freezing and drought tolerance in plants, Acta Hortic., 2001, vol. 560, p. 277. https://doi.org/10.17660/ActaHortic.2001.560.53
Stupnikova, I.V., Borovskii, G.B., and Voinikov, V.K., Seasonal changes in the composition and content of dehydrins in winter wheat plants, Russ. J. Plant Physiol., 2004,vol. 51, p. 636. https://doi.org/10.1023/B:RUPP.0000040750.10102.72
Wisniewski, M., Nassuth, A., Teulières, C., Marque, C., Rowland, J., Cao, P. B., and Brown, A., Genomics of cold hardiness in woody plants, Crit. Rev. Plant Sci., 2014, vol. 33, p. 92. https://doi.org/10.1080/07352689.2014.870408
Pagter, M., and Arora, R., Winter survival and deacclimation of perennials under warming climate: Physiological perspectives, Physiol. Plant., 2013, vol. 147, p.75. https://doi.org/10.1111/j.1399-3054.2012.01650.x
Ouyang, L., Leus, L., De Keyser, E., and Van Labeke, M.-Ch., Cold acclimation and deacclimation of two garden rose cultivars under controlled daylength and temperature, Front. Plant Sci., 2020, vol. 11, p. 1. https://doi.org/10.3389/fpls.2020.00327
Fadón, E., Herrero, M., and Rodrigo, J., Dormant flower buds actively accumulate starch over winter in sweet cherry, Front Plant Sci., 2018, vol. 9, p.1. https://doi.org/10.3389/fpls.2018.00171
Pagter, M., Hausman, J. F., and Arora, R., Deacclimation kinetics and carbohydrate changes in stem tissues of Hydrangea in response to an experimental warm spell, Plant Sci., 2011, vol. 180, p. 140. https://doi.org/10.1016/j.plantsci.2010.07.009
Zhao, L.Y., Yang, T.Y., Xing, C.H., Dong, H.Z., Qi, K.J., Gao, J.Z., Tao, S.T., Wu, J.Y., Wu, J., Zhang, S.L., and Huang, X.S., The β-amylase PbrBAM3 from pear (Pyrus betulaefolia) regulates solublesugar accumulation and ROS homeostasis in response to cold stress, Plant Sci., 2019, vol. 287, p. 110184. https://doi.org/10.1016/j.plantsci.2019.110184
Rubio, S., Dantas, D., Bressan-Smith, R., and Pérez, F.J., Relationship between endodormancy and cold hardiness in grapevine buds, J. Plant Growth Regul., 2016, vol. 35, p. 266. https://doi.org/10.1007/s00344-015-9531-8
Smith, A.M., Zeeman, S.C., and Smith, S.M., Starch degradation, Annu. Rev. Plant Biol., 2005, vol. 56, p. 73. https://doi.org/10.1146/annurev.arplant.56.032604.144257
Kasuga, J., Arakawa, K., and Fujikawa, S., High accumulation of soluble sugars in deep supercooling Japanese white birch xylem parenchyma cells, New Phytol., 2007, vol. 174, p. 569. https://doi.org/10.1111/j.1469-8137.2007.02025.x
Keunen, E., Peshev, D., Vangronsveld, J., Van Den Ende, W., and Cuypers, A., Plant sugarsare crucial players in the oxidative challenge during abiotic stress: Extending thetraditional concept, Plant Cell Environ., 2013, vol. 36, p. 1242. https://doi.org/10.1111/pce.12061
Chen, B., Zhang, B., and Mao, J., The relationship between the changing of water content and the cold resistance of grape branches, Plant Physiol. J., 2014, vol. 50, p. 535. https://doi.org/10.13592/j.cnki.ppj.2013.0441
Sun, S.H., Hu, C. G., Qi, X.J., Chen, J.Y., Zhong, Y. P., Muhammad A., Lin, M., and Fang, J.B., The AaCBF4-AaBAM3.1 module enhances freezing tolerance of kiwifruit (Actinidia arguta), Hortic. Res., 2021, vol. 8, p. 97. https://doi.org/10.1038/s41438-021-00530-1
Dai, H.B., Zhu, Z.H., Wang, Z.G., Zhang, Z.P., Kong, W.W., and Miao, Mi.M., Galactinol synthase 1 improves cucumber performance under cold stress by enhancing assimilate translocation, Hortic. Res., 2022, vol. 20, p. 9. https://doi.org/10.1093/hr/uhab063
Mo, F. L., Xue, X.P., Meng, L.J., Zhang, Y., Cui, Y.L., Liu, J.Y., Cheng, M.Z., Wang, P.W., Lv, R., Meng, F.Y., Qi, H.N., Qiu, Y.W., and Wang A.X., Genome-wide identification and expression analysis of SLAC1 gene family in tomato (Solanum lycopersicum) and the function of SlSLAC1–6 under cold stress, Sci. Hortic., 2023, vol. 313, p. 11904. https://doi.org/10.1016/j.scienta.2023.111904
Zhao, Y., Ai, J., Yang, Y.M., Wang Z.X., Liu, Y.X., He, W., and Liu, H.S., Identification of cold resistance in Vitis amurensis germplasms base on TTC staining index and logistic equation., Trans. Chin. Soc. Agric. Eng., 2018, vol. 34, p. 174. https://doi.org/10.11975/j.issn.1002-6819.2018.11.022
Xiong, J.Q., Patil, G.G., Moe, R., and Torre, S., Effects of diurnal temperature alternationsand light quality on growth, morphogenesis and carbohydrate content of Cucumis sativus L., Sci. Hortic., 2011, vol. 128, p. 54. https://doi.org/10.1016/j.scienta.2010.12.013
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J.H., and Zhu, J.K., Methods and concepts in quantifying Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status, Plant J., 2006, vol. 45, p. 523. https://doi.org/10.1111/j.1365-313X.2005.02593.x
Repo, T., Mononen, K., Alvila, L., Pakkanen, T.T., and Hänninen, H., Cold acclimation of pedunculate oak (Quercus robur L.) at its northernmost distribution range, Environ. Exp. Bot., 2008, vol. 63, p. 59. https://doi.org/10.1016/j.envexpbot.2007.10.023
Thomashow, M.F., Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1999, vol. 50, p.571. https://doi.org/10.1146/annurev.arplant.50.1.571
Schaberg, P.G., Berg, A.K., Murakami, P.F., Shane, J.B., and Donnelly, J.R., Factors influencing red expression in autumn foliage of sugar maple trees, Tree Physiol., 2003, vol. 23, p. 325. https://doi.org/10.1093/treephys/23.5.325
Gusta, L.V., Wisniewski, M., Nesbitt, N.T., and Gusta, M.L., The effect of water, sugars and proteins on the pattern of ice nucleation and propagation in acclimated anon acclimated canola leaves, Plant Physiol., 2004, vol. 135, p. 1642. https://doi.org/10.2307/4356521
Zhang, X.S., Feng, C.Y., Wang, M.N., Li, T.L., Liu, X., Plasma membrane-localized SlSWEET7a and SlSWEET14 regulate sugar transport and storage in tomato fruits, Hortic. Res., 2021, vol. 8, p.186. https://doi.org/10.1038/s41438-021-00624-w
Ruan, Y.L., Sucrose metabolism: Gateway to diverse carbon use and sugar signaling, Annu. Rev. Plant Biol., 2014, vol. 65, p. 33. https://doi.org/10.1146/annurev-arplant-050213-040251
Tao, H.X., Sun, H.Q., Wang, Y.F., Wang, X., and Guo, Y.P., Effects of water stress on quality and sugar metabolism in ‘Gala’ apple fruit, Hortic. Plant J., 2023, vol. 9, p. 60. https://doi.org/10.1016/j.hpj.2022.03.008
Funding
This work received financial support from the “Project of science and Technology Department of Jilin Province (20210101011JC)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zhao, Y., Qin, H.Y., Yang, Y.M. et al. Carbohydrate Changes during Seasonal Cold Acclimation and Deacclimation in Phloem and Xylem of Vitis Species with Different Cold Hardiness Levels. Russ J Plant Physiol 70, 158 (2023). https://doi.org/10.1134/S1021443723601908
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443723601908