Abstract
The effect of drought on the morphophysiological, biochemical, and molecular genetic parameters of plants Sedobassia sedoides (Pall.) Freitag & G. Kadereit with an intermediate C3–C4-type of photosynthesis and Bassia prostrata (L.) A.J. Scott with a C4-NADP type of photosynthesis grown at different temperatures (25 and 30°C) was studied. A decrease in the biomass, water content, and effective quantum yield (ΦPSII) of PSII, as well as an increase in the expression of the psbA gene encoding the PSII D1 protein under the action of drought, was observed in both species regardless of the growing temperature. Both species showed a decrease in the content of photosynthetic enzymes ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and phosphoenolpyruvate carboxylase (PEPC) under drought conditions at 25°С, which was accompanied by a significant increase in the expression of the rbcL and PPDK genes in S. sedoides. Acclimation of S. sedoides plants to elevated temperatures led to an increase in the activity of cyclic electron transport around PSI, to mitigation of the negative effect of drought on the light reactions of photosynthesis (reduction in NPQ) and the content of the PEPC enzyme, as well as to a shift in the ionic balance caused by a decrease in the potassium content. B. prostrata showed greater drought resistance and was characterized by greater thermolability of photosynthetic enzymes, changes in the content and ratio of which allowed this species to maintain growth in drought conditions at different temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Climate change is one of the most serious environmental problems. Extreme hot and dry periods are becoming more frequent and longer around the world and are predicted to increase further with rising temperatures [1–3]. Drought reduces the activity of photosynthesis and the content of pigments, as well as impairs the integrity of membranes and osmotic regulation, which limits the growth, development, and yield of plants [1]. A decrease in photosynthesis may be a consequence of “stomatal” or “nonstomatal” (metabolic) limitations [4]. Water deficiency leads to a decrease in noncyclic electron transport and the photochemical efficiency of PSII, often due to the degradation of the D1 protein, which is the most vulnerable among the internal components of PSII [5]. Also, osmotic stress can cause activation of cyclic electron transport (CET) of PSI [6]. At the same time, the effect of drought on the expression of genes encoding the main components of photosystems can be both stimulating [7] and suppressing [8]. A decrease in CO2 availability due to stomatal limitations in leaf tissues can lead to a decrease in the activity of the key enzyme of photosynthesis, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), depending on the intensity of drought and species differences [9]. Drought also differently affects the expression of the rbcL and RbcS genes [3, 10], and a decrease in the content of Rubisco may be, among other things, a consequence of increased protein degradation processes caused by stress conditions. It has been shown that Rubisco can be used by plants as a reserve of nitrogen/amino acids, which is excreted from chloroplasts and stored in vacuoles; under stress conditions, Rubisco is actively cleaved by proteases and sent to other organs to maintain protein synthesis [11, 12]. The phosphoenolpyruvate carboxylase (PEPC) is the second key enzyme of photosynthesis in C4-species, the change in the content and activity of which, under drought conditions, is associated with a change in the content and activity of Rubisco in different species, both upward [13] and downward [14]. It has been shown that the introduction of the C4-PEPC gene into C3-plants increases their drought resistance [15]. Another important enzyme of the C4-pathway is phosphate dikinase (PPDK), which is present in the chloroplasts and cytoplasm of both C4- and C3-plants and is involved in nitrogen assimilation, the synthesis of fatty acids, and osmotically active compounds [16]. The accumulation of this enzyme is induced by various abiotic stresses, including drought [16].
Most plants, regardless of the type of photosynthesis, show a significant ability to adapt their photosynthetic characteristics to the ambient temperature, which is called thermal acclimation. At the same time, C4-plants, in comparison with C3-species, are initially better adapted to higher temperatures [17]. An increase in growing temperature causes an increase in the optimal temperature of photosynthesis in plants and makes the photosynthetic apparatus more resistant to heat stress [18]. The increase in stability is caused by the optimization of the operation of the most vulnerable to an increase in temperature systems, which include the oxygen-releasing complex in photosystem II (PSII), the ATP generation system, and Rubisco carbon fixation due to Rubisco-activase [18], including increased activity of cyclic electron transport (CET) of PSI to maintain ATP synthesis [17]. Heat stress also activates thermosensitive enzymes and the expression of the majority of genes involved in energy and lipid metabolism, pigment biosynthesis, and photosynthesis [18]. For example, the biochemical characteristics of Rubisco can change under the influence of temperature, which contributes to the acclimation of the plant to temperature changes [19]. C4-plants have their own characteristics of biochemical limitations at elevated temperatures. It was shown that the rate of CO2 fixation of Rubisco in species with malate (NADP) C4-type of photosynthesis is higher at any temperature than in C3- and intermediate С3–С4 (С2)-species; at the same time, the rate of fixation of CO2 by Rubisco increases with an increase in temperature in all species, and the difference between species with different types of photosynthesis also increases [20, 21]. Molecular genetic studies have shown that heat stress causes a rapid reprogramming of the expression of a wide range of genes that are crucial for reducing the negative effect of temperature exposure; so far relatively little is known about the change in the expression of the plastid genome, although the components of the photosynthetic apparatus are the main targets of thermal damage [22].
The C4-photosynthesis pathway relies on a coordinated system of anatomical and biochemical traits that allow CO2 to be concentrated around Rubisco in bundle sheath cells, which prevents the Rubisco oxygenation reaction and, thereby, suppresses photorespiration, making C4-plants more successful in open and warm habitats compared to C3-species [2, 23]. It is believed that C4-photosynthesis developed gradually in C3-species through intermediate stages of C3–C4 photosynthesis [23, 24]. Four separate stages of the evolutionary transition from C3 to C4 photosynthesis (intermediate C3–C4 photosynthesis) are considered: proto-Kranz—C2 (Type I and II)—C4-like photosynthesis, in a series of which there is an increase in C4‑features [25]. At the same time, there is a point of view that C2-photosynthesis is a stable evolutionary state and does not always lead to C4-photosynthesis [26, 27]. Plants with intermediate C3–C4 photosynthesis use a photorespiratory carbon pump, or glycine shuttle, to capture CO2 released from mesophyll photorespiratory activity and transport it to the bundles sheath cells for reuse in the Calvin cycle. In this case, the activity of cyclic electron transport of PSI increases due to an increase in the need for ATP, which is necessary for the functioning of the glycine shuttle [24]. The presence of a high level of intraspecific and intrapopulation photosynthetic diversity and plasticity has been shown for different C3–C4 species, which complicates the determination of plants belonging to different types of C2-photosynthesis [23, 26, 28]. At the same time, the physiological plasticity inherent in C2-plants allows them to inhabit wide ecological ranges [27]. Despite a general preference for warmer climates, C2-plants are found in cooler regions than C4-species [23]. A comparative analysis of the adaptation of C2- and C4-plants of related species of the same family to elevated temperature and drought has not been carried out.
The aim of this work was to study the ability of plants of the C3–C4 (C2) species Sedobassia sedoides and C4-NADP species Bassia prostrata to acclimate to elevated temperatures and its effect on resistance to osmotic stress.
MATERIAL AND METHODS
Plant Material and Growing Conditions
The seeds of halophytes Sedobassia sedoides (Pall.) Freitag & G. Kadereit (Bassia sedoides (Pall.) Asch) and Bassia prostrata (L.) A. J. Scott (Kochia prostrata (L.) Schrad.) (subfamily Chenopodiaceae) were collected in natural habitats of the Caspian lowland (Volgograd oblast). Seeds were soaked in distilled water for germination. Three–four-day-old seedlings were planted on perlite soaked in 50% Hoagland’s solution. NaCl at a final concentration of 50 mM was added to Hoagland’s nutrient solution after the appearance of true leaves for optimal growth. Plants were grown in two separate chambers at 25°C and 30°C under fluorescent lamps at a flux density of PAR quanta of 200 μmol/(m2 s) at a 16-h photoperiod. After 30 days of cultivation, some plants were watered with a 15.8% PEG6000 solution for 4 days. In total, there were four groups of plants of each species: (1) control plants grown at 25°C without PEG treatment; (2) plants grown at 25°C and 4 days of PEG treatment; (3) plants grown at 30°C without PEG treatment; (4) plants grown at 30°C and 4 days of PEG treatment.
Determining Water, Proline, Sodium and Potassium Ions Contents
To determine the dry biomass, plant samples were dried at 80°C to constant weight. The water content (W) was calculated according to the formula and expressed in g H2O/g dry weight:
where FW is fresh weight and DW is dry weight.
The content of sodium and potassium ions in shoots was determined in an aqueous extract of dried samples (100 mg) on an FPA-2-01 flame photometer (AOOT ZOMZ, Russia) and expressed in mmol/g dry weight.
The content of free proline was determined using an acid ninhydrin reagent according to the Bates method [29] with modifications. Aqueous extracts of dried and ground material were used as analyzed extracts. Results were calculated per 1 g of dry weight.
Photosystem I Activity
The change in the redox potential of P700 was measured by monitoring the optical density of leaves at 820 nm using a dual-wavelength pulse modulation system ED-P700DW (Heinz-Walz, Effeltrich, Germany) in combination with PAM-101 (Heinz-Walz, Germany). The kinetics of P700 oxidation was measured under illumination with far red light (720 nm, 17.2 W/m2). The maximum oxidation of P700 was determined using a xenon gas discharge lamp (50 ms, 1500 W/m2, Heinz-Walz, Germany) in the presence of far red light.
Photosystem II Efficiency
The quantum yield of PSII fluorescence of a dark-adapted (20 min) leaf fragment was determined using a PAM fluorimeter (PAM-101, Heinz-Walz, Germany). The dark maximum quantum yield of PSII fluorescence (Fv/Fm) was measured. The measurement was carried out with additional illumination of the sample with a weak modulated red-light flux, which was carried out by an ADC (PDA-100, Walz, Germany), which converted the primary signal from the PAM-101 to a computer with a specialized software interface. The indicators were calculated based on the current value of the minimum (F0) and maximum (Fm) fluorescence of a dark-adapted leaf according to the formula
The effective quantum yield of PSII photochemistry at a given light intensity was calculated by the formula
where \(F_{{\text{q}}}^{'}\) is a photochemical quenching of fluorescence by an open reaction center of PSII and \(F_{{\text{m}}}^{'}\) is a maximum fluorescence after light adaptation.
Nonphotochemical quenching of chlorophyll fluorescence (NPQ) was calculated by the formula
Determining Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) and Phosphoenolpyruvate Carboxylase (PEPC) Proteins by Western Blotting
The total protein was extracted from 0.2–0.5 g of the aboveground part of the plant, which was crushed in liquid nitrogen, and 1–2 mL of extraction buffer containing 50 mM Tris-HCl (pH 8), 10 mM MgCl2, 0.3 mM EDTA, 2% polyvinylpyrrolidone, and 5 mM dithiothreitol. The homogenate was centrifuged at 12 000 g for 15 min at 4°C (MiniSpin centrifuge, Eppendorf, Germany). The protein content was determined by the Bradford method using bovine serum alb umin (Sigma-Aldrich, United States) as a standard.
The content of Rubisco and PEPC proteins was analyzed using enzyme immunoassay according to [30] using commercial polyclonal antibodies against the large subunit proteins (L) of Rubisco (RbcL, AS03037, Agrisera, Sweden) and PEPC (AS09458, Agrisera, Sweden). Separation of total proteins (10–15 μg of total protein per slot) was performed using 10% denaturing gel electrophoresis (SDS-PAGE) according to [31] using standard molecular weight markers (BioRad, United States). After electrophoresis, the proteins were transferred to a nitrocellulose membrane (Amersham, GE Healthcare, United Kingdom) using a wet blotting machine (BioRad, United States) according to the standard protocol. Rubisco and PEPC proteins were visualized using rabbit immunoglobulins conjugated with luminol and coumaric acid fluorescent dyes (Sigma, United States) and Retina XBE film (Germany). The intensity of bands in Western blotting was assessed using the ImageJ 1.37v program (United States) and expressed relative to the average level (n = 3) for control plants, which was taken as 100%. The analysis was carried out at least three times.
RNA Isolation and Quantitative Real Time (RT)-PCR
RNA isolation was carried out by phenol-chloroform extraction with precipitation using LiCl. RNA was extracted using a buffer mixture (0.1 M LiCl, 0.1 M Tris-HCl (pH 7.5), 1% SDS, 10 mM EDTA (pH 8)) with acidified phenol (pH 4.5) in a 1 : 1 ratio heated to 90°C in a water bath (WB-4MS, Biosan, Latvia). An extraction mixture was added to the crushed plant tissue (400–500 mg) in a 1 : 3 ratio. Chloroform (500 μL) was used to separate the fractions. Samples were centrifuged for 15 min at 12 000 g (MiniSpin, Eppendorf, Germany) at room temperature. After the third centrifugation, 10 M LiCl was added to the supernatant to a final concentration of 2.5 M LiCl and left overnight at 4°C. The next day, RNA was pelleted by centrifugation and washed once with 2 M LiCl and twice with 80% ethanol. The precipitate was dissolved in 100 μL of RNase-free water. The concentration of isolated RNA was determined using a NanoDrop 1000 spectrophotometer (ThermoScientific, United States). RNA was purified from genomic DNA according to the standard ThermoScientific protocol (United States) using DNAse I and RiboLock (ThermoScientific, United States). Reverse transcription was carried out in two stages. At the first stage, the primers for the synthesis of the first strand of the total cDNA on the RNA template (Oligo(dT)15 primer and Random(dN)10 primer (Evrogen, Russia)) were annealed for 5 min at 65°C (TT-2 thermostat, Termite, DNA-Technology, Russia). At the second stage, reverse transcriptase was performed using MMLV reverse transcriptase (Evrogen, Russia), dNTP (ThermoScientific, United States), adding RiboLock (ThermoScientific, United States). The concentration of the resulting cDNA was measured using a NanoDrop 1000 spectrophotometer (ThermoScientific, United States).
Primers for PCR (Supplementary Table S1) were selected using Pick Primers NCBI (National Center for Biotechnology Information, Bethesda, MD) with Primer Pair Specificity Checking Parameters and SnapGene Viewer (4.2.11) on nucleotide sequences available in the NCBI database: primers for the rbcL genes of Sedobassia sedoides (AY270063.1), Bassia prostrata (AY270104.1), PPDK of Bienertia sinuspersici (MK674493.1), psaA of Bassia littorea (OK539756.1) and Chenopodium quinoa (LOC32958941), psaB of C. quinoa (LOC32958940), psbA of Bassia scoparia (AY251266.1) and C. quinoa (LOC32959011), CAB of C. quinoa (LOC110735177). UBQ10 of C. quinoa (LOC110721034) and b-Tubulin of C. quinoa (XM_021890176) were used as reference genes.
The primers were checked and the amplicon size was determined using PCR (TP4-PCR-01-Tertsik, DNA-Technology, Russia) and electrophoresis in 2% agarose gel. The level of expression of the studied genes was assessed by real-time PCR (RT-qPCR) using a Light Cycler96 amplifier (Roche, Switzerland) using SybrGreen I dye (Evrogen, Russia). RT-qPCR data were analyzed using Light Cycler96 Software Version 1.1. Transcript levels are relative to control plants.
Statistical Analysis
There were at least three biological replicates in all experiments. The SigmaPlot 12.0 software was used for correlation and factorial (ANOVA) analysis. The graphs show the arithmetic mean values of the obtained values and their standard errors. Differences were considered significant at P < 0.05 (Tukey’s test). R software (version 3.6.1) was used for multivariate principal component analysis (PCA).
RESULTS
Biomass and Content of Water, Proline, and Ions
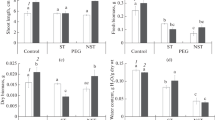
Under control conditions, plants of the annual species S. sedoides were characterized by a higher biomass than plants of the perennial species B. prostrata (Fig. 1a). A twofold decrease in dry biomass (DW) was observed in both species grown under drought conditions regardless of the growing temperature (Fig. 1a). Growing S. sedoides and B. prostrata plants at elevated temperature without PEG treatment did not lead to any changes in dry biomass accumulation compared to control plants in both species (Fig. 1a).
Accumulation of (a) dry biomass, content of (b) water, (c) proline, (d) sodium, and (e) potassium ions and (e) K+/Na+ ratio in Sedobassia sedoides and Bassia prostrata plants grown at different temperatures and short-term effect of PEG-induced drought. (1) Control plants grown at 25°C without PEG treatment; (2) plants grown at 25°C and 4 days of PEG treatment; (3) plants grown at 30°C without PEG treatment; (4) plants grown at 30°C and 4 days of PEG treatment. Different Latin letters indicate differences significant at P < 0.05.
The water content (W) in shoots of control B. prostrata plants was almost two times lower than in shoots of S. sedoides plants (Fig. 1b). Exposure of plants to drought at normal growing temperature (25°C) resulted in a decrease in water content in the shoots of both plant species by 67–70%. The water content in the shoots of S. sedoides and B. prostrata grown under conditions of elevated temperature (30°C) without PEG treatment was 20–30% lower than in the control plants, and the effect of drought at this temperature had a greater effect on the decrease in water content in the shoots of S. sedoides than in B. prostrata plants (Fig. 1b).
The content of proline (Pro) in shoots of control S. sedoides plants was 4.5 times lower than in control B. prostrata plants (Fig. 1c). Under drought conditions at 25°C, S. sedoides plants showed a 9.4-fold increase in the content of proline and a 1.4-fold increase in B. prostrata plants. During acclimation to an elevated temperature (30°С), the content of proline in S. sedoides plants increased almost twofold, while it decreased by twofold in B. prostrata plants compared to the control. Exposure to drought after acclimation to elevated temperatures resulted in an approximately twofold increase in proline content (relative to plants grown at 30°C without PEG treatment; Fig. 1c) in both species.
The content of Na+ and K+ in the shoots of control S. sedoides plants was 2.3 and 1.6 times higher than in the shoots of control B. prostrata plants, respectively (Fig. 1d, 1e). No changes in the content of Na+ and K+ in plants of either species were observed under drought conditions at 25°C as well as at elevated temperatures without PEG treatment. A significant decrease in the Na+ content under the action of drought at 30°C was observed only in B. prostrata plants (Fig. 1d), and a decrease in the K+ content was observed only in S. sedoides plants (Fig. 1e). The K+/Na+ ratio in shoots of control S. sedoides plants was 1.6 times lower than in control B. prostrata plants (Fig. 1f). Changes in the ratio of K+/Na+ ions were observed only under drought conditions at 30°C in both B. prostrata plants (1.5-fold increase relative to control plants and those grown at 30°C without PEG treatment) and S. sedoides plants (1.3-fold decrease in compared with plants grown at 25°C) (Fig. 1f).
Activity of Cyclic Electron Transport of PSI and Efficiency of PSII
Under control conditions, the activity of cyclic electron transport (CET) of PSI in S. sedoides plants was lower than in B. prostrata (Fig. 2a). Drought had no effect on CET activity at 25°C. Acclimation to an elevated temperature led to an increase in the activity of CET of PSI in S. sedoides plants, up to values characteristic of C4 species, the level of which was preserved even under the action of drought (at 30°C). In B. prostrata plants, CET activity remained constant under all types of exposure (Fig. 2a).
Photosynthetic parameters in Sedobassia sedoides and Bassia prostrata plants grown at different temperatures and short-term effect of PEG-induced drought. (a) Cyclic electron transport activity of PSI; (b) maximum quantum yield of PSII (Fv/Fm); (c) effective quantum yield of PSII at a given light intensity (ΦPSII); (d) nonphotochemical fluorescence quenching of PSII (NPQ). (1) Control plants grown at 25°C without PEG treatment; (2) plants grown at 25°C and 4 days of PEG treatment; (3) plants grown at 30°C without PEG treatment; (4) plants grown at 30°C and 4 days of PEG treatment. Different Latin letters indicate differences significant at P < 0.05.
The efficiency of the maximum quantum yield of PSII photosynthesis in plants of both species decreased when exposed to drought: significantly at 25°C in S. sedoides and at 30°C in B. prostrata, but it did not change when plants were grown at elevated temperatures without exposure to drought (Fig. 2b). The quantum yield efficiency (ΦPSII), i.e., the efficiency of PSII photochemistry at a given light intensity, significantly decreased relative to the control plants in both species under the influence of drought, regardless of the cultivation temperature (Fig. 2c). Nonphotochemical quenching of PSII fluorescence (NPQ) significantly increased under drought conditions: it is higher at 25°C in S. sedoides plants and at 30°C in B. prostrata plants (Fig. 2d).
Content of Photosynthetic Enzymes
The content of Rubisco (large subunit) decreased by 80–85% under the influence of drought in S. sedoides plants regardless of the growing temperature. In B. prostrata plants, exposure to drought at 25°C also led to an 85% decrease in the content of Rubisco. During acclimation to elevated temperatures, the content of Rubisco decreased by 20% in S. sedoides and by 45% in B. prostrata (Figs. 3a, 3b). The content of Rubisco in B. prostrata under drought conditions at elevated temperatures remained the same as when grown at elevated temperatures without drought (Figs. 3a, 3b). The content of PEPC in S. sedoides changed only under drought conditions at 25°C, while the content of PEPC in B. prostrata significantly decreased under all treatments. However, the PEPC content turned out to be two times higher in plants exposed to drought at elevated temperatures than under the action of these factors separately (Figs. 3a, 3c).
Results of Western blotting of proteins (a, b) Rubisco (large subunit), PEPC and (d) expression of rbcL (Rubisco L subunit) and (e) PPDK (pyruvate phosphate dekinase) genes in shoots of Sedobassia sedoides and Bassia prostrata plants grown at different temperatures and short-term effect of PEG-induced drought. (1) Control plants grown at 25°C without PEG treatment; (2) plants grown at 25°C and 4 days of PEG treatment; (3) plants grown at 30°C without PEG treatment; (4) plants grown at 30°C and 4 days of PEG treatment.
Expression of Photosynthetic Genes
Drought caused an eightfold increase in the accumulation of rbcL gene transcripts in S. sedoides plants and a slight increase in B. prostrata plants regardless of temperature (Fig. 3d). Acclimation to elevated temperatures resulted in a sixfold increase in the accumulation of rbcL gene transcripts in S. sedoides and a 70% increase in B. prostrata. The accumulation of PPDK gene transcripts increased sixfold upon exposure to drought and threefold upon acclimation to elevated temperatures in S. sedoides plants (Fig. 3e). In B. prostrata, the level of PPDK gene transcripts did not change under changing conditions. The level of transcripts of the psaA and psaB genes encoding apoproteins A1 and A2 of PSI, respectively, remained unchanged in all variants of the experiment in both species (Figs. 4a, 4b). The level of transcripts of the psbA gene encoding the PSII D1 protein in S. sedoides increased by an average of three times relative to the control plants and by an average of two times in B. prostrata for all types of treatment (Fig. 4c). The accumulation of transcripts of the CAB gene (chlorophyll a/b-binding protein LHCB/CAB PSII) was observed in B. prostrata under the influence of drought, regardless of temperature, while it remained unchanged in S. sedoides (Fig. 4d).
Expression of (a, b) psaA and psaB (apoproteins A1 and A2 of PSI), (c) psbA (D1 protein of PSII), and (d) CAB (chlorophyll a/b-binding protein LHCB/CAB PSII) genes in shoots of Sedobassia sedoides and Bassia prostrata plants grown at different temperatures and short-term effect of PEG-induced drought. (1) Control plants grown at 25°C without PEG treatment; (2) plants grown at 25°C and 4 days of PEG treatment; (3) plants grown at 30°C without PEG treatment; (4) plants grown at 30°C and 4 days of PEG treatment.
PCA Analysis
Multivariate principal component analysis (PCA) did not show significant differences between S. sedoides plants grown at 25°C and 30°C without exposure to drought but separated from them plants exposed to drought by the first principal component (PC1), which reflects 46.2% of the total variation (Fig. 5a). The main elements of PC1 included PSII efficiency and NPQ values as well as the content of Rubisco and proline (Table 1). The PCA also showed a clear difference between the effect of drought at 25 and 30°C on S. sedoides in the second principal component (PC2), which represents 21.87% of the total variation (Fig. 5a). The main elements of PC2 included the activity of PSI (cyclic transport), K+ content, K+/Na+ ratio, and the content of the main C4-cycle enzyme PEPC. The first two main components are enough to explain 68% of the changes from the total variation. Multivariate principal component analysis performed for B. prostrata showed no clear differences between plants under different treatments (Fig. 5b).
Multivariate principal component analysis (PCA) of physiological parameters involved in adaptation of (a) Sedobassia sedoides and (b) Bassia prostrata plants to elevated temperature and short-term PEG-induced drought. (1) Control plants grown at 25°C without PEG treatment; (2) plants grown at 25°C and 4 days of PEG treatment; (3) plants grown at 30°C without PEG treatment; (4) plants grown at 30°C and 4 days of PEG treatment.
DISCUSSION
Drought is one of the most common environmental factors limiting photosynthesis and plant growth. A decrease in biomass and water content in shoots was observed under drought conditions at 25°C in both studied species S. sedoides and B. prostrata (Fig. 1). However, differences between species in the increase in the content of proline (Fig. 1), which is widely used as a marker of osmotic stress [32], indicate a greater effect of drought on S. sedoides than B. prostrata plants. Photosynthesis is considered among the primary physiological processes that are affected by water deficiency [9]. PSII is the most sensitive to stress, which is often expressed in the degradation of the D1 protein [5, 18]. A decrease in the effective quantum yield of PSII photochemistry at a given light intensity (ΦPSII) and a 3.3-fold increase in NPQ were observed in the C3–C4 species S. sedoides, which led to a significant decrease in the maximum quantum yield of PSII (Fig. 2). An increase in NPQ values indicates a higher dissipation of light energy in the form of heat during osmotic stress [33]. At the same time, a significant increase in the expression of the psbA gene encoding the D1 protein was observed (Fig. 4). In addition, a significant effect of drought manifested in a sharp (by 85%) decrease in the content of the main enzyme of the Calvin cycle, Rubisco, in S. sedoides (Fig. 3). It is known that the response of plants to drought is species-specific and varies from minor changes in the content and activity of Rubisco to their sharp decrease [9]. At the same time, the decrease in the content of Rubisco in S. sedoides was not a consequence of a decrease in the expression of the rbcL gene; on the contrary, a sixfold increase in its expression was observed, which is typical for some C3-species under drought conditions [3, 10]. This disproportion between the content of rbcL transcripts and the Rubisco protein may be a consequence of posttranscriptional regulation [34] or an increase in protein degradation processes caused by stress conditions, when Rubisco is actively cleaved by proteases and sent to other plant organs to maintain protein synthesis [11, 12]. An increase in the expression of the PPDK gene under drought conditions may be indirect evidence of an increase in the degradation process in S. sedoides (Fig. 4) since PPDK is involved in nitrogen assimilation and can play an important role in amino-acid transport and significantly accelerate the mobilization of nitrogen from leaves [16].
Drought had a less negative effect on the efficiency of PSII in the C4-NADP species B. prostrata, which was expressed in a smaller change in dissipation (NPQ) (Fig. 2). At the same time, there was an increase in the expression of the CAB gene encoding the chlorophyll a/b-binding protein (LHCB/CAB) of PSII, the regulation of which is considered one of the important mechanisms for regulating the function of chloroplasts in response to stress factors [18]. With a decrease in the content of Rubisco similar to that in S. sedoides, the accumulation of rbcL gene transcripts in B. prostrata was significantly lower, and the expression of the PPDK gene did not change, but there was a more significant decrease in the content of PEPC (Fig. 3). The revealed differences in the reactions of PEPC and PPDK between species are probably associated with differences in their functions: in C4-species, these proteins are key photosynthetic enzymes, while they mainly perform protective functions under stress in C3-species and, possibly, in C3–C4-species [35].
Acclimation to elevated temperatures led to a slight decrease in water content in shoots, which, however, did not affect the accumulation of dry biomass in either species (Fig. 1). Growing at 30°C also did not affect the efficiency of PSII in S. sedoides and B. prostrata plants (Fig. 2), but it caused a two- to fourfold increase in the accumulation of psbA gene transcripts (Fig. 4), while the level of PSI psaA and psaB gene transcripts remained unchanged in both species. At the same time, an increase in the CET activity of PSI in the C3–C4-species S. sedoides was observed almost to the level of the C4-NADP species B. prostrata (Fig. 2). It is believed that an increase in CET at high temperature can compensate for the proton leakage of thylakoids, allowing continued ATP synthesis [17]. Cultivation at 30°C led to a decrease in the content of Rubisco in both species, but it was more significant (by 2 times) in B. prostrata (Fig. 3). This is probably caused by an increase in the rate of CO2 fixation by Rubisco with an increase in temperature, which is typical for all plant species and, in particular, for species with malate (NADP) C4-type of photosynthesis [20, 21]. The retention of biomass accumulation at the level of control plants (Fig. 1) and the results of PCA may be evidence of a nonstress-induced decrease in the content of Rubisco (Fig. 5). Thus, multivariate analysis did not show a clear separation of plants grown at 25 and 30°C without exposure to drought for both species. The absence of significant stress is also indicated by a decrease in the content of proline in B. prostrata compared with the control plants and a relatively small increase in this indicator in S. sedoides (Fig. 1). The higher level of Rubisco in S. sedoides at elevated temperatures than in B. prostrata is probably supported by a more significant increase in rbcL gene expression (Fig. 3). At the same time, the content of not only Rubisco but also the C4-enzyme PEPC decreases in B. prostrata, which makes it possible to maintain the ratio of Rubisco/PEPC that is optimal for photosynthesis.
Despite multiple evidence of a more negative effect on plants of the combined stress of elevated temperature and drought than each of these effects separately [3, 36], cultivation at 30°C in S. sedoides mitigated the negative effect of drought on PSII, which was expressed in lower energy dissipation (Fig. 2d) and neutralized the negative effect on the PEPC content (Fig. 3). However, these changes did not affect the accumulation of biomass during drought (Fig. 1), probably as a result of the same effect of drought on the Rubisco content and the efficiency of PSII, regardless of the growing temperature (Figs. 2, 3). Thus, acclimation to elevated temperatures of S. sedoides avoided the additional negative effect of drought, causing changes in the protective reactions of the photosynthesis process and in maintaining water balance. With a significant decrease in the water content in the shoots of S. sedoides caused by drought at 30°C, there was a significantly lower accumulation of proline (by 2.5 times compared with control plants) and a decrease in the content of potassium and the K+/Na+ ratio (Fig. 1, Table 1). This may indicate a decrease in the role of proline in osmoregulation and a change in the ionic balance in favor of Na+, which is more typical for halophytes [37] and, in particular, a higher accumulation of Na+ (3.5 times) relative to K+ in S. sedoides grown under control conditions (Fig. 1). It is the differences in CET activity, K+ and PEPC contents that are the main factors for a clear separation of the second main component of PSII in C3–C4 S. sedoides plants grown at different temperatures under drought conditions (Fig. 5, Table 1).
No additional negative effect of the combined effect of high temperature and drought on the accumulation of dry biomass was observed in the C4-NADP species B. prostrata as well as in the C3–C4-species S. sedoides; however, the growth maintenance mechanisms were different. Drought at 30°C led to an increase in energy dissipation and, accordingly, to a decrease in the maximum efficiency of PSII (Fig. 2), but, at the same time, it had a less negative effect on the content of photosynthetic enzymes Rubisco and PEPC (Fig. 3). In C4‑species of Chenopodiaceae, adaptation to stress conditions is associated precisely with significant biochemical adaptation: changes in the content of Rubisco and C4-cycle enzymes and activation of osmoregulation [17, 38]. The significant climatic range of C4-NADP species B. prostrata from the southern semideserts (Central Asia, Iran, Mongolia, and China) to the northern forest-steppes of Eurasia [39] is probably caused by the wide thermal lability of photosynthetic enzymes. Acclimation of B. prostrata plants to elevated temperatures led to the restoration of proline content to the level of control plants, a decrease in sodium content, and an increase in the K+/Na+ ratio under drought conditions (Fig. 1), i.e., it contributed to an increase in the role of potassium ions in the water-ion balance, which is characteristic of xerophytic species. The greater drought tolerance of the C4-NADP-species B. prostrata, compared to the C3‒C4-species S. sedoides, is evidenced by a lower water content and a higher K+/Na+ ratio under control conditions (Fig. 1) as well as the absence of clear differences after PCA (Fig. 5)
Thus, the С3–С4-species of S. sedoides proved to be less drought resistant under both growing temperature regimes. The drought negatively affected both the content of the main photosynthetic enzyme and the efficiency of PSII, causing a significant increase in the expression of the corresponding rbcL and psbA genes. At the same time, the acclimation of S. sedoides plants to an elevated temperature led to an increase in the activity of cyclic electron transport around PSI as well as to the mitigation of the negative effect of drought on the light reactions of photosynthesis and the PEPC enzyme content against the background of a shift in the ion balance towards sodium. The role of potassium ions in osmoregulation increased in C4-NADP-species B. prostrata under drought conditions at elevated temperatures. In general, B. prostrata is characterized by more thermolabile photosynthetic enzymes, changes in the content and ratio of which allow this species to maintain growth in drought conditions at different temperatures.
REFERENCES
IPCC. Future global climate: Scenario-based projections and near-term information, In: Climate change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change, Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Pean, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Huang, M., Leitzell, K., Lonnoy, E., Matthews, J.B.R., Maycock, M., Waterfield, T., Yelekci, O., Yu, R., and Zhou, B., Eds., Cambridge University Press, 2021, p. 553.
Sonmez, M.C., Ozgur, R., Uzilday, D., Turkan, I., and Ganie, S.A., Redox regulation in C3 and C4 plants during climate change and its implications on food security, Food Energy Secur., 2022, vol. 12, p. e387. https://doi.org/10.1002/fes3.387
Raja, V., Qadir, S.U., Alyemeni, M.N., and Ahmad, P., Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum, 3 Biotech., 2020, vol. 10, p. 208. https://doi.org/10.1007/s13205-020-02206-4
Ripley, B., Frole, K., and Gilbert, M., Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses, Ann. Bot., 2010, vol. 105, p. 493. https://doi.org/10.1093/aob/mcp307
Alyammahi, O. and Gururani, M.A., Chlorophyll-a fluorescence analysis reveals differential response of photosynthetic machinery in melatonin-treated oat plants exposed to osmotic stress, Agronomy, 2020, vol. 10, p. 1520. https://doi.org/10.3390/agronomy10101520
Rakhmankulova, Z.F., Shuyskaya, E.V., Prokofieva, M.Yu., Borovkov, A.M., and Voronin, P.Yu., Comparative contribution of CO2/H2O exchange components to the process of adaptation to drought in xero-halophytes from the family Chenopodiaceae with different types of photosynthesis, Russ. J. Plant Physiol., 2020, vol. 67, p. 494. https://doi.org/10.1134/S102144372003019X
Zhang, Q., Huang, J., Ke, W., Cai, M., Chen, G., and Peng, C., Responses of Sphagneticola trilobata, Sphagneticola calendulacea and their hybrid to drought stress, Int. J. Mol. Sci., 2021, vol. 22, p. 11288. https://doi.org/10.3390/ijms222011288
da Silva, M.C., Pinto, P.I.S., Guerra, R., Duarte, A., Power, D.M., and Marques, N.T., Gene transcripts responsive to drought stress identified in Citrus macrophylla bark tissue transcriptome have a modified response in plants infected by Citrus tristeza virus, Sci. Hortic., 2023, vol. 307, p. 111526. https://doi.org/10.1016/j.scienta.2022.111526
Galmés, J., Aranjuelo, I., Medrano, H., and Flexas, J., Variation in Rubisco content and activity under variable climatic factors, Photosynth. Res., 2013, vol. 117, p. 73. https://doi.org/10.1007/s11120-013-9861-y
Amoah, J.N. and Seo, Y.W., Effect of progressive drought stress on physio-biochemical responses and gene expression patterns in wheat, 3 Biotech., 2021, vol. 11, p. 440. https://doi.org/10.1007/s13205-021-02991-6
He, Y., Yu, Ch., Zhou, L., Chen, Y., Liu, A., Jin, J., Hong, J., Qi, Y., and Jiang, D., Rubisco decrease is involved in chloroplast protrusion and Rubisco-containing body formation in soybean (Glycine max.) under salt stress, Plant Physiol. Biochem., 2014, vol. 74, p. 118e124. https://doi.org/10.1016/j.plaphy.2013.11.008
Yoshitake, Y., Nakamura, S., Shinozaki, D., Izumi, M., Yoshimoto, K., Ohta, H., and Shimojima, M., RCB-mediated chlorophagy caused by oversupply of nitrogen suppresses phosphate-starvation stress in plants, Plant Physiol., 2021, vol. 185, p. 318. https://doi.org/10.1093/plphys/kiaa030
AbdElgawad, H., Avramova, V., Baggerman, G., Van Raemdonck, G., Valkenbog, D., Van Ostade, X., Guisez, Y., Prinsen, E., Asard, H., Ende, W., and Beemster, G., Starch biosynthesis is crucial for maintaining photosynthesis and leaf growth under drought stress, Authorea, 2020. https://doi.org/10.22541/au.158498002.27084474
Hussain, T., Koyro, H.-W., Zhang, W., Liu, X., Gul, B. and Liu, X., Low salinity improves photosynthetic performance in Panicum antidotale under drought stress, Front. Plant Sci., 2020, vol. 11, p. 481. https://doi.org/10.3389/fpls.2020.00481
Jeanneau, M., Vidal, J., Gousset-Dupont, A., Lebouteiller, B., Hodges, M., Gerentes, D., and Perez, P., Manipulating PEPC levels in plants, J. Exp. Bot., 2002, vol. 53, p. 1837. https://doi.org/10.1093/jxb/erf061
Yadav, S., Rathore, M.S., and Mishra, A., The pyruvate-phosphate dikinase (C4-SmPPDK) gene from Suaeda monoica enhances photosynthesis, carbon assimilation, and abiotic stress tolerance in a C3 plant under elevated CO2 conditions, Front. Plant Sci., 2020, vol. 11, p. 345. https://doi.org/10.3389/fpls.2020.00345
Yamori, W., Hikosaka, K., and Way, D.A., Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation, Photosynth. Res., 2014, vol. 119, p. 101. https://doi.org/10.1007/s11120-013-9874-6
Allakhverdiev, S.I., Kreslavski, V.D., Fomina, I.R., Los, D.A., Klimov, V.V., Mimuro, M., Mohanty, P., and Carpentier, R., Inactivation and repair of photosynthetic machinery under heat stress, in Photosynthesis: Overviews on Recent Progress and Future Perspective, New Delhi: IK International, 2012, p. 189.
Cavanagh, A.P. and Kubien, D.S., Can phenotypic plasticity in Rubisco performance contribute to photosynthetic acclimation?, Photosynth. Res., 2014, vol. 119, p. 203. https://doi.org/10.1007/s11120-013-9816-3
Sharwood, R.E., Ghannoum, O., Kapralov, M.V., Gunn, L.H., and Whitney, S.M., Temperature responses of Rubisco from Paniceae grasses provide opportunities for improving C3 photosynthesis, Nat. Plants, 2016, vol. 2, p. 16186. https://doi.org/10.1038/nplants.2016.186
Moore, C.E., Meacham-Hensold, K., Lemonnier, P., Slattery, R.A., Benjamin, C., Bernacchi, C.J., Lawson, T., and Cavanagh, A.P., The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems, J. Exp. Bot., 2021, vol. 72, p. 2822. https://doi.org/10.1093/jxb/erab090
Danilova, M.N., Kudryakova, N.V., Andreeva, A.A., Doroshenko, A.S., Pojidaeva, E.S., and Kusnetsov, V.V., Differential impact of heat stress on the expression of chloroplast-encoded genes, Plant Physiol. Biochem., 2018, vol. 129, p. 90. .https://doi.org/10.1016/j.plaphy.2018.05.023
Lundgren, M.R. and Christin, P.-A., Despite phylogenetic effects, C3–C4 lineages bridge the ecological gap to C4 photosynthesis, J. Exp. Bot., 2017, vol. 68, p. 241. https://doi.org/10.1093/jxb/erw451
Munekage, Y.N. and Taniguchi, Y.Y., A scheme for C4 evolution derived from a comparative analysis of the closely related C3, C3–C4 intermediate, C4-like, and C4 species in the genus Flaveria, Plant Mol. Biol., 2022, vol. 110, p. 445. https://doi.org/10.1007/s11103-022-01246-z
Sage, R.F., Khoshravesh, R., and Sage, T.L., From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis, J. Exp. Bot., 2014, vol. 65, p. 3341. https://doi.org/10.1093/jxb/eru180
Lundgren, M.R., C2 photosynthesis: a promising route towards crop improvement?, New Phytol., 2020, vol. 228, p. 1734. https://doi.org/10.1111/nph.16494
Walsh, C.A., Bräutigam, A., Roberts, M.R., and Lundgren, M.R., Evolutionary implications of C2 photosynthesis: how complex biochemical trade-offs may limit C4 evolution, J. Exp. Bot., 2023, vol. 74, p. 707. https://doi.org/10.1093/jxb/erac465
Rakhmankulova, Z.F., Shuyskaya, E.V., Khalilova, L.A., Orlova, Y.V., Burundukova, O.L., Velivetskaya, T.A., and Ignat’ev, A.V., Ultra- and mesostructural response to salinization in two populations of C3–C4 intermediate species Sedobassia sedoides, Russ. J. Plant Physiol., 2020, vol. 67, p. 835. https://doi.org/10.1134/S1021443720040135
Bates, L.S., Waldren, R.P., and Teare, I.D., Rapid determination of free proline for water stress studies, Plant Soil., 1973, vol. 39, p. 205. https://doi.org/10.1007/BF00018060
Pozhidaeva, E.S., Western blot hybridization, in: Molecular genetic and biochemical methods in modern plant biology, Kuznetsova, Vl.V., Kuznetsova, V.V., and Romanova, G.A., Moscow: Binom, 2011, p. 228.
Laemmli, U.K., Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 1970, vol. 227, p. 680. https://doi.org/10.1038/227680a0
Szabados, L. and Savoure, A., Proline: a multifunctional amino acid, Trends Plant Sci., 2010, vol. 15, p. 89.
Brestic, M. and Zivcak, M., PSII fluorescence techniques for measurement of drought and high temperature stress signal in plants: protocols and applications, in Molecular Stress Physiology of Plants, Rout, G.R. and Das, A.B., Eds., Dordrecht: Springer, 2013, p. 87. https://doi.org/10.1007/978-81-322-0807-5
Kusnetsov, V.V., Chloroplasts: structure and expression of the plastid genome, Russ. J. Plant Physiol., 2018, vol. 65, p. 465. https://doi.org/10.1134/S1021443718030044
Singh, J., Garai, S., Das, S., Thakur, J.K., and Tripathy, B.C., Role of C4 photosynthetic enzyme isoforms in C3 plants and their potential applications in improving agronomic traits in crops, Photosynth. Res., 2022, vol. 154, p. 233. https://doi.org/10.1007/s11120-022-00978-9
Xu, Z.Z. and Zhou, G.S., Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis, Planta, 2006, vol. 224, p. 1080. https://doi.org/10.1007/s00425-006-0281-5
Flowers, T.J. and Colmer, T.D., Plant salt tolerance: adaptation in halophytes, Ann. Bot., 2015, vol. 115, p. 327. https://doi.org/10.1093/aob/mcu267
Liu, H. and Osborne, C.P., Water relations traits of C4 grasses depend on phylogenetic lineage, photosynthetic pathway, and habitat water availability, J. Exp. Bot., 2015, vol. 66, p. 761.
Dzyubenko, N.I., Soskov, Yu.D., Khusainov, S.Kh., and Agaev, M.G., Morphology and geography of the ecotypes Kochia prostrata (L.) Schrad. from Middle Asia, Kazakhstan and Mongolia, Sel’skokhoz. Biol., 2009, vol. 44, no. 5, p. 25.
Funding
This study was supported by the Russian Foundation for Basic Research (project no. 21-54-50006YaF_a) and the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme no. 122042700044-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
STATEMENT ON THE WELFARE OF ANIMALS
The study was conducted without the use of animals and without involving people as subjects.
Additional information
Translated by M. Shulskaya
Supplementary Information
Rights and permissions
About this article
Cite this article
Shuyskaya, E.V., Rakhmankulova, Z.F., Prokofieva, M.Y. et al. Effect of Acclimation to High Temperatures on the Mechanisms of Drought Tolerance in Species with Different Types of Photosynthesis: Sedobassia sedoides (C3–C4) and Bassia prostrata (C4-NADP). Russ J Plant Physiol 70, 127 (2023). https://doi.org/10.1134/S1021443723601283
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443723601283