Abstract
The interactions of branches in relation to the transports of IAA export activity (IEA) were studied in pea plants of the semidwarf cv. Adagumsky having a strong apical dominance (AD) and the dwarf cv. Porta with a weakened AD. In a model system of two-branched seedlings, the branches of cv. Adagumsky competitively suppressed the growth and IEA of each other, but that is not the case with cv. Porta. The root supply with GA3 inhibits the outgrowth of axillary buds at basal node 2 in cv. Porta seedlings, thus enhancing AD, and led to establishing a 1.5 fold different IEA in the shoots between two-branched and one-branched seedlings, making it similar to cv. Adagumsky. Thus, the emerging role of GAs as a systemic signal in regulation of AD and correlative inhibition is suggested. Molecular mechanisms of GA involvement in these processes are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The essence of the phenomenon of apical dominance (AD) is that the lateral buds, which are laid in the leaf axils (axillary meristems), stay dormant in the presence of the functional apical bud of the main shoot and start to grow when this bud is removed [1]. Despite its long-term study, the topic of plant branching regulation still remains at the center of attention of plant biologists. To date, a great body of experimental material has been accumulated on the mechanisms of AD control as well as genes involved in the regulation of dormancy/growth of lateral meristems [1]. Studying the topic of AD and branching is not only of academic interest, but may also be of practical importance for increasing plant productivity [2].
Experiments on the replacement of the apical bud with lanolin paste with auxin, started more than 85 years ago, demonstrated the leading role of the apical auxin in inhibiting shoot branching [1]. IAA, synthesized in young apical leaves, is polarly transported down the stem by membrane auxin efflux carriers of the family PINFORMED (PIN) [3], while the IAA itself does not penetrate lateral buds [1]. To explain the auxin distant effect on the lateral meristem growth, two main mechanisms were suggested. One of the mechanisms, called the auxin-transport hypothesis, suggests that the auxin polar flow in the main stem somehow inhibits the outflow of IAA from the lateral buds thereby inhibiting their growth [1]. Another mechanism implies the participation of some mobile compounds in AD acting as auxin secondary messengers. Cytokinins (CKs) were considered initially as such substances [1, 4] supplemented later with strigolactones (SLs) discovered more recently [5]. Both hormones have the ability to move acropetally along the xylem, while CKs activates, and SLs, on the contrary, inhibits the bud growth [1]. Generally, auxin inhibits CK synthesis [4] but activates SL synthesis [6]. A simplified outline of the interactions between hormones in the regulation of AD is shown in Fig. 1.
IAA and its control of secondary messengers, regulators of apical dominance and correlative inhibition, cytokinins (CKs) and strigolactones (SLs). Feedback loops between IAA and CKs, participation of SLs are presented. The scheme is made on the basis of literature data [1, 4–6, 8, 10, 11, 13, 16–19, 29]. Post-transcriptional interaction designations: (*) stabilization/destabilization, (**) receptor activation. ARF—AUXIN RESPONSE FACTOR; Aux/IAA—transcriptional repressor of auxin-inducible genes; AXR1—AUXIN-RESISTANCE protein 1; CCD7/MAX3/RMS5 and CCD8/MAX4/RMS1—carotenoid cleavage dioxygenases of Arabidopsis/pea; CKX— cytokinin oxidase; D53/SMXL—SUPPRESSOR OF MAX2 1-LIKE protein; PIF—PHYTOCHROME-INTERACTING FACTOR protein; TIR1/AFB— TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX PROTEIN, auxin receptor; type B RR—a positive type B RESPONSE REGULATOR of cytokinin response.
Auxin signaling begins with the interaction of the hormone with the receptor, where auxin acts as a molecular glue bringing together AUXIN/INDOLE-3-ACETIC ACID INDUCIBLE (Aux/IAA) proteins, transcriptional repressors of auxin-inducible genes, and the auxin receptor TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX (TIR1/AFB) proteins [6]. TIR1/AFB is a substrate-recognizing subunit (F-box protein) of the SCF E3-ubiquitin ligase complex that triggers polyubiquitination and promotes the 26S proteasome-mediated degradation of the Aux/IAA proteins. Thus, the auxin-promoted removal of Aux/IAA allows AUXIN RESPONSE FACTORs (ARFs) to activate early auxin-responsive gene transcription [6] (Fig. 1). Auxin up-regulates an expression of the key enzymes for SLs synthesis, two carotenoid cleavage dioxygenases CCD7/MAX3/RMS5 and CCD8/MAX4/RMS1, possibly acting through auxin-induced degradation of the transcriptional repressor IAA12/BDL, thereby activating presumed ARF5/MP [6] (Fig. 1). Arabidopsis shows auxin-dependent regulation of expression of CCD7/MAX3/RMS5 and CCD8/MAX4/RMS1, which corresponds to the presence of auxin-response cis-elements in the promoter region of these genes (4 and 2 copies of TGTCTC ARF-binding fragments, respectively). Relatively recently in pea, an auxin receptor TIR1/AFB that is involved in the activation of SLs synthesis and belongs to the AFB4,5/RMS2 clade of auxin co-receptors was identified [6]. Surprisingly, it was found that auxin signaling triggered by receptors of the AFB4/AFB5 clade does not involve the IAA12/BDL-dependent pathway, and it was concluded that these receptors are unlikely to be the only auxin receptors specifically involved in mediating the auxin-dependent SL synthesis [6].
Adenosine phosphate-ISOPENTENYLTRANSFERASE genes (IPTs), encode the key enzymes for CK synthesis. In Arabidopsis, from all AtIPTs gene family (9 genes) [7], only AtIPT3 expression is up-regulated by decapitation [8]. In pea, among the 6 IPT genes [9] only PsIPT1,2 respond to decapitation by increased expression [4]. Thus AtIPT3 gene function can be considered to be similar to those of pea auxin-responsive PsIPT1 and PsIPT2 genes. In Arabidopsis, treatment of the inflorescences of wild-type plants with IAA induced ARF3 expression peaked at 4 h of treatment, and mutations in ARF3 lead to ectopic CK biosynthesis [10]. Therefore, ARF3/ETTIN could presumably be a negative regulator of AtIPT3 expression (Fig. 1). ARF3 is an unusual ARF lacking the Aux/IAA-interacting Phox/Bem1 domain [10]. ARF3 coordinates with AGAMOUS, homeotic gene, encodes a flower-specific MADS domain transcription factor to repress the expression of IPT3, IPT5, IPT7 and LONELY GUY (LOG) enzymes, with the last converting CK ribotides in active free basis [10]. ARF3 directly represses the expression of IPT family genes, IPT3, IPT5, and IPT7, and indirectly represses LOG family genes, both of which encode enzymes required for cytokinin biosynthesis [10]. The IPT3 promoter lacks the TGTCTC, auxin-response cis-element, and high ARF3 enrichment was detected at the IPT3 and IPT7 promoter regions but not at the coding regions, demonstrating that ARF3 targets IPT3 and IPT7 [10]. Notably, the recently discovered SLs as AD enhancers are slightly ahead of CK with respect to the molecular mechanisms of IAA dependent synthesis, and the details of how auxin inhibits IPT gene expression are not yet clear [1].
In addition to the opposite effect of CKs and SLs in AD these two classes of hormones interact antagonistically with each other at the level of synthesis and signaling. In rice, SL synthesis genes in roots and nodes are suppressed by CKs and in Arabidopsis, CKs suppress auxin-induced expression of CCD8/MAX4/RMS1 gene [1] (Fig. 1). In turn, SLs can activate the degradation of CKs. Recent data obtained using rice suggest the possibility that SL negatively regulates cytokinin signaling by inducing one of several CK oxidases (CKXs), namely OsCKX9 [11] (Fig. 1), making CKs as antagonists of SLs at the level of synthesis and metabolism. On the other hand, it was shown that in roses and Jatropha curcas, CKs suppress the expression of the MAX2/RMS4/D3 F-box protein required for SL signaling [12, 13] (Fig. 1). First target protein in SL signaling is the SUPPRESSOR OF MAX2 1-LIKE6,7,8 (D53/SMXL6,7,8), chaperonin-like class I Clp ATPase, which interact with SL-activated receptor D14/AtD14/RMS3, and this complex forms a substrate for ubiquitination by the SCF E3 ubiquitin ligase containing a substrate docking F-box protein MAX2/RMS4/D3 [1, 5, 6]. Both D14 and D53 are ubiquitinated and degraded through the 26S proteasome-mediated pathway as a result of the interaction with SCFMAX2 [6] (Fig. 1). Therefore, CKs SLs are antagonists to each other, having the ability to mutually reduce each other’s activity. We can conclude that the combined regulation of the auxin-dependent synthesis of SLs and CKs and the crosstalk of their metabolism and signaling ultimately creates a complex auxin-CK-SL network (Fig. 1).
The ability of a shoot to transport auxin is strongly correlated with its growth activity and this correlation is striking, but the mechanism by which auxin moving in one shoot might prevent auxin transport in the opposite shoot is not clear [1]. CKs and SLs behave as antagonists with respect to IAA synthesis, important for sustain lateral bud development [14]. Data on Arabidopsis show that IAA synthesis follows the scheme of tryptophan—indole-3-pyruvic acid—IAA [6, 15, 16]. TRYPTOPHAN AMINOTRANSFERASE related (TAA/TAR) proteins convert tryptophan to indole-3-pyruvic acid and YUCCA (YUC) flavin monooxygenase-like enzymes convert indole-3-pyruvic acid to IAA by the YUC proteins [6, 15, 16]. Recently, it was revealed that SLs repress IAA levels in the stem at least in part by a rapid down-regulation of transcript levels of IAA biosynthesis genes, such as TAR2 and YUC1, through D53/SMXL6,7,8 proteins, which are destabilized in the process of SL reception [6] (Fig. 1). A promoting effect of CK treatment was observed on the auxin synthesis genes RhYUC1 and RhTAR1 in the rose buds [13]. In Arabidopsis, CKs have been shown to induce expression of the IAA17/AXR3 gene, and an increase in the rate of IAA biosynthesis requires the stabilization of the IAA17/AXR3 protein (axr3-1 mutation) and/or increased level of IAA17/AXR3 expression [17, 18]. In the axr3-1 mutant having the iaa17/axr3 protein resistant to degradation, both the IAA pool size and auxin biosynthetic rates were increased in comparison to the wild type and these were not altered by CK treatment. According to Jones et al. [17], CK activation of IAA synthesis may consist of the stabilization of IAA17/AXR3 proteins in a feedback loop mechanism regulating IAA levels and biosynthesis rates (Fig. 1). CK also inhibit the expression of AUXIN-RESISTANCE protein 1 (AXR1) [19] which is a subunit of the E1 enzyme homolog that participates in the RUB (related to ubiquitin) modification of Cullin subunit of SCF E3 ubiquitin ligase complex thereby activating it. Hence, in this pathway CKs can also stabilize Aux/IAA proteins by preventing their ubiquitin-proteasome degradation [19] and thus damping auxin signaling, and, as a consequence, interrupts the negative feedback control of IAA synthesis [17] (Fig. 1). Also the transcriptional factors belonging to WRKY family are able to bind to the TAR1 gene promoter, activating the expression, and can be induced by CK [14, 18] (Fig. 1). In Arabidopsis root, the transcription of both TAA1 and YUC8 can be promoted by CK possibly via PHYTOCHROME-INTERACTING FACTOR (PIF4) protein belonging to the bHLH transcriptional factors which is required for this upregulation, with YUC8 and TAA1 being direct targets of PIF4 [16] (Fig. 1). The above mentioned involvements of CKs in IAA synthesis regulation occur most likely through canonical CK signaling pathway, in which CKs activate the receptor hybrid two-component histidine kinases and then the hot phosphate is transmitted by a multi-step phosphorelay to the members of type B RESPONSE REGULATORs (type B RRs, in Arabidopsis—ARR1,2,10-14,18-21) [16, 18, 19]. The phosphorylated type B RRs promote transcription of the different early CK response genes, including negative regulators of CK activity such as CKXs and type A RRs (negative regulators of CK signaling) [8, 18, 19]. It is not clear whether the IAA17/AXR3 [17], WRKY [14, 18] and PIFs [16] dependent pathways are independent of each other in CK promoted activation of IAA synthesis. Therefore, between the IAA on the one hand and the SLs and CKs on the other, a negative feedback loop is eventually formed, that is especially important when studying the effect of correlative inhibition (CI) occurring between developing shoots on the same plant.
Earlier work pointed out that GAs enhance AD, and GA synthesis/signaling mutants have a low-branching phenotype [5]. It has been suggested that the action of GAs in AD and CI is based on their regulation of IAA polar transport [5]. In Arabidopsis, it was found that the shoot stems in GA mutants have a reduced capacity for polar IAA transport [20]. The authors also found that GAs is not a major positive regulator of the PIN gene transcription, although on pea Bai and DeMason [21] was shown that GAs are capable of enhancing PsPIN1 expression due to the presence of two types of GA-regulatory elements, one 16 P-box and one TATC-box, in the promoter. The auxin transport impairment in GA mutants can be explained by a decrease in PIN auxin efflux facilitator abundance and that GA stabilize PIN proteins to the plasma membrane (PM) [20]. Further work was focused on the effect on PIN cycling of GA-induced Ser/Thr kinase, PINOID (PID), which targets PIN by phosphorylating its central, hydrophilic domain [22]. In pea, GA-regulatory elements, one 16 P-box, and one TATC-box, were also found in the PsPK2/PID promoter [21]. Facts were found that contradicted the assumption that GAs are able to activate basipetal IAA transport in the stem, which is obviously related to the specificity of the object of study (lateral root formation and leaf vasculature development). Thus, PID-promoted phosphorylation of PIN inversely caused transcidosis and displacement of PIN to the apical part of the plasma membrane [3]. It was also consistent with this GA influence that in the Arabidopsis seedlings the downregulation of PID by CKs causes an increase in basipetal auxin transport, although it is not known whether transcriptional regulation of PID plays a role in regulating its kinase activity [19]. Thus, contradictory results were obtained with regard to GA-dependent PIN phosphorylation as a mechanism of basipetal IAA transport activation in the stem. It was recently reported that GA balances the protein traffic between the vacuole degradation route and recycling back to the cell surface [23]. Low GA levels promote vacuolar delivery and degradation of multiple cargos, including PIN proteins, whereas high GA levels promote their recycling to PM which thus supports basal localization of auxin effux carriers PINs [23]. GA signaling is based on destabilization of DELLA proteins which regulate many physiological processes [24]. GA-induced DELLA protein degradation is mediated by the SCF E3 ubiquitin ligase complex containing a substrate docking F-box protein SLY1/GID2 [24]. GA is recognized by the GA receptor GID1 and the formation of the GA-GID1-DELLA complex promotes the recruitment of DELLA protein by the SCFSLY1/GID2 complex. This triggers polyubiquitination and degradation of DELLA proteins, allowing the expression of GA response genes [24]. A role of DELLA signaling pathway in GA-regulated PIN trafficking has been detected, but protein biosynthesis is not required. This GA cellular action occurs through DELLA proteins that regulate the microtubule arrangement presumably through their interaction with prefoldins [23, 24]. Interestingly, the effect of GAs on the cycling of PIN transport proteins brings them slightly closer but opposite to SLs which have an ability to counteract the accumulation of PIN1 on PM specifically by triggering their rapid depletion in a clathrin-dependent process that is likely to be endocytosis [5]. The presented literature data on the regulation of auxin transport show one of the first possible ways in which GAs could be involved in the regulation of AD and CI.
In the previous study with two-branched (2-B) pea seedlings as a model system, we provided the evidence that CI between the shoots/branches is a direct consequence of the interactions between IAA and CK, therewith xylem-CK (X-CK) appears to control both growth and IAA export out of branches [25]. Apparently, the levels of X-CK are established in hypocotyl, a stem analog in our model, rather than in root, as a result of IAA homeostasis in hypocotyl, which is dynamically maintained due to the auxin-regulated CK synthesis that controls the X-CK levels and hence regulates the delivery of IAA from shoots to hypocotyl thereby keeping its IAA level stable. The experiments conducted on several pea cultivars revealed that the occurrence of CI is cultivar-specific and attributed to the characteristic feature of the IAA-CK interaction in accordance with the second messenger model, but not relating to SL activity [26]. In contrast to semidwarf cv. Adagumsky and tall cv. Torsdag, a weakened AD and the absence of CI in в 2-B system of pea seedlings was shown in dwarf cvs. Porta and Térèse [26], which are GA-deficient [27]. In the present study it was succeeded to confirm that GAs are involved in regulation of AD and CI. It was shown that the overall level of IAA export from shoots did not correlate with GA status. Therefore, the absence of a relationship between IAA transport and its effect on AD and CI showed another way in which GAs are involved in plant branching, which may be related to modifications of IAA-CK interactions [26].
MATERIALS AND METHODS
Plant material. The pea (Pisum sativum L.) seeds of the wild-type cvs. Adagumsky (semidwarf), and Porta (dwarf) were used.
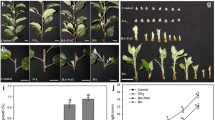
Production of two-, one-branched and branchless pea plants. Seeds were soaked and germinated for 3 days at 25°С in the dark as described by Kotov and Kotova [25]. On the 4-th day, epicotyl was removed from cotyledonary node to induce the growth of two axillary buds at this node and the seedlings were raised in tap water at 20 ± 1°С under fluorescent light, providing an intensity of 135 μmol/(m2 s), with a 14-h day/10-h night photoperiod. Four days later, the seedlings bearing two equal growing cotyledonary shoots (2-B plants) with the typical shoot size of 6 mm were selected and continued to grow under the same conditions. To obtain one-branched (1-B) plants, one or two shoots were removed in the 8-day-old 2-B plants. Two days after, the samples of shoot diffusates were taken in the 10-day-old plants (Fig. 2).
Bud length measurements. Axillary buds were measured at basal node 1, 2 and 3 in 12-day-old seedlings, using a horizontal calibrated stereomicroscope. The mean bud length value ± SE were determined from 15 biological replicates for each treatment.
Hormonal treatments. To make a 100 mM stock concentration of GA3 (Serva, Germany) it was dissolved in DMSO and was kept at –20°С. For cv Porta, GA3 was added to the hydroponic culture solution to give a final concentration of 20 μM for 6-day old seedlings or 10 μM for the 8-day-old 2-B and 1-B plants.
Estimation of IAA export activity in the cotyledonary shoots. Export of IAA out of shoot apices IAA) was measured by diffusate method as described by Kotov and Kotova [25, 26]. Each shoot tip with the internode below was cut off and its basal end was placed individually into 120–150 µL of distilled water (Fig. 2). The samples were incubated in a humid box for 2 h at 20 ± 1°С in darkness, and then separately for each sample, the IAA-containing diffusates from shoot tips were stored at –20°C before analysis, while the shoot tips were cut off from the underlying internode and weighed. The diffusates from 5–6 shoot tips with the similar weight (not exceed ±5 mg) were pooled in a combined probe and subjected to ELISA for measuring diffusible IAA as described by Kotov and Kotova [25, 26]. The correlation of IAA amount with the averaged shoot tip weight was calculated by linear regression analysis (SigmaPlot 11 for Windows 7) conducted on the 4–7 data points for each treatment. The IAA export activity (IEA) of shoots was expressed as the amount of diffusible IAA in the shoot tip having a standard weight of 45 mg, and calculated using the linear regression equations in SigmaPlot 11 with standard error of the estimate (±SEE).
ELISA of IAA. Antiserum against IAA was produced by immunization of rabbits either with, respectively, with BSA coupled with IAA through indolic nitrogen, allowing to analyze free IAA without preliminary methylation [25]. Antigens for the sensibilisation of polystyrene plates were synthesized substantially by a method similar to that of immunogen production with using ovalbumin as a carrier protein [25]. Competitive indirect ELISA was performed on 96-well microtiter plates (Costar # 9018, United States) and, each sample was tested in six wells as described by Kotov and Kotova [25, 26]. For the detection of primary antibodies, the horseradish peroxidise conjugated with anti-rabbit sheep IgG (Medgamal, Russia) was used and visualized by ortho-phenylene diamine. The absorbance was read at 492 nm with an ELISA plate reader (MR 580 Dynatech, United States). Logit-log transformations, standard curves, and results were calculated by SigmaPlot 11 for Windows 7 (Systat Software Inc.) and Microsoft Office Excel 2003 described by Kotov and Kotova [25]. All curves fitted to linear regression models with a high correlation coefficient (r2 = 0.977–0.998), and the linear ranges of standard calibration curve were from 3 to 100 nM IAA. The non-specific interference in diffusates samples was tested by adding known amounts of a hormone standard into the sample [25] and the added/found ratio did not exceed 100 ± 15%.
Statistical analysis. Where comparisons were made between two treatments (Figs. 3, 4), data were subjected to one-way ANOVA using SigmaPlot 11 for Windows 7. The variance was analyzed by Tukey’s significant difference, with the level of significance set to P < 0.001; P < 0.01; P < 0.05.
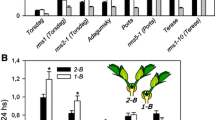
The length of axillary buds at basal node 1, 2 and 3 in 12-day-old seedlings of pea cvs. Adagumsky (semidwarf) and Porta (dwarf). From day 6, some cv. Porta plants were treated hydroponically with 20 μM GA3. Nodes were counted acropetally from the cotyledonary node as node 0. Data are means ± SE (n = 15). Asterisks indicate statistically significant differences (***P < 0.001; *P < 0.05) between the axillary buds of cv. Adagumsky plants and the relative buds of cv. Porta plants.
IAA export activity (IEA) out of excised cotyledonary shoot tip in 10-day-old two-shoot (2-B) (2) and one-shoot (1-B) (1) plants (see Fig. 1) of pea cvs. Adagumsky (semidwarf) and Porta (dwarf). 2-B plants were prepared by removing epicotyl from 4-day-old seedlings to induce growth of two axillary buds at the cotyledonary node. At days 8, 1-B plants were produced by removing one branch in some 2-B plants and some plants of cv. Porta were treated hydroponically with 10 μM GA3. IEA was measured two days later, on days 10 and calculated with standard error of the estimate (±SEE). Asterisks indicate statistically significant differences (**P < 0.01), comparing 1-B plants with 2-B plants.
RESULTS
Gibberellins are involved in the Control of Apical Dominance in Pea
Comparison of the size of basal axillary buds in seedlings of pea semidwarf cv. Adagumsky and dwarf cv. Porta showed their significant difference, especially with respect to node 2 bud (Fig. 3). The data indicate the loss of dormancy of node 2 bud and its outgrowth in intact seedlings of dwarf cv. Porta, which suggests a decreased AD in this case. Earlier, when comparing the basal axillary buds of tall cv. Torsdag and dwarf cv. Térèse seedlings, a similar correlation between node 2 bud outgrowth and the dwarf phenotype was established, due to the le mutation in cv. Térèse [27]. In pea, the LE gene encodes GA3 oxidase, which is involved in the synthesis of active GA1 from the inactive precursor GA20 [21], and thus determines GA deficiency and dwarfism. This is consistent with the fact that GA-deficient mutants displayed higher shoot branching [5].
The root supply with 10 μM GA3 inhibits the outgrowth of axillary buds at basal node 2 in cv. Porta seedlings, thus enhancing AD, and making it similar to cv. Adagumsky (Fig. 3). This is consistent with the previous data showing that GA3 treatment enhances AD [5]. Selective release of node 2 buds by GA deficiency is not yet clear, but is obviously specific to the action of GA. In contrast, in SL-deficient mutants of tall cv. Torsdag (rms1) and dwarf cvs. Porta (rms5) and Térèse (rms1), growth activation is observed in node 1–3 axillary buds, with SL-deficient mutants in dwarf GA-deficient backgrounds displaying stronger branching than in tall GA-sufficient backgrounds [26]. Thus, our data obtained on different cultivars are consistent with the fact that in pea, GA-and SL-deficient double mutants displayed stronger branching than single mutants, suggesting that GAs act independently of SLs to repress branching [5]. Earlier, a paradoxical result was shown in SLs mutants, where SLs enhance AD in intact pea seedlings but have no effect on CI in the 2-B seedling system [25]. So, AD may not correlate with CI and we further tested the effect of GA3 on CI in a 2-B system.
Deficiency in Gibberellins is Responsible for Loss of Competition between Shoots in Two-Shoot Pea Plants
In cv. Porta, the root supply with 10 μM GA3 led to establishing a different IEA in the shoots between 2-B and 1-B plants similar to that observed in cv. Adagumsky (Fig. 4). In our experiments with 2-B pea plants, GA3 treatments did not lead to an increase in shoot IEA and obviously, these results do not confirm the ability GA to induce AD and CI through alterations in auxin transport [5, 20]. Therefore, in cv. Porta the shoots do not sense each other in 2-B seedlings and this fact is directly related to GA deficiency (Fig. 4). The systemic action of GA3 changes this effect.
We can speculate that this effect could be due to the influence of GA on the lateral transfer of IAA in the hypocotyl, making the IAA transport channels from the two shoots independent of each other [28]. This version, however, does not agree well with the previously obtained results on CK transport in cv. Porta in 2-B seedlings. Thus, removal of one of the shoots did not significantly affect X-CK levels [26], whereas in this case, X-CK levels should have greatly increased due to decapitation of one of the two independent channels, which, analogous to the shootless variant [26], should have caused a significant increase in total CK synthesis/transport, which was not detected. Also, the effect of GA action in this case cannot be explained by the presumed activation of polar IAA transport [5, 20, 23], because IAA export from GA-deficient cv. Porta shoots after GA3 treatment was no higher than before the treatment (Fig. 4). Thus, the apparent cause of GA-dependent shoot competition is a modification of the IAA-CK interaction, the character of which was suggested earlier [26] and in which GA involvement was shown in the present work and further discussed.
DISCUSSION
SLs have previously been shown to enhance AD, but it was paradoxical to find that SLs had no effect on CI between shoots in a 2-B model system of pea seedlings [25, 26]. Although SLs inhibit IAA synthesis [6] and transport [5, 14], reduce CK synthesis [29] and activate CK catabolism [11], apparently these modifications do not dramatically alter the IAA-CK interaction on which CI is based [25, 26]. In cv. Adagumsky, feeding experiment showed that treatment with 1 µM BA eliminates CI, with IEA in 1-B shoots virtually unchanged and in 2-B shoots increased to the level as that in 1-B shoots [25]. This result implies that the occurrence of CI is a consequence of CK deficiency, the appearance of which seems to be determined by a mechanism based on CK-IAA interaction with the presence of a constant “IAA threshold level” (see below) which on/off CK synthesis in the axial organ of the main shoot (in the 2-B system this is the hypocotyl) [25]. Thus, the number of shoots per plant creates an X-CK deficiency that directly controls IAA synthesis in the shoots. Now it was shown that another class of hormonal regulators, GAs, also enhance AD (Fig. 3), but, unlike SLs, are also able to influence CI by transforming the non-competitive shoot system in 2-B GA-deficient pea shoots into a system with competing shoots (Fig. 4). Thus, since GA signaling is based on DELLA proteins [24], we will further consider the causes of CI loss between shoots in GA-deficient cv. Porta, basing on the possible influence of DELLA proteins on IAA-CK interaction shown in Fig. 5. Interesting, CKs, Suc, but not Glu, can stabilize the DELLA protein REPRESSOR OF GA (RGA) [15].
IAA and its control of secondary messengers, regulators of apical dominance and correlative inhibition, cytokinins (CKs) and strigolactones (SLs). The basic regulatory framework (see Fig. 1) was supplemented with gibberellin-(GA) and sugar-related potential regulatory circuits, in accordance with the described results and current literature [1, 12, 14–16, 18, 21, 31, 33–37]. Feedback loops between IAA and CKs, participation of SLs, gibberellins (GAs) and sugars (Sug) are presented. Post-transcriptional interaction designations: (*) stabilization/destabilization, (**) receptor activation, (***) interaction through sink activity. ARF—AUXIN RESPONSE FACTOR; Aux/IAA—transcriptional repressor of auxin-inducible genes; AXR1—AUXIN-RESISTANCE protein 1; CCD7/MAX3/RMS5 and CCD8/MAX4/RMS1—carotenoid cleavage dioxygenases of Arabidopsis/pea; CKX—cytokinin oxidase; D53/SMXL—SUPPRESSOR OF MAX2 1-LIKE protein; DELLA—gibberellin signaling protein; PIF—PHYTOCHROME-INTERACTING FACTOR protein; TIR1/AFB—TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX PROTEIN, auxin receptor; type B RR—a positive type B RESPONSE REGULATOR of cytokinin response.
Comparison of IAA/CK balance in dwarf cv. Porta (without CI) and semidwarf cv. Adagumsky (with CI) highlighted two differences that could cause GA-dependent loss of CI [26]. Firstly, it is the export of IAA from the shoots, which remains unchanged when the number of shoots on the plant is reduced. The level of IAA export is a reflection of the rate of IAA synthesis and thus the participation of DELLA proteins could be related to the regulation of this process. In Arabidopsis, the transcription of early auxin synthesis genes TAA1 and YUC8 can be induced by PIF4 [16] (Fig. 5). In turn, DELLA proteins are known to deactivate PIFs proteins by binding to them and inhibit their expression activity [30] (Fig. 5). Thus, GAs signaling based on the degradation of DELLA proteins can actually activate the synthesis of IAA [16]. However, GA3 treatment of dwarf cv. Porta 2-B seedlings rather slightly reduced IAA export from shoots (Fig. 4), which does not support this pathway of GA involvement in the regulation of IAA synthesis in our model system. Other data on Arabidopsis say that the enhanced Glu induction of IAA biosynthesis in the pif1 pif3 pif4 pif5 line indicates that the PIF proteins act as negative regulators of sugar (Sug)-induced IAA biosynthesis. In support of this explanation, the PIF5-OE line showed a severely reduced capacity for Glu induction of IAA biosynthesis [15]. Thus, this leaves the possibility that DELLA proteins blocking the PIFs [30] may play a positive role in Glu-activated IAA synthesis [15].
Another putative GA-dependent way to stabilize IAA export from shoots is based on the data obtained in Arabidopsis regarding the mechanism of CK-induced IAA synthesis. As already mentioned, CKs may positively controls IAA biosynthesis by inducing expression of the IAA17/AXR3 gene which activates IAA synthesis in its self-regulation circuit through the Aux/IAA-ARF loop [17, 18] (Fig. 5). Recently it was found IAA17/AXR3 to be able directly interact with DELLA protein RGA-like3 (RGL3) and increased its protein stability, with RGL3 stabilized IAA17/AXR3 protein through inhibiting the interaction of auxin recetor TIR1 and IAA17 by competitively binding to IAA17/AXR3 [31] (Fig. 5). Thus, GA deficiency that stabilize DELLA proteins [24] may activate in part IAA synthesis in CK-independent manner and this is consistent with the absence of correlative inhibition of IAA export from shoots in cv. Porta (Fig. 4). As an alternative, some other mechanisms can be suggested. The expression of AtIPT::GUS was detected in the base of outgrowing lateral branch in Arabidopsis [7]. This proposes the autonomous synthesis in outgrowing lateral shoots. In Jatropha curcas, GA3 treatment of nodal stems and axillary buds significantly down-regulated the expression of the JcIPT3 and JcLOG1 [12] (Fig. 5). Hence, conversely GA deficiency may substantially promote CK synthesis in the branches themselves. The assumptions discussed above require further experimental verification with respect to the effect of GAs on CI in the 2-B system of pea seedlings.
The second interesting feature of the IAA/CK balance that differs dwarf cv. Porta and semidwarf cv. Adagumsky is that the change in IAA export from shoots when they are removed and, accordingly, the change in auxin status in the hypocotyl does not change CKs synthesis/level/transport so dramatically [26]. This peculiarity of hormonal regulation of cv. Porta is a reflection of the fact that in this case the inhibition of CKs synthesis in the hypocotyl when one of the shoots is removed is apparently not based on CK feedback from IAA coming from the remaining shoot and stopping this synthesis [25, 26]. For cv. Adagumsky, a model of regulation of CK synthesis on/off was adopted, when the level of IAA in the hypocotyl must reach a threshold level, with these level being unchanged in the 2-B and 1-B systems of pea seedlings. Following the “IAA threshold level” concept in GA-deficient plants, these levels appear to be different in 2-B and 1-B seedlings [26]. The most attractive idea is that the IAA-CK interaction in this case occurs without involving auxin from the shoots and is based not on the regulation of CKs synthesis by the amount of IAA but on the sensitivity to it. There are two facts which can support the involvement of GAs in the suggested IAA-CK interaction. Firstly, in potato cv. Désirée it has been shown that CKs can increase sensitivity to IAA by increasing the expression level of the potato auxin receptor genes, StTIR1a,b,c and StAFB4,6 [32] (Fig. 5). Secondly, sensitivity to CK under GA-deficient conditions may be enhanced by the fact that DELLA proteins RGA1-3 can increase the expression of type B ARR1 (Fig. 5), which has been shown in Arabidopsis root meristems [33]. Thus, there is a possibility of CK signaling activation through the DELLA proteins, and thus a theoretical possibility of active participation of CK signaling in auxin signaling. The above-mentioned hypothesis of GA-dependent drift of the “IAA threshold level” in the regulation of CKs synthesis requires further experimental confirmation.
So far, we have followed the hormonal model of CI regulation, but now there is more and more evidence about the importance of Sug as a kind of hormones and regulators of AD [14]. Most likely, the function of GAs in AD could be attributed to its role in increasing source-sink relation [34]. It is known that GA increase the sink the strength which is defined as the competitive ability of an organ (in our case this is the apical bud) to receive or attract assimilates [34] (Fig. 5). GAs stimulate nutrient transport, increasing phloem unloading, or acting on metabolism and compartmentalization of Suc [34], or activate growth through cell expansion and division that governs sink size [34]. Strong arguments in favor of the nutritive model of AD have been provided [14]. GAs affect sucrose-6-phosphate synthase enhancing Suc synthesis and thus is involved in phloem loading of sucrose and also can activates symplasmic supply routes through GA-inducible 1,3-β-glucanases that hydrolyze callose at sieve plates and plasmodesmata [35]. Further, apoplastic unloading is also under the influence of GA, through its role in increasing vacuolar or extracellular invertase activity [34, 36]. Thus, we should revisit the hormonal model of CI and AD in terms of the signaling of Sug, whose allocation is regulated by the sink-source relation and in whose regulation GA plays an important role by enhancing sink-strength and, consequently, stimulating the competitive relationships of the growing organs. Changes in the IAA/CK balance and the character of IAA-CK interaction may well be a secondary effect of GA-induced distribution of Sug, whose signaling influences the synthesis/signaling of auxins and CKs [14].
Recent studies have shown that Sug can both induce the synthesis of CKs and IAA [1, 14]. The possibility for the regulation of CK synthesis through nutritive mechanism can be supported by data in Arabidopsis where AtIPT3 gene expression was found to be able to up-regulate by Glu [18] (Fig. 5). Also in the rose Suc stimulated the expression of RhIPT3,5 and the accumulation of CKs in the nodes [14]. It has been reported that Suc feeding to detached stems of potato etiolated shoots promotes the accumulation of CKs [37]. As for IAA synthesis, Sug were found to regulate gene expression of AXR3/IAA17 [18] (Fig. 5), which is presumably involved in the control of IAA synthesis [17]. In nodal explants of roses, Suc in axillary buds upregulated early auxin synthesis genes, RhTAR1 and RhYUC1 [14] (Fig. 5). Suc activated the RhTAR1 and RhYUC1 genes possibly through the WRKY transcription factor SUCROSE SIGNALLING1 (RhSUSI1), orthologous to the barley sucrose-inducible transcription factor SUSIBA2 [14]. In the rose, RhSUSI1 is induced by Suc in the buds and is able to bind to the RhTAR1 gene promoter, activating its expression [14]. In Arabidopsis, Suc and CK increased an abundance of YUC8 and TAA1 which are direct targets of PIF4 [15, 16] (Fig. 5). Importantly, there are many evidences indicating that a positive feedback loop exists between CKs and Sug, when CKs in the growing organs attracts Sug which in turn up-regulate the local CK synthesis [1, 14, 37] (Fig. 5). Manipulation of the sink-size of the main pea shoot apex by removing part of the young leaves showed that removal of large leaflets auxin-independently increases the level of nodal CKs, which in turn correlated with the growth of nodal axillary buds [38]. Thus, the role of CK as a possible integrator of sink-source and auxin signals in the regulation of AD.
Crosstalk between CK and Sug signalings is an important issue in understanding the mechanism of CI and AD. In a short-term 3-hour experiment on detached cv. Adagumsky shoots, 1% Suc was shown to double the IEA, whereas 10 µM BA had no effect [25]. This result is questionable because in a long-term feeding experiment (2 days) on intact 2-B seedlings, 10 µM BA increased IEA 2-fold [25]. It is not yet possible to interpret these results unambiguously without additional experiments that will have to resolve the nature of the Sug-CK interaction, namely, whether CK action on IEA stimulation is mediated by activation of the Sug influx [37, 39] or Sug are capable of activating CK signaling leading to IEA stimulation [1] (Fig. 5).
It cannot be excluded, that the sink-source mechanism of CI might explain the mutual influence of lateral shoots at the level of their IEA in our 2-B model system acquiring an sink-source autonomy. However, comparing the two facts that Sug can promote IAA synthesis [14, 17] and that GA can increase the flow of Sug to growing organs [34, 35] and thus theoretically increase IAA levels, we come to a contradiction with our experimental data. On the contrary, GA-deficient cv. Porta shoots synthesize and export not less IAA, but even slightly more than after GA3 treatment (Fig. 4). This discrepancy can be resolved by the fact that GA deficiency in the tobacco plant everexpressed AtGA2-oxidase was accompanied by an increase in chlorophyll content and clearly higher PS II Fv/Fm quantum yield [40]. These results suggest that young shoots in GA-deficient plants may be self-sufficient with respect to photosynthetic products, which is the obvious reason for their lack of correlative interaction. Further study is needed to determine whether CI in 2-B pea plants is related to IAA-CK interaction or both CI and changes in IAA and CK content/transport could be attributed to a competition for available assimilates between growing shoots in the same plant.
REFERENCES
Kotov, A.A., Kotova, L.M., and Romanov, G.A., Signaling network regulating plant branching: recent advances and new challenges, Plant Sci., 2021, vol. 307, art. ID 110880. https://doi.org/10.1016/j.plantsci.2021.110880
Wang, L. and Zhang, Q., Boosting rice yield by fine-tuning SPL gene expression, Trends Plant Sci., 2017, vol. 22, p. 643. https://doi.org/10.1016/j.tplants.2017.06.004
Adamowski, M. and Friml, J., PIN-dependent auxin transport: action, regulation, and evolution, Plant Cell, 2015, vol. 27, p. 20.
Tanaka, M., Takei, K., Kojima, M., Sakakibara, H., and Mori, H., Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance, Plant J., 2006, vol. 45, p. 1028.
Rameau, C., Bertheloot, J., Leduc, N., Andrieu, B., Sakr, S., and Foucher, F., Multiple pathways regulate shoot branching, Front. Plant Sci., 2015, vol. 5, p. 741. https://doi.org/10.3389/fpls.2014.00741
Ligerot, Y., de Saint Germain, A., Waldie, T., Troadec, C., Citerne, S., Kadakia, N., Pillot, J.P., Prigge, M., Aubert, G., Bendahmane, A., Leyser, O., Estelle, M., Debellé, F., and Rameau, C., The pea branching RMS2 gene encodes the PsAFB4/5 auxin receptor and is involved in an auxin-strigolactone regulation loop, PLoS Genet., 2017, vol. 13, p. e1007089. https://doi.org/10.1371/journal.pgen.1007089
Miyawaki, K., Matsumoto-Kitano, M., and Kakimoto, T., Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate, Plant J., 2004, vol. 37, p. 128.
Müller, D., Waldie, T., Miyawaki, K., To, J.P., Melnyk, C.W., Kieber, J.J., Kakimoto, T., and Leyser, O., Cytokinin is required for escape but not release from auxin mediated apical dominance, Plant J., 2015, vol. 82, p. 874.
Azarakhsh, M., Kirienko, A.N., Zhukov, V.A., Lebedeva, M.A., Dolgikh, E.A., and Lutova, L.A., KNOTTED1-LIKE HOMEOBOX 3: a new regulator of symbiotic nodule development, J. Exp. Bot., 2015, vol. 66, p. 7181. https://doi.org/10.1093/jxb/erv414
Zhang, K., Wang, R., Zi, H., Li, Y., Cao, X., Li, D., Guo, L., Tong, J., Pan, Y., and Jiao, Y., AUXIN RESPONSE FACTOR3 regulates floral meristem determinacy by repressing cytokinin biosynthesis and signaling, Plant Cell, 2018, vol. 30, p. 324. https://doi.org/10.1105/tpc.17.00705
Duan, J., Yu, H., Yuan, K., Liao, Z., Meng, X, Jing, Y., Liu, G., Chu, J., and Li, J., Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice, Proc. Natl. Acad. Sci. U.S.A., 2019, vol. 116, p. 14319. https://doi.org/10.1073/pnas.1810980116
Ni, J., Zhao, M.L., Chen, M.S., Pan, B.Z., Tao, Y.B., and Xu, Z.F., Comparative transcriptome analysis of axillary buds in response to the shoot branching regulators gibberellin A3 and 6-benzyladenine in Jatropha curcas, Sci. Rep., 2017, vol. 7, p. 11417. https://doi.org/10.1038/s41598-017-11588-0
Roman, H., Girault, T., Barbier, F., Péron, T., Brouard, N., Pěnčík, A., Novák, O., Vian, A., Sakr, S., Lothier, J., Le Gourrierec, J., and Leduc, N., Cytokinins are initial targets of light in the control of bud outgrowth, Plant Physiol., 2016, vol. 172, p. 489. https://doi.org/10.1104/pp.16.00530
Barbier, F., Péron, T., Lecerf, M., Perez-Garcia, M.D., Barrière, Q., Rolčík, J., Boutet-Mercey, S., Citerne, S., Lemoine, R., Porcheron, B., Roman, H., Leduc, N., Le Gourrierec, J., Bertheloot, J., and Sakr, S., Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrid, J. Exp. Bot., 2015, vol. 66, p. 2569. https://doi.org/10.1093/jxb/erv047
Ljung, K., Nemhauser, J.L., and Perata, P., New mechanistic links between sugar and hormone signaling networks, Curr. Opin. Plant Biol., 2015, vol. 25, p. 130. https://doi.org/10.1016/j.pbi.2015.05.022
Di, D.W., Wu, L., Zhang, L., An, C.W., Zhang, T.Z., Luo, P., Gao, H.H., Kriechbaumer, V., and Guo, G.Q., Functional roles of Arabidopsis CKRC2/YUCCA8 gene and the involvement of PIF4 in the regulation of auxin biosynthesis by cytokinin, Sci. Rep., 2016, vol. 6, p. 36866. https://doi.org/10.1038/srep36866
Jones, B., Gunneras, S.A., Petersson, S.V., Tarkowski, P., Graham, N., May, S., Dolezal, K., Sandberg, G., and Ljung, K., Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction, Plant Cell, 2010, vol. 22, p. 2956.
Kushwah, S. and Laxmi, A., The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana, Plant, Cell Environ., 2014, vol. 37, p. 235. https://doi.org/10.1111/pce.12149
Brenner, W.G. and Schmülling, T., Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organspecific responses, BMC Plant Biol., 2012, vol. 12, p. 112.
Willige, B.C., Isono, E., Richter, R., Zourelidou, M., and Schwechheimer, C., Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana, Plant Cell, 2011, vol. 23, p. 2184. https://doi.org/10.1105/tpc.111.086355
Bai, F. and DeMason, D.A., Hormone interactions and regulation of Unifoliata, PsPK2, PsPIN1, and LE gene expression in pea (Pisum sativum) shoot tips, Plant Cell Physiol., 2006, vol. 47, p. 935.
Huang, F., Zago, M.K., Abas, L., van Marion, A., Galván-Ampudia, C.S., and Offringa, R., Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport, Plant Cell, 2010, vol. 22, p. 1129.
Salanenka, Y., Verstraeten, I., Löfke, C., Tabata, K., Naramoto, S., Glanc, M., and Friml, J., Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane, Proc. Natl. Acad. Sci. U.S.A., 2018, vol. 115, p. 3716.
Yoshida, H., Ueguchi-Tanaka, M., and Matsuoka, M., Regulatory networks acted upon by the GID-DELLA system after perceiving gibberellins, in The Enzymes, Vol. 35: Signaling Pathways in Plants, Mashida, Y., et al., Eds., Amsterdam: Elsevier, 2014, vol. 35, ch. 1, p. 1. https://doi.org/10.1016/B978-0-12-801922-1.00001-4
Kotov, A.A. and Kotova, L.M., Auxin–cytokinin interactions in the regulation of correlative inhibition in two-branched pea seedlings, J. Exp. Bot., 2018, vol. 69, p. 2967. https://doi.org/10.1093/jxb/ery117
Kotov, A.A. and Kotova, L.M., Correlative inhibition between branches in two-branched pea seedlings is cultivar-dependent, J. Plant Growth Regul., 2019, vol. 38, p. 132. https://doi.org/10.1007/s00344-018-9821-z
Morris, S.E., Turnbull, C.G.N., Murfet, I.C., and Beveridge, C.A., Mutational analysis of branching in pea. Evidence that Rms1 and Rms5 regulate the same novel signal, Plant Physiol., 2001, vol. 126, p. 1205.
Bennett, T., Hines, G., van Rongen, M., Waldie, T., Sawchuk, M.G., Scarpella, E., Ljung, K., and Leyser, O., Connective auxin transport in the shoot facilitates communication between shoot apices, PLoS Biol., 2016, vol. 14, p. e1002446. https://doi.org/10.1371/journal.pbio.1002446
Dun, E.A., de Saint Germain, A., Rameau, C., and Beveridge, C.A., Antagonistic action of strigolactone and cytokinin in bud outgrowth control, Plant Physiol., 2012, vol. 158, p. 487.
Leivar, P. and Monte, E., PIFs: systems integrators in plant development, Plant Cell, 2014, vol. 26, p. 56. https://doi.org/10.1105/tpc.113.120857
Shi, H., Liu, W., Wei, Y., and Ye, T., Integration of auxin/indole-3-acetic acid 17 and RGA-LIKE3 confers salt stress resistance through stabilization by nitric oxide in Arabidopsis, J. Exp. Bot., 2017, vol. 68, p. 1239. https://doi.org/10.1093/jxb/erw508
Kolachevskaya, O.O., Lomin, S.N., Arkhipov, D.V., and Romanov, G.A., Auxins in potato: molecular aspects and emerging roles in tuber formation and stress resistance, Plant Cell Rep., 2019, vol. 38, p. 681.
Moubayidin, L., Perilli, S., Dello Ioio, R., Di Mambro, R., Costantino, P., and Sabatini, S., The rate of cell differentiation controls the Arabidopsis root meristem growth phase, Curr. Biol., 2010, vol. 20, p. 1138.
Iqbal, N., Nazar, R., Khan, M.I.R., Masood, A., and Khan, N.A., Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions, Curr. Sci., 2011, vol. 100, p. 998.
Rinne, P.L., Paul, L.K., Vahala, J., Kangasjärvi, J., and van der Schoot, C., Axillary buds are dwarfed shoots that tightly regulate GA pathway and GA-inducible 1,3-β-glucanase genes during branching in hybrid aspen, J. Exp. Bot., 2016, vol. 67, p. 5975.
Rabot, A., Portemer, V., Péron, T., Mortreau, E., Leduc, N., Hamama, L., Coutos-Thévenot, P., Atanassova, R.S., and Le Gourrierec, J., Interplay of sugar, light and gibberellins in expression of Rosa hybrida vacuolar invertase 1 regulation, Plant Cell Physiol., 2014, vol. 55, p. 1734. https://doi.org/10.1093/pcp/pcu106
Salam, B.B., Barbier, F., Danieli, R., Teper-Bamnolker, P., Ziv, C., Spıíchal, L., Aruchamy, K., Shnaider, Y., Leibman, D., Shaya, F., Weissberg, M.C., Gal-On, A., Jiang, J., Ori, N., Beveridge, C., and Eshel, D., Sucrose promotes stem branching through cytokinin, Plant Physiol., 2021, p. 1. https://doi.org/10.1093/plphys/kiab003
Kotov, A.A. and Kotova, L.M., The contents of auxins and cytokinins in pea internodes as related to the growth of lateral buds, J. Plant Physiol., 2000, vol. 156, p. 438.
Roitsch, T. and Ehneb, R., Regulation of source/sink relations by cytokinins, Plant Growth Regul., 2000, vol. 32, p. 359.
Biemelt, S., Tschiersch, H., and Sonnewald, U., Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants, Plant Physiol., 2004, vol. 135, p. 254. https://doi.org/10.1104/pp.103.036988
ACKNOWLEDGMENTS
We thank Dr. Catherine Rameau (INRA Centre de Versailles-Grignon, France) very much for providing the seeds of pea wild-type cv. Porta.
Funding
The research was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (no. 121033000137-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving humans or animals performed by any of the authors.
Additional information
Abbreviations: 2-B and 1-B—two- and one-branched plants, respectively; AD—apical dominance; ARF—AUXIN RESPONSE FACTOR; Aux/IAA—AUXIN/INDOLE-3-ACETIC ACID INDUCIBLE protein, a transcriptional repressor of auxin-inducible genes; CCD7/MAX3/RMS5 and CCD8/MAX4/RMS1—carotenoid cleavage dioxygenases of Arabidopsis/pea; CI—correlative inhibition; CK—cytokinin; CKX—cytokinin oxidase; D14/AtD14/RMS3—rice/Arabidopsis/pea strigolactone receptor; D53/SMXL—SUPPRESSOR OF MAX2 1-LIKE protein, first target protein in strigolactone signaling; DELLA—a conserved DELLA domain in GA signaling proteins; IEA—IAA export activity; IPT—adenosine phosphate-ISOPENTENYLTRANSFERASE; LOG—Lonely Guy, enzyme which convert cytokinin nucleotides in free basis; MAX2/RMS4/D3—the Arabidopsis/pea/rice F-box protein, a component of SCF E3 ubiquitin ligase complex; PID—Ser/Thr kinase PINOID; PIF—PHYTOCHROME-INTERACTING FACTOR protein; PIN—membrane auxin efflux carriers of the family PINFORMED; PM—plasma membrane; RGA— REPRESSOR OF GA, DELLA protein; SEE—standard error of the estimate; SL—strigolactone; Sug—sugars; TAA/TAR—TRYPTOPHAN AMINOTRANSFERASE related protein; TIR1/AFB—TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F‑BOX PROTEIN, auxin receptor; type B RR—a positive type B RESPONSE REGULATOR of cytokinin response; X-CK—xylem cytokinin; YUC—YUCCA, flavin monooxygenase-like enzyme.
Rights and permissions
About this article
Cite this article
Kotov, A.A., Kotova, L.M. In What Way Do Gibberellins Increase Apical Dominance and Correlative Inhibition?. Russ J Plant Physiol 69, 22 (2022). https://doi.org/10.1134/S102144372202008X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S102144372202008X