Abstract
Late blight of potato (Solanum tuberosum L.) caused by the oomycete Phytophthora infestans (Mont.) de Bary was studied in respect to the disease resistance induced by Bacillus subtilis Cohn (strains 26D and 11VM) and B. thuringiensis Berliner (strains B-5351 and B-6066) bacteria. The content of hydrogen peroxide, activities of antioxidant (catalases and peroxidases) and hydrolytic (proteases and amylases) enzymes, together with the gene expression of inhibitors of hydrolases, as the parameters related to the resistance, were assessed. The 15-day-old plants of a cv. Rannyaya Rosa susceptible to late blight were derived from microtubers. The plants were sprayed with bacterial suspension (108 cells/mL) of one of the mentioned strains of B. subtilis or B. thuringiensis. After 5 days, part of the plants was inoculated with P. infestans zoospores (105 spores/mL). After 6, 24, or 48 h postinoculation, the plants were fixed for biochemical analyses. It was found that either B. subtilis or B. thuringiensis reduced the late blight severity on the potato leaves; the effect depended on the particular bacterial strain applied. This was apparently a consequence of H2O2 accumulation and increased expression of the genes encoding protease and amylase inhibitors. The transcriptional activity of the genes of hydrolase inhibitors was stimulated by B. subtilis and B. thuringiensis to different extents. This suggests the existence of different strain-dependent pathways of establishment of bacteria-induced potato resistance to P. infestans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Late blight is the most devastating disease of potato crop affecting almost any part of the plant: leaves, stems, tubers, flowers, and berries. The infection entails the losses, which are expressed in decrease in mass and quality of tubers, death of the infected vegetating shoots during tuber formation, and massive rot of the stored tubers. The causal agent of the disease is the oomycete Phytophthora infestans (Mont.) de Bary, which is a hemibiotrophic pathogen. It starts to behave as a biotroph at early stages of the disease and later turns to the necrotrophic manner of nutrition. The host-plant is inoculated with zoospores that penetrate through the lenticels, stomata, and wounds of the tissues. The zoosporangia can germinate to form the hyphae immediately infecting the plant or produce numerous zoospores capable of germination [1].
Making crops more robust against pathogens is an urgent task of contemporary plant growing. In this regard, the most prospective strategy includes microbiological approaches exploiting potentials of plants and soil-born microbes. The ecologically safe formulations, protecting plants from biotic and abiotic stresses, are often based on Plant Growth-Promoting Bacteria (PGPB) [2]. Such bacteria of the Bacillus genus appear to be especially promising because of their high efficiency, long-lasting vital capacity, and widespread natural antagonism to diverse phytopathogenic fungi. Many Bacillus-based bioformulations have been designed to protect plants from phytopathogens and are regarded as prospective agents controlling plant diseases and pests [3].
The microorganism-based bioformulations are peculiar in that they nonspecifically activate plant defense systems. Some representatives of Bacillus bacteria are known to carry out an endophytic lifestyle within plant tissues. They suppress growth and development of causal agents of plant diseases by means of secretable hydrolytic enzymes. In addition, their discharge of low-molecular-weight lipopeptides triggers the generation of hydrogen peroxide and activates oxylipin signaling defense system regulating activity of inhibitors of proteinases [4]. The crop-protecting action of the formulations based on Bacillus spp. may be mediated by H2O2, which is involved in the increased gene expression of PR-proteins. Presumably, Bacillus bacteria promote ROS generation that initiates transduction of the signals initiating, in turn, different defense responses. It was reported that plant treatment with Bacillus subtilis forwards an establishment of an induced systemic resistance (ISR), which is mediated by jasmonic acid [5]. Meanwhile, the bacteria of this genus can promote alternative systemic acquired resistance (SAR), which functions through the salicylate signaling pathway [6]. Despite a good deal of evidence on suppression of phytopathogens by Bacillus bacteria, the actual mechanism of the corresponding plant resistance remains unclear. It was suggested that the specificity of defense responses caused by Bacillus bacteria might depend on the nature of the particular producer organism [3].
The purpose of this work was to examine the effects of different strains of B. subtilis and B. thuringiensis on the content of hydrogen peroxide, the activities of antioxidative and hydrolytic enzymes, and the transcriptional activity of the genes of hydrolase inhibitors in potato plants in relation to the resistance of the plants to the late blight caused by the oomycete P. infestans.
MATERIALS AND METHODS
Objects. The plants of potato (Solanum tuberosum L.) were derived from microtubers of the cv. Rannyaya Rosa susceptible to late blight. The microtubers were planted into a container with TerraVita soil (Nord Palp, Russia) to a depth of 3–4 cm. The soil contained high-moor peat decomposed to different extents, purified bank sand, pearlite, complex mineral fertilizer, and biohumus (pH 6.0–6.5). The plants were grown for 15 days in a growth chamber before application of bacteria.
The bacterial cultures of Bacillus thuringiensis Berliner (B-5351 and B-6066 strains) were obtained from the All-Russia Collection of Industrial Microorganisms. The B. subtilis Cohn bacteria of the 26D strain were isolated from the commercial bioformulation Phytosporin-M (Bashinkom, Russia) and those of the 11VM strain were purchased from the collection of the Skryabin Institute of Biochemistry and Physiology of Microorganisms of Russian Academy of Sciences. The bacteria were maintained on a liquid Luria-Bertani medium for 72 h followed by dilution of the suspension with distilled water to a desired concentration.
The culture of the late blight causal oomycete (Phytophthora infestans (Mont.) de Bary, Bashkirskii isolate) was taken from the collection of the Institute of Biochemistry and Genetics.
Treatment of plants with bacteria, inoculation with pathogen, and assessment of disease symptoms. The plants were treated with a suspension culture of one bacterial strain (final titer of 108 cells/mL, 5 mL/plant). After 5 days, part of the plants was inoculated with a P. infestans spore suspension (106 spores/mL, 5 mL/plant).
The disease symptoms were quantitatively scored in 5 days postinoculation as the size of affected area (percentage of a total area of the lamina). The leaves were photographed, and their digital photo images were analyzed with the ImageJ program (National Institutes of Health, Bethesda, United States).
In 6, 24, and 48 h after the late blight inoculation, the control and infected plants were fixed in liquid nitrogen to be stored at –80°C until biochemical analyses.
Assay for hydrogen peroxide. The leaves were disrupted in 25 mM Na-phosphate buffer (PB), pH 6.2, at a 1 : 3 ratio (w/v), and the homogenate was centrifuged at 10 000g for 20 min in a 5415R centrifuge (Eppendorf, Germany). Content of H2O2 was assayed in the supernatant using xylenol orange dye [7]. The reagent contained 0.074% of Mohr’s salt in 5.81% sulfuric acid and 0.009% xylenol orange in 1.82% sorbitol at a 1 : 100 ratio. The optical density of the reaction products was measured at 560 nm at a Biospek-Mini spectrophotometer (Shimadzu, Japan).
Assay for catalase (CAT) activity. Plant tissue was homogenized in 50 mM PB, pH 7.8, at a 1 : 10 ratio (w/v). The homogenate was centrifuged at 12 000g for 10 min followed by measurements of the enzymatic activity [8]. The reaction was initiated by mixing 0.1 mL supernatant (water in the control) with 0.2 mL of 0.03% H2O2. After 10 min of the reaction, it was ceased by addition of 1 mL of 4% ammonium molybdate with a subsequent measurement of A410. The activity of catalase (EC 1.11.1.6) was calculated by the following formula:
where E is catalase activity (mol H2O2/L); Аwat and Аsup are optical densities of the samples containing water or supernatant, respectively; V is volume of the added sample (0.1 mL); t is incubation time (600 s); and K is molar extinction coefficient of the complex of H2O2 with ammonium molybdate (22.2 × 103 mol–1 cm–1).
Peroxidase (PO) activity was assayed in the cytoplasmic fraction. Leaf pieces were disrupted in 10 mM PB, pH 6.2, at a 1 : 3 ratio (w/v), and the homogenate was centrifuged at 12 000g for 25 min. The activity of peroxidase (EC 1.11.1.7) was determined in the supernatant by a micromethod [9] based on an oxidation of the PO substrate o-phenylenediamine. Optical density was read at a Benchmark Microplate Reader spectrophotometer (BioRad, United States) at 490 nm. The change in the optical density after 1 min of the reaction was taken as one unit of the enzymatic activity.
Estimating transcriptional activity of the genes of potato inhibitors of protease and amylase. Total RNA was isolated from the plants with TRIzol according to the protocol of the manufacturer (Molecular Research Center, United States). To prepare cDNA based on the m-RNA of the tested samples, the reverse transcription reaction was carried on using M-MuLV reverse transcriptase according to the manufacturer’s protocol (Sintol, Russia). The accumulation of transcripts of the genes of amylase inhibitor (GenBank number XM006351484) and those of amylase inhibitor (number JX683427) (Supplementary Materials, Table 1) were analyzed by a classic real-time PCR at an iCycler iQ5 Real-Time PCR Detection System (Bio-Rad, United States). The intercalating SYBR Green I dye (Sintol) was used. Changes in the transcriptional activity (number of mRNA copies of each gene) were derived from a level of normalized expression using the software of an iCycler iQ5 Real-Time Detection System (Bio-Rad). The histograms report the rates of gene expression as compared with the initial point representing the untreated control.
Activities of amylases and proteases were assayed by hydrolysis of starch or BSA, respectively [10]. These substrates, taken at 1% final concentration, were immobilized in 4% PAAG. The samples possessing the enzymatic amylolytic and proteolitic activities were applied onto PAAG and were incubated at 37°C for 20 min followed by staining with Lugol’s iodine or Coomassie G-250 solution, respectively. The enzymatic activities were determined densitometrically by calibration curves drawn using standard preparations of amylase from Aspergillus niger or bovine tripsin (Sigma, United States). The activities were expressed as µmol substrate/(g protein min). The protein content in the samples was estimated by Bradford.
Statistics. The experiments included five biological replications. The biochemical parameters and transcriptional activities were measured at least three times. The histograms represent sample means with their 95% confidence intervals. To estimate significance of sample means' differences, the analysis of variance followed by the Duncan’s multiple range test (at 95% reliability level) were carried out with the Statistica 13 program. The significantly differing means are indicated with different letters on the histograms.
RESULTS AND DISCUSSION
Effect of Different Strains of B. subtilis and B. thuringiensis on Resistance of Potato Plants to P. infestans Infection
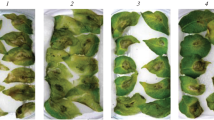
On the infected plants of the susceptible cv. Rannyaya Rosa, a difference was observed in a late blight severity between the untreated control and the plants pretreated with B. subtilis or B. thuringiensis bacteria (Fig. 1). The control leaves were affected by 65 ± 15%. This index was significantly lower in the bacteria-treated counterparts: down to 20 ± 11 and 28 ± 11%, respectively, for the B. subtilis 26D and 11VM strains. For the B. thuringiensis, B-6066 and B-5351 strains, the corresponding values were 36 ± 13 and 41 ± 15%. In general, the resistance of potato to late blight was promoted by all the tested strains but to different extent. B. subtilis 26D was the most efficient in agreement with the results obtained on tubers [11] and in vitro culture [12] of S. tuberosum.
Late blight severity on potato leaves pretreated with different strains of Bacillus subtilis or Bacillus thuringiensis and inoculated with Phytophthora infestans. (1) Untreated control; (2) B. subtilis 26D; (3) B. subtilis 11VM; (4) B. thuringiensis B-5351; (5) B. thuringiensis B‑6066. Different letters indicate significantly differing means.
Effect of Different Strains of B. subtilis and B. thuringiensis on Hydrogen Peroxide Content in Potato Plants Infected with P. infestans
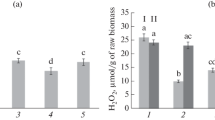
Some mechanisms of the Bacillus-induced potato resistance to late blight may be related to changes in the hydrogen peroxide level in plant tissues. We actually found that the H2O2 concentration progressively increased in the plants that were treated with B. subtilis but not inoculated with P. infestans. The plants treated with the B. subtilis 11VM and B. thuringiensis B-6066 strains demonstrated the largest difference from the control plants after 48 h postinoculation with the pathogen (Fig. 2).
Content of hydrogen peroxide in potato plants pretreated with different strains of B. subtilis or B. thuringiensis and/or inoculated with P. infestans. (1) Untreated uninoculated control; (2) B. subtilis 26D; (3) B. subtilis 11VM; (4) B. thuringiensis B-5351; (5) B. thuringiensis B-6066; (6) P. infestans; (7) B. subtilis 26D + P. infestans; (8) B. subtilis 11VM + P. infestans; (9) B. thuringiensis B-5351 + P. infestans; (10) B. thuringiensis B-6066 + P. infestans. Time after inoculation: (1) 6 h; (2) 24 h; and (3) 48 h. Different letters indicate significantly differing means corresponding to equal postinoculation time. Asterisks indicate significantly differing means corresponding to a counterpart of treatment but different postinoculation times.
The effect might be due to antistress properties of metabolites of B. subtilis. Endophyitic bacteria of the Bacillus genus are known to activate antioxidant enzymes of the plant [13]. Meanwhile, we revealed that the H2O2 level significantly increased in the potato leaves at the early stage of infection (6 h postinoculation) if the leaves had been pretreated with bacteria, especially B. subtilis 26D and B. thuringiensis B-6066 (Fig. 2). However, in 48 h postinoculation, this parameter was more or less uniform in the plants treated with different bacterial strains and did not differ from the untreated infected control.
The resistance of potato to P. infestans is determined to a large extent by a hypersensitive response. This implies changes in the hydrogen peroxide concentration in the plant tissues in response to the pathogen’s ingress. In this regard, H2O2 can be considered as an essential signaling agent that is involved in transduction of intracellular signals controlling gene expression and activation of defense systems of the plant. In turn, accumulating H2O2 increases cytosolic concentration of calcium ions fulfilling important functions of signal transduction into the plant genome [14]. Presumably, B. subtilis and B. thuringiensis bacteria favor the early ROS generation and initiate transmission of the signals triggering different defense systems. Bacteria of Bacillus genus elevate plant resistance to pathogens not only due to secretion of antibiotics but also because of the accumulation of phenols together with pro- and antioxidant enzymes in the infection site [15]. Apparently, PGPB sensitize plant tissues to penetration of pathogens and prepare the plant defense system to perform early defense reactions subsequently.
Effect of Different Strains of B. subtilis and B. thuringiensis on Activities of Antioxidant Enzymes in Potato Plants Infected with P. infestans
During pathogenesis, many metabolic processes can modulate the level of hydrogen peroxide in the plant tissues. To a large extent, these changes are associated with the changes in activities of antioxidant enzymes. Catalase is one of the essential constituents of an enzymatic antioxidant system [16]. In our experiments, healthy plants exhibited an enhanced CAT activity only after their treatment with B. subtilis 26D (Fig. 3). In the untreated infected plants, the activity decreased in the course of the infection process. In the plants pretreated with B. thuringiensis B-6066 or, especially, B. subtilis 11VM, the catalase activity significantly increased over the 24-h postinoculation period.
Activity of catalase in potato plants pretreated with different strains of B. subtilis or B. thuringiensis and/or inoculated with P. infestans. (1) Untreated uninoculated control; (2) B. subtilis 26D; (3) B. subtilis 11VM; (4) B. thuringiensis B-5351; (5) B. thuringiensis B-6066; (6) P. infestans; (7) B. subtilis 26D + P. infestans; (8) B. subtilis 11VM + P. infestans; (9) B. thuringiensis B-5351 + P. infestans; (10) B. thuringiensis B-6066 + P. infestans. Time after inoculation: (1) 6 h, (2) 24 h, and (3) 48 h. Different letters indicate significantly differing means corresponding to equal postinoculation time. Asterisks indicate significantly differing means corresponding to a counterpart of treatment but different postinoculation times.
CAT activity can be considerably modified by signaling molecules. In this regard, hydrogen peroxide is both signal and substrate for this enzyme. The influence of H2O2 on the activity of plant catalases is ambiguous. For example, peroxide, depending on its concentration, either inhibits [17] or stimulates [18] the CAT activity in wheat seedlings. Salicylic acid is also capable of CAT inhibition. This effect is involved in the hypersensitive reaction and can further activate the CAT gene expression and enhance the enzyme synthesis [19].
Another enzyme dealing with hydrogen peroxide is peroxidase. It is involved in both generation and elimination of H2O2. The chief functions of PO include protection of a plant organism from a harmful action of ROS and direct participation in the differentiation processes of tissues and organs of higher plants.
In our experiments with healthy plants, only the ones pretreated with B. subtilis 11VM manifested an activation of PO in comparison with the untreated control (Fig. 4). Inoculation with P. infestans elevated the PO activity as against the healthy counterparts after 24 h postinoculation. In the infected plants, all the tested bacterial strains of B. subtilis and B. thuringiensis increased the PO activity in comparison with the control as the inoculation with the pathogen alone.
Activity of peroxidase in potato plants pretreated with different strains of B. subtilis or B. thuringiensis and/or inoculated with P. infestans. (1) Untreated uninoculated control; (2) B. subtilis 26D; (3) B. subtilis 11VM; (4) B. thuringiensis B-5351; (5) B. thuringiensis B-6066; (6) P. infestans; (7) B. subtilis 26D + P. infestans; (8) B. subtilis 11VM + P. infestans; (9) B. thuringiensis B‑5351 + P. infestans; (10) B. thuringiensis B-6066 + P. infestans. Time after inoculation: (1) 6 h, (2) 24 h, and (3) 48 h. Different letters indicate significantly differing means corresponding to equal postinoculation time. Asterisks indicate significantly differing means corresponding to a counterpart of treatment but different postinoculation times.
The important trait of peroxidase is the ability of switching to catalase activity finally preventing accumulation of surplus H2O2. This feature was reported for several apoplastic forms of PO [20]. In addition, the extensive multigenic family of typical class III peroxidases of the plants participates in the cell wall reinforcement due to oxidative reactions between proteins and phenols. As a result, lignin sediments in the cell walls making them more resistant to pathogens’ hydrolases. In the potato plants interacting with Bacillus bacteria, the H2O2 level might be controlled by decrease in the CAT activity or by modulation of the PO activity.
Effect of Different Strains of B. subtilis and B. thuringiensis on Transcriptional Activity of the Genes of Inhibitors of Protease and Amylase and on Activities of These Enzymes in Potato Plants Infected with P. infestans
The essential feature of plant–pathogen interactions, allowing of an assessment of pathogenic effects, is represented by hydrolytic enzymes of the pathogen. They destroy the cell walls of the plants and promote its penetration into host’s tissues [21]. The plant responds to this attack by synthesis of inhibitors of such enzymes [22].
We found that treatments of potato plants with any tested strains of B. subtilis or B. thuringiensis bacteria, as well as an infection with P. infestans, stimulated accumulation of the transcripts of the amylase inhibitor gene in comparison with the control counterpart. In the infected plants pretreated with B. thuringiensis B-5351 or B-6066 strains, the transcription level of the amylase inhibitor gene was significantly higher than in the noninfected plants. The transcription activity of the protease inhibitor gene also increased, and the maximal effect occurred upon the treatment with B. thuringiensis B-6066 of either healthy or infected plants (Fig. 5). It seems that infection of potato with P. infestans may initiate de novo synthesis of the inhibitors capable of suppression of activities of amylase and proteinase enzymes. It is known that the content of hydrolase inhibitors rises in planta at the expense of synthesis of novel forms of inhibitors rather than accumulation of constitutive compounds [23]. The inhibitors of proteases and amylases are the most common in the plants [20].
Transcriptional activities of the inhibitor genes of amylase and protease in potato plants pretreated with different strains of B. subtilis or B. thuringiensis and/or inoculated with P. infestans. (1) Untreated uninoculated control; (2) B. subtilis 26D; (3) B. subtilis 11VM; (4) B. thuringiensis B-5351; (5) B. thuringiensis B-6066; (6) P. infestans; (7) B. subtilis 26D + P. infestans; (8) B. subtilis 11VM + P. infestans; (9) B. thuringiensis B-5351 + P. infestans; (10) B. thuringiensis B-6066 + P. infestans. Time after inoculation was 48 h. Different letters indicate significantly differing means corresponding to a particular gene.
Amylolytic activity is characteristic of most taxonomical groups of plant pathogens; these enzymes are almost always constitutive proteins. However, amylase is absent in oomycetes, including the Phytophthora genus. These pathogens use the corresponding enzymes of the host to decompose starch and activate their biosynthesis in the colonized plant tissues [24]. Presumably, the increased transcriptional activity of highly-specific amylase inhibitors, which is triggered by bacterial metabolites, hinders growth and development of P. infestans in the plant tissues.
Our studies revealed significantly lower activity of amylases in the healthy plants treated with B. thuringiensis B-5351 than in the control. Meanwhile, this parameter markedly increased after the potato treatment with B. subtilis 11VM (Fig. 6). In the infected plants, the protease activity significantly decreased if they had been pretreated with B. thuringiensis B-6066.
Activities of (1) amylases and (2) proteases in potato plants pretreated with different strains of B. subtilis or B. thuringiensis and/or inoculated with P. infestans. (1) Untreated uninoculated control; (2) B. subtilis 26D; (3) B. subtilis 11VM; (4) B. thuringiensis B-5351; (5) B. thuringiensis B-6066; (6) P. infestans; (7) B. subtilis 26D + P. infestans; (8) B. subtilis 11VM + P. infestans; (9) B. thuringiensis B-5351 + P. infestans; (10) B. thuringiensis B-6066 + P. infestans. Time after inoculation was 48 h. Different letters indicate significantly differing means corresponding to one particular enzyme.
The high proteolytic activity in the infected tissues not only supplies amino acids for growth and development of the pathogenic microbe but can also neutralize protective proteins of potato, such as lectins inhibiting hydrolases. Thus, the extracellular metal-proteinase of phytopathogenic bacterium Erwinia carotovora (Jones) Waldee cleaves lectin, which is involved in protection of potato [25]. Apparently, the increased gene transcriptional activity of the protease inhibitor aims at suppression of inhibitors of exogenous proteases and favors disease resistance in potato. The key role in initiation of formation of inhibitors of protective proteases belongs to the membrane receptor systemin—the protein of approximately 160 kDa molecular mass [26]. In response to wounding, it causes membrane depolarization accompanied by opening ionic channels and sharp increase in an intracellular content of calcium ions. These events lead to activation of MAP-kinase and phospholipase. Some associated reactions yield jasmonic acid, which possibly acts as an activator of gene transcription of defense proteins [27].
It is suggested that PGPB synthesize and secrete exometabolites that are specific to particular bacterial strain. These compounds comprise peptides with antibiotic properties and universal signaling molecules—ethylene, salicylic, and jasmonic acids [28]. For example, treatment of pepper plant with B. cereus BS107 confers them resistance to bacterial rot caused by Xanthomonas axonopodis pv. vesicatoria. The establishment of the resistance involves the genes of protective PR proteins, which are activated upon pathogenesis. Of them, PR-1 is inducible by salicylic acid, PR-4 and PR-10 by jasmonic acid and ethylene, while others are induced by H2O2 [29]. Treatment of tomato plants with B. subtilis BEB-DN stimulates the expression of some genes governing ISR. Among them, the genes of inhibitors suppressing proteases and enzymes of lignin synthesis are the most active [30].
Our results infer that the mobilization of defense systems afforded by several strains of Bacillus bacteria in potato plants depends on accumulation of hydrogen peroxide and activation of antioxidant enzymes and hydrolase inhibitors. The revealed difference in the rate of stimulation of transcriptional activities of the genes of hydrolase inhibitors caused by different strains of B. subtilis and B. thuringiensis suggests the existence of species- and strain-dependent pathways of induction of potato resistance to late blight.
REFERENCES
Novotel’nova, N.S., Pystina, K.A., and Golubeva, O.G., Fitoftorobye griby (Sem. Phytophthoraceae) (Phytophthora Fungi of Family Phytophthoraceae), Leningrad: Nauka, 1974.
Verma, P., Yadav, A.N., Kumar, V., Singh, D.P., and Saxena, A.K., Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crop improvement, in Plant-Microbe Interactions in Agro-Ecological Perspectives, Singh, D.P., Singh, H.B., and Prabha, R., Eds., Singapore: Springer-Verlag, 2017, p. 543.
Melent’ev, A.I., Aerobnye sporoobrazuyushchie bakterii Bacillus Cohn v agroekosistemakh (Aerobic Sporulating Bacteria Bacillus Cohn in Agricultural Ecosystems), Moscow: Nauka, 2007.
Yarullina, L.G., Kasimova, R.I., Kuluev, B.R., Surina, O.B., Yarullina, L.M., and Ibragimov, R.I., Comparative study of bunt pathogen resistance to the effects of fungicides in callus co-cultures Triticum aestivum with Tilletia caries, Agric. Sci., 2014, vol. 5, p. 906. https://doi.org/10.4236/as.2014.510098
Veselova, S.V., Nuzhnaya, T.V., and Maksimov, I.V., Role of jasmonic acid in interaction of plants with plant growth promoting rhizobacteria during fungal pathogenesis, in Jasmonic Acid: Biosynthesis, Functions and Role in Plant Development, Morrison, L., Ed., New York: Nova Science, 2015, ch. 3, p. 33.
Cawoy, H., Mariutto, M., Henry, G., Fisher, C., Vasilyeva, N., Thonart, P., Dommes, J., and Ongena, M., Plant defense stimulation by natural isolates of bacillus depends on efficient surfactin production, Mol. Plant Microbe Interact., 2014, vol. 27, p. 87. https://doi.org/10.1094/MPMI-09-13-0262-R
Bindschedler, L.V., Minibayeva, F., Gardner, S.L., Gerrish, C., Davies, D.R., and Bolwell, G.P., Early signaling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+, New Phytol., 2001, vol. 151, p. 185. https://doi.org/10.1046/j.1469-8137.2001.00170.x
Yarullina, L.G., Ibragimov, R.I., Tsvetkov, V.O., Yarullina, L.M., and Shpirnaya, I.A., Tsitokhimicheskie i biokhimicheskie metody issledovaniya mikroorganizmov–vozbuditelei boleznei rastenii: uchebnoe posobie (Cytochemical and Biochemical Study Methods of Microorganisms–Plant Pathogens: Manual), Ufa: Bashkir. Gos. Univ., 2016.
Maksimov, I.V., Sorokan’, A.V., Burkhanova, G.F., and Abizgildina, P.P., Regulation of peroxidase activity under the influence of signaling molecules and Bacillus subtilis 26D in potato plants infected with Phytophthora infestans, Appl. Biochem. Microbiol., 2014, vol. 50, p. 173. https://doi.org/10.1134/S0003683814020136
Tsvetkov, V.O., Shpirnaya, I.A., Maksutova V.O., and Ibragimov, R.I., Determination of amylase and protease activity using substrates immobilized in polyacrylamide gel, Izv. Ufimsk. Nauchn. Tsentra, Ross. Akad. Nauk, 2018, no. 3-5, p. 81.
Yarullina, L.G., Akhatova, A.R., and Kasimova, R.I., Hydrolytic enzymes and their proteinaceous inhibitors in regulation of plant–pathogen interactions, Russ. J. Plant Physiol., 2016, vol. 63, p. 193. https://doi.org/10.1134/S1021443716020151
Yarullina, L.G., Sorokan, A.V., Burkhanova, G.F., and Tsvetkov, V.O., Signal regulation of activity of protective proteins in potato plants in vitro with the defeat potato late blight, Theor. Appl. Ecol., 2019, vol. 4, p. 136. https://doi.org/10.25750/1995-4301-2019-4-136-141
Berg, G., Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture, Appl. Microbiol. Biotehnol., 2009, vol. 84, p. 11. https://doi.org/10.1007/s00253-009-2092-7
Chanda, B., Xia, Y., Mandal, M.K., Sekine, K.T., Gao, Q.M., and Selote, D., Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants, Nat. Genet., 2011, vol. 43, p. 421. https://doi.org/10.1038/ng.798
White, J.F. and Torres, M.S., Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol. Plant, 2010, vol. 138, p. 440. https://doi.org/10.1111/j.1399-3054.2009.01332.x
Mittler, R., Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 2002, vol. 7, p. 405. https://doi.org/10.1016/S1360-1385(02)02312-9
Bakalova, S., Nikolova, A., and Wedera, D., Isoenzyme profiles of peroxidase catalase and superoxide dismutase as affected by dehydration stress and ABA during germination of wheat seeds, J. Plant Physiol., 2004, vol. 30, p. 64.
Kolupaev, Yu.E. and Karpets, Yu.V., Formirovanie adaptivnykh reaktsii rastenii na deistvie abioticheskikh stressorov (Development of Adaptive Plant Reactions on the Effect of Abiotic Stress), Kyiv: Osnova, 2010.
Guan, L.M. and Scandalios, J.G., Hydrogen-peroxide-mediated catalase gene expression in response to wounding, Free Radical Biol. Med., 2000, vol. 28, p. 1182. https://doi.org/10.1016/S0891-5849(00)00212-4
Minibayeva, F., Kolesnikov, O., Chasov, A., Beckett, R.P., Lüthje, S., Vylegzhanina, N., Buck, F., and Bottger, M., Wound-induced apoplastic peroxidase activities: their roles in the production and detoxification of reactive oxygen species, Plant, Cell Environ., 2009, vol. 32, p. 497. https://doi.org/10.1111/j.1365-3040.2009.01944.x
Maksimov, I.V., Sorokan’, A.V., Cherepanova, E.A., Surina, O.B., Troshina, N.B., and Yarullina, L.G., Effects of salicylic and jasmonic acids on the components of pro/antioxidant system in potato plants infected with late blight, Russ. J. Plant Physiol., 2011, vol. 58, p. 299. https://doi.org/10.1134/S1021443711010109
Yarullina, L.G., Kasimova, R.I., Akhatova, A.R., Maksimov, I.V., Ibragimov, R.I., and Umarov, I.A., Qualitative and quantitative changes of potato tuber proteome under the influence of signal molecules and infection with Phytophthora infestans, Appl. Biochem. Microbiol., 2016, vol. 52, p. 71. https://doi.org/10.1134/S0003683816010154
Lastochkina, O.V., Aliniaeifard, S., Seifikalhor, M., Yuldashev, R., Pusenkova, L., and Garipova, S., Plant growth-promoting bacteria: biotic strategy to cope with abiotic stresses in wheat, in Wheat Production in Changing Environments. Responses, Adaptation and Tolerance, Hasanuzzaman, M., Nahar, K., and Hossain, A., Eds., Singapore: Springer-Verlag, 2019, p. 579. https://doi.org/10.1007/978-981-13-6883-7_23
Gappa-Adachi, R., Yano1, K., Takeuchi, S., Morita, Y., and Uematsu, S., Phytophthora blight of southern star (Oxypetalum caeruleum) caused by Phytophthora palmivora in Japan, J. Gen Plant Pathol., 2012, vol. 78, p. 39. https://doi.org/10.1007/s10327-011-0351-9
Feng, T., Nyffenegger, C., Højrup, P., Vidal-Melgosa, S., Yan, K., Fangel, J.U., Meyer, A.S., and Kirpekar, F., Characterization of an extension-modifying metalloprotease: N-terminal processing and substrate cleavage pattern of Pectobacterium carotovorum, Appl. Microbiol. Biotechnol., 2014, vol. 98. P. 10077. https://doi.org/10.1007/s00253-014-5877-2
Gancheva, M.S., Malovichko, Y.V., Poliushkevich, L.O., Dodueva, I.E., and Lutova, L.A., Plant peptide hormones, Russ. J. Plant Physiol., 2019, vol. 66, p. 171. https://doi.org/10.1134/S1021443719010072
Vasyukova, N.I. and Ozeretskovskaya, O.L., Jasmonate-dependent defense signaling in plant tissues, Russ. J. Plant Physiol., 2009, vol. 56, p. 581. https://doi.org/10.1134/S102144370905001X
Schoonbeek, H.-J., Jacquat-Bovet, A.C., Mascher, F., and Métraux, J.P., Oxalate-degrading bacteria can protect Arabidopsis thaliana and crop plants against Botrytis cinerea, Mol. Plant Microbe Interact., 2007, vol. 20, p. 1535. https://doi.org/10.1094/MPMI-20-12-1535
Yang, J.W., Yu, S.H., and Ryu, C.-M., Priming of defense related genes confers root-colonizing Bacilli-elicited induced systemic resistance in pepper, Plant Pathol. J., 2009, vol. 25, p. 389. https://doi.org/10.5423/PPJ.2009.25.4.389
Valenzuela-Soto, J.H., Estrada-Hernández, M.G., Laclette, E.I., and Délano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development, Planta, 2010, vol. 231, p. 397. https://doi.org/10.1007/s00425-009-1061-9
ACKNOWLEDGMENTS
The equipment of Biomika Center of Collective Use (Department of Biochemical Research and Nanobiotechnology of the Agidel Regional Center of Collective Use) and that of the CODINK Unique Scientific Installation was used.
Funding
The work was partially carried out through State Task no. AAA-A21-121011990120-7 with support of the Russian Foundation for Basic Research and the Belarusian Republican Foundation for Fundamental Research (project no. 20-516-00005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by A. Aver’yanov
Abbreviations: CAT—catalase; ISR—induced systemic resistance; PB—phosphate buffer; PGPB—plant growth-promoting bacteria; PI—proteinase inhibitors; PO—peroxidase; PR-proteins—pathogenesis-related proteins; SAR—systemic acquired resistance.
Supplementary Information
Rights and permissions
About this article
Cite this article
Yarullina, L.G., Tsvetkov, V.O., Burkhanova, G.F. et al. Effect of Bacillus Bacteria on Hydrogen Peroxide Content and Gene Expression of Hydrolase Inhibitors in Potato Plants Infected with Phytophthora infestans (Mont.) de Bary. Russ J Plant Physiol 68, 1257–1264 (2021). https://doi.org/10.1134/S1021443721060194
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721060194