Abstract
The joint effect of Bacillus subtilis 26D endophytic bacteria and chitooligosaccharides (COSs) on the resistance of potato plants (Solanum tuberosum L.) to the late blight causative agent Phytophthora infestans (Mont.) De Bary was studied. A twofold decrease in the area of late-blight lesions on potato leaves was revealed during the joint presowing treatment of minitubers with B. subtilis bacteria (108 cells/mL) with COSs (1 mg/L). Treatment with COSs was found to have similar protective effect on potato plants, but this was not observed with the use of only bacteria. The mechanisms of increased potato-plant resistance to P. infestans were associated with the activation of catalase, peroxidase, and hydrolases (amylase and protease) inhibitors, the accumulation of hydrogen peroxide, and transcripts of genes encoding PR proteins: amylase inhibitor, basic protective protein (PR-1), chitinase (PR-3), protease inhibitor (PR-6), peroxidase (PR-9). The revealed activation of the gene expression of the main antimicrobial protein PR-1 (a marker of the development of systemic acquired resistance) and PR-6 (a marker of the development of induced systemic resistance) under the influence of combined treatment with B. subtilis and COSs indicates that the development of protective reactions in potato plants to the late blight pathogen in this case occurs synergistically, with the participation of various signaling pathways, in which B. subtilis prime protective genes, and COSs act as a trigger for their expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The primary task of crop production at present is the use of effective plant-protection products that are safe for the environment and humans. Around the world, there is a growing success for this direction in its competition with chemical plant-protection products [1]. It is assumed that biological preparations activate the physiological and biochemical processes involved in the formation of a nonspecific plant response to external stress. Thus, plant treatment with preparations of nonpathogenic rhizobacteria increases the sensitivity of genes involved in systemic acquired resistance (SAR) or induced systemic resistance (ISR) during subsequent infection with pathogens [2].
Biological preparations based on the endophytic bacteria Bacillus subtilis, which suppress the growth and development of pathogens and stimulate plant growth and their resistance to unfavorable environmental factors, are widely used for plant protection [3]. The increase in plant resistance to pathogens under the influence of such preparations is associated with the ability of B. subtilis bacteria to produce various metabolites with antiviral, antibacterial, and antifungal effects [4]. It has been shown that the lipopeptides produced by bacteria as signaling molecules promote the development of ISR as a result of transduction of H2O2 generation and the lipoxygenase-signaling cascade [5].
In this regard, it is very important to increase the effectiveness of microbiological preparations to protect food crops from various phytopathogens. It has been shown that chitin derivatives are compatible with microbiological preparations, enhancing and prolonging their action [6]. Chitosan and chitooligosaccharides (COSs) are active elicitors of plant immunity that are used to increase the biological preparation activity [7]. It is assumed that COSs bind to specific receptors on cell membranes, launching the trigger mechanisms of genome activation [8]. Among them, the genes responsible for the synthesis of regulatory proteins involved in transcription and information transmission are characterized by a rapid and high level of expression [9]. As elicitors of plant-defense reactions, COSs are involved in the induction of ROS accumulation, including hydrogen peroxide [10], in the synthesis of antibiotics [11] and hydrolytic and antioxidant enzymes [12].
Late blight, which is caused by the oomycete Phytophthora infestans (Mont) de Bary, is one of the most common potato diseases in Russia, and the fight against it is currently an important task. It has been shown that metabolites of B. subtilis [13], like COSs [14], reduce the degree of damage to Solanum tuberosum by the late-blight pathogen P. infestans. Understanding the mechanisms of the formation of a protective plant response by biological preparations in combination with elicitors may significantly increase the effectiveness of environmentally friendly approaches on potato protection and expand the range of their application.
The purpose of the present work was to study the inducing effect of B. subtilis bacteria in combination with COSs on the formation of defense reactions in potato plants upon infection with the late-blight pathogen.
MATERIALS AND METHODS
Object of study. The experiments were performed on Solanum tuberosum potato plants (Chishminskaya experimental station of the Bashkir Research Institute of Agriculture, Ufa, Russia) grown from microtubers of the susceptible variety Rannyaya rosa. The tubers were planted in containers with soil (TerraVita, Nord Pulp, high-moor peat of various degrees of decomposition, purified river sand, perlite, complex mineral fertilizers, vermicompost, pH of 6.0–6.5) to a depth of 3–4 cm. The plants were grown on a light platform with a photoperiod of 16 h (illumination 8000–10 000 lux) at 20–22°C.
B. subtilis 26D bacteria from the collection of the Institute of Biochemistry and Genetics of the Ufa Federal Research Center of the Russian Academy of Sciences (Ufa, Russia) were cultivated in lysogeny-broth (LB) medium for 24 h, and the suspension was then diluted with distilled water to the required concentration.
A culture of P. infestans oomycete from the collection of the Institute of Biochemistry and Genetics of the Ufa Federal Research Center of the Russian Academy of Sciences (Ufa, Russia) was used to infect the plants. The pathogen was grown on potato dextrose agar for 7 days after reisolation from infected potato minitubers to restore the aggressiveness of the pathogen. The surface of P. infestans isolate colonies was covered with distilled water and kept at 4°C for 30 min. The sporangium concentration was assessed with a Fuchs-Rosenthal Counting Chamber. The spore suspension was diluted to a titer of 1 × 105 spore/mL.
Tuber treatment prior to planting. The tuber surfaces were sterilized, washed with running water, and dried. Some of them were sprayed with a suspension of B. subtilis 26D (108 cells/mL), a solution of COSs (1 mg/mL), or a mixture of bacteria with COSs (2 mL per 1 microtuber). In the control, microtubers were treated with distilled water. In accordance with method described in [15], COSs with an average molecular weight of 7.5 kDa and a degree of acetylation of 65% were obtained in the laboratory.
Fifteen days after germination, some of the plants were sprayed with 5 mL of P. infestans spore suspension with 1 × 105 spore/mL. Untreated plants and plants not infected with late blight, as well as untreated and infected plants (for comparison with infected samples) were used as control plants. The H2O2 content and the activity of catalase, peroxidase, hydrolytic enzymes, and their inhibitors, as well as the transcriptional activity of PR protein genes, were determined in the leaves 24 and 72 h after infection. The development of the disease was assessed by the percentage of the affected area of the leaf blade (level of damage) on the seventh day after plant infection with P. infestans. The leaves were photographed, and the images were analyzed with the ImageJ software (National Institutes of Health (NIH), United States).
Determination of H2O2 content. To determine H2O2 content, we used the modified method described in [16]. The leaves were homogenized in a mortar at 4°C in 25 mM phosphate buffer (PB) with a pH of 6.2 in a 1 : 3 ratio and centrifuged for 10 min at 10 000 g and 4°C with a 5415R microcentrifuge (Eppendorf, Germany). The H2O2 content in the supernatant was determined with xylenol orange. The reagent contained 0.074% Mohr’s salt (Fe2(NH4)2SO4 (99.997% purity) in 5.81% sulfuric acid and 0.009% xylenol orange in 1.82% sorbitol (in a ratio of 1 : 100). The reaction mixture was incubated for 40 min at room temperature, and the optical density was then measured at 560 nm with a LS 55 fluorescent spectrometric cell (Perkin Elmer, United States) against a control containing water instead of the sample. The concentration of hydrogen peroxide was determined with a previously constructed calibration curve.
Determination of the catalase activity (CAT). To determine the catalase activity (EC 1.11.1.6) with the modified method described in [17], the plant tissue was homogenized at 4°C in 50 mM PB (pH 7.8) in a ratio of 1 : 10. After centrifugation at 10 000 g and 4°C in a 5415R microcentrifuge (Eppendorf, Germany), the supernatant was used to analyze the enzyme activity. The reaction was initiated by the addition of the supernatant to 65 mM hydrogen peroxide in 50 mM PB (pH 7.8), and the mixture was incubated at room temperature for 2 min. The reaction was stopped by the addition of 32.4 mM ammonium molybdate. Distilled water was added to the control sample instead of the supernatant. The intensity of the developed color was measured on a Perkin Elmer LS 55 spectrophotometer at 410 nm. The catalase activity was calculated with the formula U = (Ac – Ae)/(KVT), where Ac and Ae are the absorption of the control (containing water instead of the sample) and experimental samples, respectively; V is the sample volume, 0.1 mL; T is the incubation time, 600 s; and K is the coefficient of H2O2 molar absorption equal to 22.2 × 103 mol–1 cm–1. The CAT activity was expressed in units/mg protein.
Determination of peroxidase activity (PA). The modified method described in [18] was used to determine the peroxidase activity (EC 1.11.1.7). The leaves were homogenized at 4°C in 10 mM PB, at a pH of 6.2. The ratio of the mass of the leaf sample to the volume of PB was 1 : 3. The homogenate was centrifuged for 20 min at 10 000 g and 4°C with a 5415R centrifuge. The peroxidase activity of the supernatant was determined with the micromethod of oxidation of the substrate with 20 mM orthophenylenediamine with 10 mM hydrogen peroxide. The color development was stopped with 4 N H2SO4. The absorbance of the solution was measured at 490 nm with a Perkin Elmer LS 55 spectrophotometer. The unit of enzyme activity was the change in the absorbance of the solution in 1 min. The PA activity was expressed in units/mg protein.
Activity of amylases, proteases, and their inhibitors. The activity of amylases, proteases, and their inhibitors was determined by the rate of hydrolysis of immobilized starch and BSA, respectively [19]. Enzyme substrates with a final concentration of 1% were immobilized in 4% polyacrylamide gel (PAG). Solutions with enzymatic activity were applied to PAG, incubated for 20 min at 37°C, and then stained with Lugol’s solution or Coomassie G-250. The enzyme activity was determined via densitometry with calibration curves constructed with standard preparations of Aspergillus niger amylase and bovine trypsin (Sigma, United States). The enzymatic activity was expressed in μmol of the substrate/g protein min. During the determination of the inhibitory activity, the inhibitor preparations were added to standard solutions of enzymes. The inhibitory activity was determined as the amount of change in enzymatic activity.
Determination of protein content. The protein content in the samples was determined according to the Bradford method [20], with BSA as a standard.
Determination of the transcriptional activity of genes of PR-proteins. Plant RNA was isolated with the use of Trizol (Molecular Research Center, Inc., United States). A weighed portion of the leaves was homogenized in liquid nitrogen. To obtain cDNA based on the mRNA of the studied samples, a reverse transcription reaction was performed with M-MuLV reverse transcriptase according to the manufacturer’s protocol. Analysis of the accumulation of the transcripts of the genes PR-1 (GenBank accession no. AY050221), PR-3 (GenBank accession no. U49970), PR-5 (GenBank accession no. AY737317), PR-6 (GenBank accession no. JX683427), PR-9 (GenBank accession no. M21334), and the gene of the amylase inhibitor (GenBank accession no. XM006351484) was performed via quantitative real-time polymerase chain reaction (PCR) with SYBR Green I dye (Synthol, Russia) and the CFX Connect Real-Time System (Bio-Rad, United States). The cDNA was diluted fivefold and used directly as a template.
The changes in the gene transcriptional activity (the number of mRNA copies for each gene) were assessed relative to the reference gene St_act (housekeeping gene, actin, GenBank number X55749) with the CFX Connect Real-Time System software (Bio-Rad, United States). The data were analyzed with the Lasergene software package (DNASTAR, Inc, United States).
Statistical processing. The experiments were carried out in five biological replicates for biochemical parameters and 15 for transcriptional activity. The histograms show the sample means and their 95% confidence intervals. The differences in the studied parameters were analyzed with the Kruskal–Wallis test in the Statistica 8 software (Statsoft, United States). Reliably different values are denoted by different letters.
RESULTS AND DISCUSSION
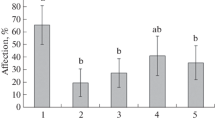
Influence of B. subtilis and COSs on potato plant resistance to infection by P. infestans and the H2O2 content. Analysis of the development of the late-blight pathogen on potato leaves showed that the presowing treatment of potato tubers with B. subtilis suspension, COSs, and their mixture favorably affected the protective potential of plants (Figs. 1 and 2a). In the control, the level of leaf damage was 85 ± 7%. Pretreatment of plants with bacteria reduced leaf infestation to 70 ± 5%, while the use of a combination of B. subtilis and COSs led to a decrease to 50 ± 6%. The results showed that the addition of COSs to bacteria significantly increased the plant tissue resistance to infection with the late-blight pathogen. It was previously shown that the addition of chitin to bacteria of the genus Bacillus increased the resistance of cotton plants to wilt [21] and strawberries to powdery mildew [22].
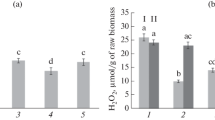
Level of infestation of potato leaves (a,% of the leaf blade area) and the H2O2 content (b) 24 h after infection with P. infestans: 1, control; 2, treatment with B. subtilis; 3, COSs; 4, B. subtilis + COSs. I, uninfected; II, plants infected with P. infestans. Reliably different values are denoted by different letters.
It is known that the earliest response of a plant organism to the introduction of a pathogen is the generation of ROS, an oxidative burst that triggers a cascade of subsequent defense reactions. It is associated with an increase in the concentration of free radicals (superoxide \({\text{O}}_{2}^{ - }\), hydroxyl OH·) and hydrogen peroxide in plant tissues. It has been shown that H2O2 is involved in the initiation of the hypersensitivity reaction and lignification processes and possesses antimicrobial activity [23]. The mechanisms of increased potato resistance to P. infestans infection caused by the bacteria B. subtilis in combination with COSs can also be associated with an increase in the H2O2 content in plant tissues [24]. Studies have shown that the H2O2 content was lower in all treatment options for uninfected plants than in the control (Fig. 2b). This was probably due to the ability of Bacillus bacteria to induce the activity of antioxidant enzymes [25]. In infected plants treated with B. subtilis 26D, COSs, and B. subtilis + COS, the H2O2 concentration in the leaves increased markedly as compared to the infected control plants. In the variant of treatment with bacteria in combination with COSs, the level of hydrogen peroxide in the leaves of infected potato plants increased by two times in comparison with the control already 24 h after infection (Fig. 1b, 4).
It is known that potato resistance to the late-blight pathogen P. infestans is largely determined by the development of the hypersensitive response (HR). This implies changes in the concentration of H2O2 in plant tissues in response to the introduction of a pathogen. Therefore, H2O2 can be considered the most important molecule involved in the transmission of intracellular signals that regulate gene expression and the activation of plant defense systems. An increase in the H2O2 level causes an increase in the concentration of calcium ions in the cytosol, which play an important role in the transmission of signaling information to the plant genome [26]. As elicitors, COSs contribute to the generation of H2O2 during the development of defense reactions to pathogens with different types of trophicity [14]. It is known that chitin, chitosan, and their oligomers are active immunostimulants. Combined treatment with the bacteria B. subtilis and COSs probably promoted the formation of earlier and more intense defense reactions after contact with the pathogen due to the rapid accumulation of hydrogen peroxide at the initial stages of the infectious process.
Influence of B. subtilis and COSs on the activity of antioxidant enzymes in potato leaves during P. infestans infection. The change in the H2O2 concentration in plant tissues during infection occurs mainly as a result of changes in the activity of antioxidant enzymes. The most important antioxidant enzyme is catalase (CAT). In all variants, a decrease in CAT activity was observed in uninfected plants as compared to control plants (Fig. 3a). A significant increase in CAT activity was observed over 24 h in infected plants pretreated with B. subtilis in combination with COSs (Fig. 3a, 4).
It is known that the CAT activity can be significantly modified by hydrogen peroxide, which is not only a signaling molecule but also a CAT substrate. At the same time, this effect on CAT activity in plants is ambiguous. For example, H2O2, depending on the concentration, either inhibited [27] or stimulated the activity of CAT in wheat seedlings [28].
An enzyme involved in both the generation and utilization of H2O2 is peroxidase (PO). The main function of PO is protection of the plant organism from oxidative stress, as well as direct participation in lignification processes.
In the present work, the PO activity was lower in all variants of pretreatment than that in the control variant 24 h after the inoculation in uninfected plants (Fig. 3b). It should be noted that only treatment with B. subtilis in combination with COSs caused an increase in PO activity in infected plants as compared to the infected control.
An important feature of PO is the ability to switch to catalase activity, which prevents the formation of excess H2O2. This phenomenon, in particular, was revealed for several forms of apoplastic peroxidases [29]. The addition of COSs to the B. subtilis culture may have promoted an earlier and more intense accumulation of H2O2 in infected plant tissues. In this case, both CAT and PO were involved in the regulation of the H2O2 content in potato plants at the early stages of the infectious process.
Influence of B. subtilis and COSs on the activity of hydrolases and their inhibitors in potato plants during P. infestans infection. The main instrument of the influence of the pathogen on plants is the hydrolytic enzymes, which destroy plant cell walls and ensure tissue penetration [30]. The plant defense response is accompanied by the synthesis of inhibitors of these enzymes [31].
As shown in Fig. 4, an increase in the activity of amylases and proteases occurs within 24 h in infected potato plants (Fig. 4, 1). In all treatment variants, the activity of amylases and proteases in leaves of uninfected plants also significantly increased or did not differ from the control experiment. It should be noted that the activity of both amylases and proteases decreased in infected plants pretreated with bacteria and COSs, especially when treated with a combination of B. subtilis and COSs, as compared to the infected control (Fig. 4, 4).
It is known that amylolytic activity is characteristic of representatives of most taxonomic groups of plant pathogens and that these enzymes are almost always represented by constitutive proteins. However, amylase is absent in oomycetes, in particular, in representatives of the genus Phytophthora, which use potato enzymes to break down starch, thereby activating their biosynthesis in affected tissues [32]. It can be assumed that a decrease in the level of amylases under the action of bacterial metabolites and COSs prevents the growth and development of P. infestans in plant tissues.
The high proteolytic activity in infected tissues not only provides amino acids for the growth and development of pathogenic microorganisms but can also neutralize the protective proteins of potatoes, such as lectins (hydrolase inhibitors). Thus, it was shown that the extracellular metalloproteinase of the phytopathogenic bacterium Erwinia carotovora (Jones) Waldee cleaves potato lectin, which is involved in plant protection [33].
Studies have shown that the activity of amylase inhibitors decreased in infected untreated plants (Fig. 5a, 1), which is a probable reason for the high level of amylases in infected potato tissues (Fig. 4a, 1). A similar tendency was typical of protease inhibitors in infected, untreated plants (Fig. 5b, 1). However, in plants pretreated and infected with P. infestans, the activity of amylase and protease inhibitors was higher than that in untreated and infected control plants. The most significant differences were characteristic for the combination of B. subtilis with COSs (Figs. 5, 4). This suggests that enzyme inhibitors can be synthesized in potato plants de novo in response to P. infestans infection, which can suppress the activity of amylases and proteases [31].
Activity of (a) amylase and (b) protease inhibitors in potato leaves infected with P. infestans (1, control), treated with Bacillus subtilis 26D (2), COSs (3), and B. subtilis + COSs (4). I, uninfected; II, plants infected with P. infestans. Reliably different values are denoted by different letters.
Influence of B. subtilis and COSs on the transcriptional activity of PR-protein genes in potato plants during P. infestans infection. The activation of plant defense reactions after contact with pathogens can occur through various signaling pathways, which is expressed in a change in the expression levels of genes encoding PR proteins. It is known that the protective effect of preparations based on Bacillus bacteria is due to the triggering of ISR [34], the developmental marker of which is the expression of the PR-6 gene (protease inhibitor).
However, the formation of resistance to pathogens under the influence of Bacillus bacteria can also develop as SAR, the developmental marker of which is the expression of the PR-1 gene [35]. As shown in Fig. 6a, infection and treatment with B. subtilis, COSs, and their mixture stimulated the accumulation of PR-1 gene transcripts, and the PR-1 gene was most intensely expressed in infected plants.
Influence of B. subtilis 26D (2) and COSs (3) and B. subtilis + COSs (4) on the relative number of transcripts of PR proteins: PR-1 (main protective protein), PR-3 (chitinase), PR-5 (thaumatin-like protein), PR-6 (protease inhibitor), PR-9 (peroxidase), Ai (amylase inhibitor) in uninfected plants (I) and plants infected with P. infestans (II) 24 h after infection (1, control). The transcriptional activity was measured relative to the reference gene St_act (housekeeping gene, actin). Reliably different values are denoted by different letters.
It should be noted that treatment with bacteria in combination with COSs, as well as treatment with COSs, had a significant effect on the increase in the expression level of the chitinase (PR-3) gene in infected plants (Fig. 6b). Among the extracellular hydrolases that can lyse the cell wall and hyphae of fungi, chitinases are of the greatest interest [12]. In most cases, chitinases are inducible enzymes, which are formed in the presence of a specific substrate. In our studies, the combination of bacteria with COSs promoted an increase in the transcriptional activity of the chitinase gene. It was shown that the expression of the PR-3 gene was significantly higher in potato cultivars resistant to Pectobacterium carotovorum than in susceptible plants [36].
The expression of the thaumatin-like protein (PR-5) gene was significantly increased in infected plants treated only with B. subtilis bacteria (Fig. 6). It is assumed that the activity of proteins of the PR-5 family is associated with an increase in membrane permeability [37]. It was shown that the strain B. cereus BS107 affected the formation of pepper plant resistance to bacterial rot Xantomonas axonopodis pv. vesicatoria and that genes PR-1, PR-4, and PR-10 were involved in this process [38].
It should be noted that a high level of transcriptional activity of the PR-6 protease inhibitor gene was observed in infected plants pretreated with COSs and a combination of bacteria with COSs (Fig. 6b, 3 and 4). It was shown that the treatment of tomato plants with the B. subtilis strain BEB-DN led to an increase in the expression of PR-6 and lignin synthesis enzymes genes [39]. It is believed that the induction of plant resistance mediated by bacteria that stimulate growth is not based on the direct activation of the expression of PR-protein genes but develops by means of priming, through the generation of ROS and redox-sensitive transcription factors and genes of PR-proteins [40]. This was confirmed in the present work.
Treatment with bacteria, COSs, and their mixture, as well as infection with P. infestans, reduced the accumulation of transcripts of amylase inhibitor gene in potato plants in comparison with infected control plants (Fig. 6). It is interesting that when infected plants were cotreated with both B. subtilis and COSs, the level of transcriptional activity of the amylase inhibitor gene increased by more than two times as compared to the control (Fig. 6a, 4). This probably provided an increase in the activity of amylase inhibitors in plant tissues (Fig. 5a, 4).
An increase in the content of hydrolase inhibitors in a plant usually occurs not due to an increase in the concentration of constitutive compounds but due to the synthesis of new forms of inhibitors [28]. In our experiments, the accumulation of transcripts of genes of protease and amylase inhibitors and an increase in the activity of their protein product in potato leaves after tuber treatment with the mixture of B. subtilis and COSs resulted in the suppression of the activity of exogenous hydrolases, which contributed to an increase in potato resistance to P. infestans.
In infected plants treated with B. subtilis bacteria in combination with COSs, the expression of the peroxidase gene (PR-9) significantly increased (Fig. 6). Plant peroxidases play a key role in protecting plants from pathogens, participating in the synthesis of antimicrobial compounds, and strengthening the plant cell wall via the formation of lignin, which correlates with their resistance [39].
CONCLUSIONS
Thus, combined treatment with B. subtilis 26D and COSs led to a decrease in the level of infestation of potato leaves by the causative agent of late blight. The mechanisms of increased resistance of potato plants to P. infestans are associated with the activation of catalase, peroxidase, hydrolases (amylase and protease) inhibitors, the accumulation of hydrogen peroxide and transcripts of genes encoding PR-proteins: amylase inhibitor, basic protective protein (PR-1), chitinase (PR-3), protease inhibitor (PR-6), peroxidase (PR-9). The revealed activation of the expression of the main antimicrobial proteins PR-1 (a marker of the development of systemic acquired resistance) and PR-6 (a marker of the development of induced systemic resistance) genes under the influence of combined treatment with B. subtilis and COSs indicated that the development of protective reactions in potato plants to the pathogen causing late blight in this case occurred synergistically, with the participation of various signaling pathways in which B. subtilis primed protective genes and COSs acted as a trigger for their expression.
REFERENCES
Zalila-Kolsi, I., Ben Mahmoud, A., Hacina, A., Sellami, S., et al., Microbiol. Res., 2016, vol. 192, pp. 148–158.
Burkhanova, G.F., Veselova, S.V., Sorokan’, A.V., Blagova, D.K., Nuzhnaya, T.V., and Maksimov, I.V., Appl. Biochem. Microbiol., 2017, vol. 53, no. 3, pp. 346–352. https://doi.org/10.18699/VJ19.561
Verma, P., Yadav, A.N., Kumar, V., Singh, D.P., and Saxena, A.K., in Plant–Microbe Interactions in Agro-Ecological Perspectives, Singh, D.P., Singh, H.B., and Prabha, R., Eds., Singapore: Springer, 2017, vol. 2, pp. 543–580.
Berg, G., Appl. Microbiol. Biotehnol., 2009, vol. 84, pp. 11–18. https://doi.org/10.1007/s00253-009-2092-7
Yarullina, L.G., Kasimova, R.I., Kuluev, B.R., Surina, O.B., Yarullina, L.M., and Ibragimov, R.I., Agricult. Sci., 2014, vol. 5, pp. 906–912. https://doi.org/10.4236/as.2014.510098
Pavlyushin, V.A., Tyuterev, S.L., Popova, E.V., Novikova, I.I., Bykova, G.A., and Domnina, N.S., Biotekhnologiya, 2010, no. 4, pp. 69–80.
Krasnobaeva, I.L., Kovalenko, N.M., and Popova, E.V., Vestn. Zashchity Rast., 2020, vol. 103, no. 4, pp. 233–240.
Yin, H., Li, Y., Zhang, H.Y., Wang, W.X., Lu, H., et al., Int. J. Plant Sci., 2013, vol. 174, no. 4, pp. 722–732. https://doi.org/10.1086/669721
Rush, T.A., Puech-Pages, V., Bascaules, A., et al., Nat. Commun., 2020, vol. 11, p. 3897. https://doi.org/10.1038/s41467-020-17615-5
Kawano, T., Sahashi, N., Takahashi, K., Uozumi, N., and Muto, S., Plant Cell Physiol., 1998, vol. 39, no. 7, pp. 721–730. https://doi.org/10.1093/oxfordjournals.pcp.a029426
Wang, X.Q., Zhao, D.L., Shen, L.L., Jing, C.L., and Zhang, C.S., in Role of Rhizospheric Microbes in Soil, Meena, V.S., Ed., Singapore: Springer, 2018, vol. 1, pp. 225–250. https://doi.org/10.1007/978-981-10-8402-7-9
Zhuravleva, N.V. and Luk’yanov, P.A., Vestn. Dal’nevost. Otd. Ross. Akad. Nauk, 2004, vol. 3, pp. 76–86.
Yanchevskaya, T.G., Grits, A.N., Kolomiets, E.I., Romanovskaya, T.V., Yarullina, L.G., Ibragimov, R.I., and Tsvetkov, V.O., Appl. Biochem. Microbiol., 2018, vol. 54, no. 3, pp. 324–330. https://doi.org/10.1134/S0003683818030158
Yarullina, L.G., Sorokan’, A.V., Burkhanova, G.F., Cherepanova, E.A., and Maksimov, I.V., Appl. Biochem. Microbiol., 2018, vol. 54, no. 5, pp. 528–534. https://doi.org/10.1134/S0003683818050174
Maksimov, V.I. and Smirnova, Yu.V., Biotekhnologiya, 1993, no. 10, pp. 26–30.
Jiang, Z.Y., Woollard, A.C.S., and Wolff, S.P., FEBS Lett., 1990, vol. 268, pp. 69–71.
Hadwan, M.H. and Abed, H.N., Data Brief, 2016, vol. 6, pp. 194–199. https://doi.org/10.1016/j.dib.2015.12.012
Fornera, S. and Walde, P., Anal. Biochem., 2010, vol. 407, no. 2, pp. 293–295. https://doi.org/10.1016/j.ab.2010.07.034
Tsvetkov, V.O., Shpirnaya, I.A., Maksutova, V.O., and Ibragimov, R.I., Izv. Ufimsk. Nauchn. Tsentra Ross. Akad. Nauk, 2018, nos. 3–5, pp. 81− 85.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, nos. 1–2, pp. 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Rajendran, L. and Samiyappan, R., Plant Pathol. J., 2008, vol. 7, no. 1, pp. 1–12. https://doi.org/10.3923/ppj.2008.1.12
Abdel-Kader, M.M., El-Mougy, N.S., Aly, M.D., and Lashin, S.M., Int. J. Agric. For., 2012, vol. 2, no. 2, pp. 8–48. https://doi.org/10.5923/j.ijaf.20120202.07
Bolwell, G.P., Bindschedler, L.V., Blee, K.A., Butt, V.S., Davies, D.R., Gardner, S.L., Gerrish, C., and Minibayeva, F., J. Exp. Bot., 2002, vol. 53, no. 372, pp. 1367–1376. PMID: 11997382
Pfannschmidt, T., Brautigam, K., Wagner, R., Dietzel, L., and Schroter, Y., Ann. Bot., 2009, vol. 103, pp. 599–607. https://doi.org/10.1093/aob/mcn081
White, J.F. and Torres, M.S., Physiol. Plant., 2010, vol. 138, pp. 440–446. https://doi.org/10.1111/j.1399-3054.2009.01332.x
Galvez-Valdivieso, G., Fryer, M.J., Lawson, T., Slattery, K., Truman, W., Smirnoff, N., et al., Plant Cell, 2009, vol. 21, pp. 2143–2162. https://doi.org/10.1105/tpc.108.061507
Bakalova, S., Nikolova, A., and Wedera, D., J. Plant Physiol., 2004, vol. 30, pp. 64–77. https://doi.org/10.1556/AAgr.56.2008.2.1
Luna, C.M., Pastori, G.M., Driscoll, S., Groten, K., Bernard, S., and Foyer, C.H., J. Exp. Bot., 2005, vol. 56, pp. 417–432. https://doi.org/10.1093/jxb/eri039
Minibayeva, F., Kolesnikov, O., Chasov, A., Beckett, R.P., Luthje, S., Vylegzhanina, N., Buck, F., and Bottger, M., Plant, Cell Environ., 2009, vol. 32, p. 497. https://doi.org/10.1111/j.1365-3040.2009.01944.x
Maksimov, I.V., Sorokan’, A.V., Cherepanova, E.A., Surina, O.B., Troshina, N.B., and Yarullina, L.G., Russ. J. Plant Physiol., 2011, vol. 58, no. 2, pp. 299–306.
Yarullina, L.G., Kasimova, R.I., Ibragimov, R.I., Akhatova, A.R., Umarov, I.A., and Maksimov, I.V., Appl. Biochem. Microbiol., 2016, vol. 52, no. 1, pp. 71–78. https://doi.org/10.1134/S0003683816010154
Gappa-Adachi, R., Yano, K., Takeuchi, S., Morita, Y., and Uematsu, S., J. Gen. Plant Pathol., 2012, vol. 78, p. 39. https://doi.org/10.1007/s10327-011-0351-9
Feng, T., Nyffenegger, C., Hojrup, P., Vidal-Melgosa, S., Yan, K., Ulrik, FangelJ., Meyer, A.S., and Kirpekar, F., Prt. Appl. Microbiol. Biotechnol., 2014, vol. 98, no. 24, p. 10077. https://doi.org/10.1007/s00253-014-5877-2
Wang, T., Liang, Y., Wu, M., Chen, Z., et al., Chin. J. Chem. Eng., 2015, vol. 23, no. 4, pp. 744–754. https://doi.org/10.1016/j.cjche.2014.05.020
Gimenez-Ibanez, S. and Solano, R., Front. Plant Sci., 2013, vol. 4, no. 72. https://doi.org/10.3389/fpls.2013.00072
Tret'yakova, O.M. and Evtushenkov, A.I., Tr. BGU, 2011, vol. 6, no. 1, pp. 163–167.
Vasyukova, N.I. and Ozeretskovskaya, O.L., Russ. J. Plant Physiol., 2009, vol. 56, no. 5 pp. 581–590.
Yang, J.W., Yu, S.H., and Ryu, C.M., Plant Pathol. J., 2009, vol. 25, no. 4, pp. 389–399. https://doi.org/10.5423/PPJ.2009.25.4.389
Valenzuela-Soto, J.H., Estrada-Hernandez, M.G., Laclette, E.I., and Delano-Frier, J.P., Planta, 2010, vol. 231, pp. 397–410. https://doi.org/10.1007/s00425-009-1061-9
Martinez-Medina, A., Flors, V., Heil, M., Mauch-Mani, B., Corne, M.J., Pieterse, C.M.J., et al., Trends Plant Sci., 2016, vol. 21, pp. 818–822. https://doi.org/10.1016/j.tplants.2016.07.009
ACKNOWLEDGMENTS
The work was performed on the equipment of the Biomics Center for Collective Use (Department of Biochemical Research and Nanobiotechnology of the Agidel Regional Center for Collective Use) and the Unique Scientific Installation Kodink.
Funding
The work was performed partially as a government task (state registration number AAAA-A21-121011990120-7), with the financial support of the Russian Foundation for Basic Research and the Belarusian Republican Foundation for Fundamental Research within the framework of scientific project no. 20-516-00005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Bulaev
Rights and permissions
About this article
Cite this article
Yarullina, L.G., Burkhanova, G.F., Tsvetkov, V.O. et al. Stimulation of the Protective Mechanisms of Solanum tuberosum by the Bacteria Bacillus subtilis and Chitooligosaccharides upon Infection with Phytophthora infestans. Appl Biochem Microbiol 58, 166–174 (2022). https://doi.org/10.1134/S0003683822020168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822020168