Abstract
The ability to accumulate nickel (Ni) was compared in hyperaccumulator Noccaea сaerulescens F.K. Mey and excluder Thlaspi arvense L. after a short-term (1, 2, or 3 days) and long-term (8 weeks) exposure. T. arvense and four accessions of N. сaerulescens (La Calamine (LC), Saint Félix de Palliéres (SF), Monte Prinzera (MP), and Lellingen (LE)) were grown on a half-strength Hoagland`s solution in the presence of 25 µM Ni(NO3)2 (N. сaerulescens and T. arvense) and 250 µM Ni(NO3)2 (N. сaerulescens; T. arvense for only 1–3 days). Metal content in the roots and shoots was determined by atomic absorption spectroscopy. The Ni content per unit mass in the roots and shoots of N. сaerulescens in most cases did not differ significantly after the short-term incubation. At 25 µM Ni in the nutrient solution, its content in the roots of LC plants after 2–3 days of incubation was lower than in T. arvense, whereas Ni content in the shoots of these plants was similar. In the plants of other accessions of N. сaerulescens, Ni content in the roots and shoots in most cases was higher than in T. arvense. At 250 µM Ni, the differences in metal content in the roots were insignificant, and its content in the shoots in all the accessions of the hyperaccumulator was much higher than in the excluder. The Ni translocation factor was higher in N. сaerulescens than in T. arvense and exceeded unity only in the plants of MP accession. After the long-term exposure, the Ni translocation factor was higher than 1 in plants of all accessions of N. сaerulescens and decreased in the following order: MP ≈ LC > LE ≥ SF; in T. arvense, it did not exceed 0.3. Upon both long-term and short-term exposure, the ability to accumulate Ni by N. сaerulescens plants of different accessions generally increased in the following order: LC < SF < LE < MP. However, minor changes were observed depending on the duration of exposure and Ni concentration in the medium. Thus, considerable differences in the ability to accumulate Ni among the plants of different accessions of hyperaccumulator N. сaerulescens became apparent as early as during the first days of exposure to Ni and hardly depended on the duration of incubation or metal concentration in the medium. The obtained data confirm the assumption about a constitutive or genetically predetermined ability of plants of different N. сaerulescens accessions to accumulate Ni.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
One of the important objectives of current ecological plant physiology is the investigation of the mechanisms responsible for plants’ ability to selectively accumulate metals. The present-day classification recognizes two contrasting groups of plants: excluders where metals are chiefly accumulated in the root system and accumulators that accumulate them predominantly in the above-ground organs [1]. Among accumulators, there is a group of hyperaccumulating species in which the content of zinc (Zn) or manganese (Mn) exceeds 1% of the shoot dry weight; the content of nickel (Ni), cobalt (Co), copper (Cu), or selenium (Se) is above 0.1% of the shoot dry weight; or the content of cadmium (Cd) or arsenic (As) exceeds 0.01% of the shoot dry weight [2]. Altogether, approximately 500 species of hyperaccumulators are presently known; the majority of them are hyperaccumulators of Ni belonging to the families Asteraceae, Brassicaceae, Buxaceae, Euphorbiaceae, Flacourtiaceae, Rubiaceae, and Violaceae, which are capable of accumulating in the shoots more than 1000 mg Ni/kg dry weight [2–4].
Hyperaccumulator plants combine high tolerance to one or several metals with the ability to accumulate them in the shoots, with the metal content in the shoots of these species manifold exceeding the metal contents in the root systems of the excluders or in the environment [2, 4]. It is assumed that tolerance to metals and ability to hyperaccumulate them are under independent genetic control [3, 5, 6], and the genes responsible for hyperaccumulation and tolerance are not species-specific; more likely, they are differentially expressed in hyperaccumulators and excluders [3, 7].
The ability to accumulate metals in plant shoots arose repeatedly in the course of evolution not only in different families of angiosperms [8] but also in different tribes and genera within the same family. For instance, the ability to accumulate Ni evolved at least six times in the family Brassicaceae, the richest in hyperaccumulator species, and the ability to hyperaccumulate Zn evolved at least thrice [4]. Hyperaccumulator Noccaea сaerulescens (formerly Thlaspicaerulescens) from the Brassicaceae family is considered a model species for the investigation of metal hyperaccumulation [6, 9–11] and is widely used for the studies of physiological, genetic, ecological, and evolutionary aspects of this phenomenon [3, 4, 7]. In spite of the monophyletic origin of all the representatives of genus Noccaea [10], the ability of different hyperaccumulator species from this genus to accumulate Ni, Zn, and Cd may considerably differ, which can largely account for their different metal tolerance [4, 12]. Noccaea goesingensis is a hyperaccumulator of Ni, N. praecox hyperaccumulates Zn, and N. сaerulescens is capable of accumulating three metals: Ni, Zn, and Cd, which is a rare case among the representatives of metallophyte flora [3, 4].
Not only different species of hyperaccumulators but also different accessions of the same hyperaccumulator may considerably vary in the ability to accumulate metals and in tolerance to their toxic effect [6, 13–19]. Taking into consideration that different accessions belong to the same species, it is important to understand the reasons for their varying tolerance and ability to accumulate metals. In many respects, such differences may be associated with the evolution of certain populations of the same species on different types of soil [20]. Under natural conditions, the greatest content of Cd and Zn is often observed in the populations growing on calamine soils rich in Zn, Cd, and Pb, while the highest levels of Ni are often observed in the populations from serpentine soils rich in Ni, Со, and Cr [6]. In earlier experiments performed in hydroponics, we showed that N. caerulescens plants of Lellingen (LЕ) accession originating from non-metalliferous soils accumulated more Zn but were less tolerant than the plants of accessions Saint Félix de Palliéres (SF) and La Calamine (LC) originating from calamine soils. At the same time, the plants of accession Monte Prinzera (MP) from serpentine soils were notable not only for a much higher tolerance to Ni than other examined accessions but also for its highest accumulation [18]. The same as in the case of Ni and Zn, LС plants accumulated less Cd; this probably partially accounted for their higher tolerance to Cd as compared with other accessions. Plants of SF accession also originating from calamine soils showed not only a high tolerance to Cd but also its greatest accumulation, which is probably associated with more efficient mechanisms of Cd detoxification [19]. The obtained results point to different mechanisms of and reasons for metal tolerance in the plants of different accessions of N. caerulescens, and close correlation between Ni and Zn content in N. сaerulescens plants [6, 21] confirms the hypothesis that transport pathways of these metals in the plant are non-specific.
In numerous papers published earlier and summarized in reviews [2–4, 7, 12, 22–24], the content of metals was usually determined in plants growing in natural habitats or in laboratory conditions after a long-term exposure to respective metal salts. Different researchers obtained numerous results; however, it is not known how fast hyperaccumulators can accumulate metals and whether or not the differences between the accessions remain upon a short-term and long-term exposure to the metals. These issues must be clarified in order to corroborate the hypothesis that representatives of different accessions of the same hyperaccumulator species possess a constitutive ability to accumulate certain metals. Therefore, the main goal of this work was to compare the ability to accumulate Ni by plants of different accesisons of hyperaccumulator N. сaerulescens and excluder Thlaspi arvense, a widespread ruderal species from the family Brassicaceae, upon a short-term or long-term exposure of plants to different Ni concentrations (25 and 250 µM).
MATERIALS AND METHODS
Plant material and growing conditions. The seeds of hyperaccumulator Noccaea сaerulescens F.K. Mey (formerly Thlaspi caerulescens J. & C. Presl) were collected from natural or their derived laboratory populations: accessions La Calamine (LC, Belgium) and Saint Félix de Palliéres (SF, France) originated from calamine soils rich in Zn, Cd, and Pb; accession Monte Prinzera (MP, Italy) originated from serpentine soils rich in Ni, Со, and Cr; and accession Lellingen (LE, Luxemburg) originated from non-metalliferous soils. The seeds of hyperaccumulator N. сaerulescens and excluder Thlaspi arvense L. were germinated in Petri dishes on filter paper moistened with tap water during two weeks at 20°С in a dark thermostat. The seedlings were transferred to 1-L pots (four seedlings per pot) with half-strength Hoagland`s solution and grown for eight weeks in a climate chamber (23/18°C day/night, 14-h-long light period). The Hoagland`s solution consisted of the following components: KNO3 (3 mM); Ca(NO3)2 (2 mM); NH4HPO4 (1 mM); MgSO4 (0.5 mM); KCl (1 µM); H3BO3 (25 µM); ZnSO4 (2 µM); MnSO4 (2 µM); CuSO4 (0.1 µM); (NH4)6Mo7O24 (0.1 µM); Fe(Na)EDTA (20 µM). The pH of the medium was adjusted to 5.25 using MES(2 mM)/KОН [18]. Nutrient solution was renewed once a week. The plants were grown in the presence of 25 µM Ni(NO3)2 (N. сaerulescens and T. arvense) and 250 µM Ni(NO3)2 (N. сaerulescens) during eight weeks. Control plants were grown on half-strength Hoagland`s solution without Ni. After 8‑week-long growing, some control plants of N. сaerulescens and T. arvense were transferred to half-strength Hoagland`s solution supplemented with Ni(NO3)2 at the concentrations of 25 or 250 µM and incubated for 1, 2, or 3 more days. The concentrations of Ni(NO3)2 had been selected in previous experiments with plants growing under similar conditions [18].
Determination of nickel content. The content of Ni was determined in the roots, leaf petioles, and leaf blades. Before the analysis, the roots were desorbed in 20 mM EDTA for 10 min at room temperature and then rinsed in distilled water. Plant material was collected after eight weeks of growing, dried at 80°С for 24 h in a drying oven to the fixed weight and weighed. Samples dried to the fixed weight were subjected to wet digestion at 140°С for 7 h in Teflon bombs after adding the mixture of 65% HNO3 and 37% HCl (4 : 1, v/v). Quantitative analysis was carried out in accordance with a standard procedure using a Perkin Elmer 1100В atomic absorption spectrophotometer (Perkin Elmer, the Netherlands). The assays were performed in triplicate. On the basis of the obtained data, we calculated the translocation factor: the ratio between Ni content in the shoots and roots.
Statistical analysis. The experiments were repeated three times. Each replication comprised three plants per type of treatment. Quantitative data were statistically analyzed using one-way ANOVA. The data are shown as the means and their standard errors.
RESULTS
By the end of the experiment, the plants had a developed rosette without signs of chlorosis or necrosis at all the types of treatment.
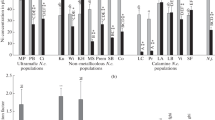
After the short-term incubation, the content of Ni per unit mass in the roots and shoots of N. сaerulescens in most cases did not show considerable differences (Figs. 1a–1c, 2a–2c). In LE plants after 2–3 days of incubation at 25 µM Ni(NO3)2 (Figs. 1b, 1c) and in SF and LC plants after 1–2 days of exposure to 250 µM Ni(NO3)2 (Fig. 2b), the Ni content was higher in the roots than in the shoots.
Ni content (mg/kg dry wt) in the roots (1), petioles (2) , and leaf blades (3) of the excluder Thlaspi arvense (Т.а.) and four accessions of the hyperaccumulator Noccaea сaerulescens (N.c.) (MP, LE, SF, and LC) after 1 (a), 2 (b), 3 (c) days, and 8 weeks (d) of incubation on half-strength Hoagland`s solution supplemented with 25 µM Ni(NO3)2. Significantly different values of Ni content are designated with different letters (at P < 0.05, one-way ANOVA). The analysis was carried out separately for the roots, petioles, and leaf blades. Significant differences between Ni contents in the roots and shoots are marked with asterisks: * at P < 0.05; ** at P < 0.01; and *** at P < 0.001 (one-way ANOVA).
Ni content (mg/kg dry wt) in the roots (1), petioles (2), and leaf blades (3) of the excluder Thlaspi arvense (Т.а.) and four accessions of the hyperaccumulator Noccaea сaerulescens (N.c.) (MP, LE, SF, and LC) after 1 (a), 2 (b), 3 (c) days, and 8 weeks (d) of incubation on half-strength Hoagland`s solution supplemented with 250 µM Ni(NO3)2. Significantly different values of Ni content are designated with different letters (at P < 0.05, one-way ANOVA). The analysis was carried out separately for the roots, petioles, and leaf blades. Significant differences between Ni contents in the roots and shoots are marked with asterisks: * at P < 0.05; ** at P < 0.01; and *** at P < 0.001 (one-way ANOVA).
At a low Ni concentration in the nutrient solution, the content of the metal in the roots of LC plants after 2–3 days of incubation was lower than in the excluder T. arvense, and the content of Ni in the shoots of these plants was similar (Figs. 1b, 1c). At the same time, the Ni content in the roots and shoots of plants of other accessions under study was much higher than in T. arvense (Figs. 1a–1c), except for SF plants, wherein the content of Ni in the roots on the second and third days of incubation did not significantly differ from its level in the roots of T. arvense. At a high concentration, the differences in the content of Ni in the roots were marginal, whereas the Ni content in the shoots of the hyperaccumulator of all the accessions under study was much higher than in the excluder (Figs. 2a–2c). The Ni content per unit mass in the leaf blades in most cases was lower than in the petioles. However, there were no significant differences between Ni contents in leaf perioles and leaf blades in MP plants after 1–3 days of incubation at both Ni concentrations (Figs. 1a–1c, 2a–2c) or in LC plants on the third day and in SF plants on the second and third days of exposure to 25 µM Ni(NO3)2 (Figs. 1b, 1c). After the long-term incubation, the content of Ni in the leaf blades of N. сaerulescens was higher than in the petioles and roots (Figs. 1d, 2d).
At both concentrations (25 and 250 µM) of Ni(NO3)2, the Ni content increased by the third day of incubation both in the roots and shoots of the excluder and hyperaccumulator as compared with that after 1-day-long incubation (Figs. 1a–1c, 2a–2c). The LC plants were an exception; therein, at a concentration of 25 µM Ni(NO3)2, the content of metal per unit mass did not change significantly in the roots and leaf petioles and increased in the leaf blades (Figs. 1a–1c).
Upon a short-term exposure, the translocation factor (the ratio between Ni content in the shoots and roots) was reliably higher than 1 only in N. сaerulescens, accesison MP, which grows under natural conditions on serpentine soils rich in Ni (Fig. 3). In N. сaerulescens plants of other accessions, naturally growing on calamine soils rich in Zn, Cd, and Pb (SF and LC) and on non-metalliferous soils (LE), the Ni translocation factor was lower than 1 but higher than in the excluder T. arvense, except for the SF and LC plants on the second day of incubation at 250 µM Ni(NO3)2. Upon a long-term incubation, the values of Ni translocation factor considerably increased in the plants of all the accessions and especially in LC plants. After 8-week-long exposure, the Ni translocation factor was higher than 1 in plants of all the examined accessions of N. сaerulescens and decreased in the following order: MP ≈ LC > LE ≥ SF. Under all the types of treatment, the lowest values of the translocation factor were observed in the excluder T. arvense (Fig. 3).
The ratio between Ni content in the shoots and roots (translocation factor) in the excluder Thlaspi arvense (Т.а.) and four accessions of the hyperaccumulator Noccaea сaerulescens (N.c.) (MP, LE, SF, and LC) after 1 (1), 2 (2), 3 (3) days, and 8 weeks (4) of incubation on half-strength Hoagland’s solution supplemented with 25 (a) or 250 μM (b) Ni(NO3)2. Only plants of the hyperaccumulator N. сaerulescens were grown in the presence of 250 µM Ni(NO3)2 for 8 weeks since this Ni concentration was toxic for the excluder upon a long-term exposure. Significantly different values of translocation factor are designated with different letters (at P < 0.05, one-way ANOVA). The analysis was carried out separately for each time point.
The obtained data point to considerable differences in the rate of and ability to accumulate Ni not only between the excluder and the hyperaccumulator but also among different accessions of the hyperaccumulator manifesting already during the first days of incubation. After both short-term and long-term incubation, the highest Ni content was found in the MP plants (Figs. 1, 2). As early as after 3-day-long incubation at 250 µM Ni(NO3)2, the content of metal in the shoots and roots of plants belonging to this accession exceeded 1000 mg/kg dry weight (Fig. 2c). The most pronounced increase in Ni accumulation with time was also observed in the MP plants upon the short-term incubation (Figs. 1a–1c, 2a–2c). For N. сaerulescens plants of different accessions, the ability to accumulate Ni generally increased in the following order: LC < SF < LE < MP; however, the results of the statistical analysis show that minor changes may occur depending on the duration of incubation and concentration of Ni in the medium (Figs. 1, 2).
DISCUSSION
Our results obtained in this and other works [15, 16, 18, 19], as well as the data of other researchers [6, 13, 14, 17], clearly show that not only excluders and hyperaccumulators but also the plants of the same species belonging to different accessions may considerably differ in their ability to accumulate metals. The plants from all the accessions of N. сaerulescens can accumulate Zn, whereas hyperaccumulation of Ni more often occurs in plant accessions which evolved on serpentine soils rich in Ni, Со, and Cr [6, 18, 25]. The content of Ni in MP plants that grow in natural conditions on serpentine soils was much higher than in plants of accessions SF and LC originating from calamine soils rich in Zn, Cd, and Pb (Figs. 1, 2). The obtained data confirm the hypothesis that the ability to accumulate certain metals developed in plants of different accessions in the course of continued evolution on a certain type of soil [20] and that it depends on the level of available metal in the soil [6]. Moreover, MP is the only accession out of the examined ones wherein stimulation of growth was observed at a low concentration of Ni [18]. Stimulation of growth at a low concentration of Ni in the solution may well account for the decrease in the Ni content per unit mass in MP plants upon the long-term incubation (as compared with short-time exposure).
The content of Ni in the plants of LE accession originating from non-metalliferous soils was often lower than in the plants of MP accession but higher than in LC and SF accessions originating from calamine soils. However, after eight weeks of incubation at low Ni concentration, its content in the representatives of the accession from non-metalliferous soil was even slightly higher than in plants of accessions originating from calamine and serpentine soils (Figs. 1, 2). In spite of the ability of plants from non-metalliferous soils to accumulate Ni at its low concentrations in the medium, their tolerance to the toxic effect of Ni is less pronounced, which is also confirmed by earlier observations [6, 18].
The content of metal in plants grown in hydroponic culture may be higher than in plants growing in natural conditions even when the level of available metal is the same. For instance, N. сaerulescens plants from calamine and non-metalliferous soils grown in hydroponic culture accumulated identical amounts of Zn, but its amount was three times higher than in the plants grown on the soil [6]. This depends on soil heterogeneity, uneven distribution of the metal along the soil profile, and on a different ratio between macro- and microelements. At the same time, when the content of Zn in the shoots of N. сaerulescens was identical, the distribution of the metal within plant tissues was similar, which makes it possible to extrapolate the results from research done using plants grown in laboratory conditions to the plants from natural populations [26]. Therefore, it is correct to compare the accumulation of metals only if the data were obtained under similar conditions. As a result, it is difficult to compare the data obtained by different researchers. In our experiments, the ability to accumulate Ni in N. сaerulescens plants of different accessions increased in the following order: LC < SF < LE < MP (Figs. 1, 2), which agrees in general with the data obtained earlier under identical conditions upon a long-term exposure of plants to Ni at other Ni(NO3)2 concentrations in the medium [18]. Similarity between the series obtained at different concentrations of the metal in solution suggests that the capability of plants of different accessions to accumulate Ni weakly depends on its concentration in the solution. However, at high concentrations, this order may change owing to the metal toxic effects.
In MP plants, the translocation factor exceeded unity as early as after 24 h from the start of exposure, whereas this did not happen in the other accessions even after 3-day-long incubation (Fig. 3). Since the translocation factor shows the ratio between the content of metal in the shoots and roots, one may conclude that the accession naturally growing on serpentine soils has highly effective transport systems ensuring Ni translocation into the above-ground organs during the first hours of exposure. In plants of other accessions, this ability is implemented later and the plants of hyperaccumulator N. сaerulescens belonging to all the accessions under study showed translocation factor above unity by the eighth week (Fig. 3). In this regard, it is interesting to examine the plants of LC accession originating from calamine soils. The plants of this accession practically did not accumulate Ni during the first two days of incubation (Fig. 1), which points to a low rate of metal uptake, whereas the values of translocation factor in the accessions LC and MP were very close after eight weeks of exposure (Fig. 3). The reasons for this phenomenon are not clear. However, it is obvious that the values of the translocation factor not only vary in different accessions, but they can also vary with time and depending on metal concentration in the solution, which is confirmed by the results obtained by other researchers [17]. Excluders have much lower values of translocation factor due to more efficient mechanisms of metal binding and detoxification in the roots mainly as a result of Ni accumulation in the vacuoles of root cortical cells [15, 22].
Physiological mechanisms responsible for hyperaccumulation and ensuring a varying ability of plants of different species and accessions to accumulate metals are actively investigated at the moment. However, the mechanisms involved in uptake and translocation of Ni (in contrast to that of Zn and Cd) are poorly known. It is known that hyperaccumulators are notable for: (1) more efficient uptake of metal ions; (2) greater mobility of metals in root tissues resulting in less efficient (than in excluders) metal transport into the vacuoles of root cells; (3) higher rate of metal loading into the xylem; and (4) efficient mechanisms of metal detoxification in the vacuoles of the above-ground organs, which taken altogether promotes the maintenance of homeostasis [22].
Comparison of the ability of N. сaerulescens plants belonging to different accessions to accumulate Ni and Zn has revealed similar patterns [18]. Similarity of the patterns detected for different metals points to the existence of non-specific pathways of metal uptake and translocation in plants. Transport of Ni in N. сaerulescens is supposed to occur via non-specific transporters of Zn or Fe, with Zn transporters ensuring more efficient uptake of Ni by the cell than Fe transporters [11]. This may account for a strong correlation between accumulation of Ni and Zn in N. сaerulescens [6, 21]. Expression of the ZIP 10 and IRT1 genes that encode proteins participating in the translocation of Zn and Fe, respectively, considerably increased under the Ni effects. Together with the enchanced Ni uptake under Zn and Fe deficiency, this confirms the viewpoint that there exist non-specific mechanisms responsible for uptake and transport of metals [11].
Different abilities to accumulate Ni detected in the accessions may be accounted for the varying expression of the genes encoding metal transporters [4]. Elevated expression of ZNT1 and ZNT2 genes encoding transporters located on the plasma membrane and involved in Ni transport into the leaf cells was observed in the plants of MP accession growing in a natural population and in hydroponic culture, which may account for their ability to accumulate Ni [17]. On the contrary, the level of expression of ZTP1 gene encoding a transporter participating in the translocation of Zn from the cytoplasm to the lumen of the organelles or its efflux from the cell and belonging to the СDF family was higher in N. сaerulescens accessions from calamine soils (as compared with accessions from serpentine soils) [13]. This probably accounts for the different level of accumulation of Zn in these plants.
An important role in metal translocation belongs to metal chelators, such as histidine (for Ni and Zn) and nicotianamine (for Ni and Zn) [7, 15, 16, 23]. Our experiments have shown that pre-treatment with histidine stimulated loading of Ni and Zn into the xylem only in the hyperaccumulator N. caerulescens but not in the excluder T. arvense. A higher rate of Ni and Zn loading into the xylem vessels of N. caerulescens is associated with a higher endogenous content of free histidine in the roots of N. caerulescens as compared with T. arvense [15, 16]. A constitutively higher level of histidine in the roots and less efficient translocation of histidine complexes with Ni and Zn into the vacuoles of root cells of N. caerulescens as compared to the excluder T. arvense suggest that the formation of complexes of Ni and Zn with histidine restricts their transport into the vacuoles of root cells and ensures a more efficient loading of metals into xylem vessels in N. caerulescens [15, 16]. In spite of considerable differences in the ability to accumulate different metals and tolerance to them, plants of different accessions of N. caerulescens did not show noticeable differences in the endogenous content of free histidine in their roots or shoots [15]. Therefore, a higher level of endogenous histidine in the roots is related to the mechanisms responsible for the phenomenon of hyperaccumulation but does not account for differences in the tolerance and ability to accumulate metals revealed in the plants of different accessions of the same hyperaccumulator species.
Loading of Zn and Cd into the xylem involves a P‑type ATPase НМА4 located on the plasma membrane; the level of its genes’ expression is high owing to an increase in the number of their copies in hyperaccumulators [4, 5, 27, 28]. The number of the copies of the same gene may differ not only in different species [5, 29] but also in different accessions of the same species [30]. The level of expression of NcHMA4 in Puente Basadre (PB) accession of hyperaccumulator N. caerulescens originating from serpentine soils rich in Ni was lower than in the accesions Prayon (Pr), SF, and Saint Laurent le Minier (SLM) (formerly Ganges) originating from calamine soils rich in Zn. This correlates with the presence of four copies of NcHMA4 in the SLM and SF plants, three copies in Pr plants, and only two copies in PB plants [30]. Moreover, in Ni‑treated plants of LC accession originating from calamine soils, the expression of HMA4 gene was much higher than in MP plants originating from serpentine soils [17]. However, even in the case of similar level of expression of НМА4, the ability to accumulate Zn and Cd may differ between the accessions of N. caerulescens. Therefore, the high level of expression of the genes encoding metal transporters is not the only factor determining the ability to hyperaccumulate metals. The mechanism of loading of Ni or its complexes into the xylem is to be elucidated. However, it is obvious that multiplication of the genes encoding metal transporters is the major process of microevolution contributing to the phenomenon of hyperaccumulation.
The ability to accumulate metals by different accessions of hyperaccumulator N. сaerulescens became apparent from the first day of incubation and did not significantly change with time. This confirms the viewpoint that representatives of different accessions possess a constitutive or genetically predetermined ability to accumulate metals, which is fulfilled in a short time. Particularly, this ability makes it possible for plants originating from serpentine soils to accumulate more than 1000 mg Ni/kg dry wt as early as on the third day of incubation (Figs. 1c, 2c). However, this does not rule out the involvement of inducible mechanisms. This assumption is confirmed by the changes in the expression level of some genes upon exposure to Ni [17]. For instance, the control plants of accessions LC and MP showed a high constitutive expression of NRAMP3 and NRAMP4 genes encoding vacuolar transporters as well as that of НМА4, while the expression of ZNT1 and ZNT2 genes was higher in LC than in МР. After a month-long exposure to 10 µM Ni, MP plants showed an induction of the expression of ZNT1, ZNT2, and NRAMP 4, while in LC plants only the expression of НМА4 was induced by Ni [17]. However, it is not known how fast these changes may occur and whether or not they may become apparent in 24 h after the start of incubation, thus distinctly affecting metal accumulation. One may assume that, upon a short-term exposure, an important contribution is made by constitutive mechanisms operating in hyperaccumulators, which account for the phenomenon of hyperaccumulation.
Thus, the plants of different accessions of hyperaccumulator N. сaerulescens considerably differ in the ability to accumulate Ni; these differences are visible as early as during the first days of exposure to Ni and are quite independent of the duration of incubation or of metal concentration in the medium. However, in spite of the evident progress made in this area, little is known about the mechanisms governing selective accumulation of metals in the roots of excluders and in the shoots of hyperaccumulators and regulating various capacities of plants belonging to different accessions of the same species to accumulate metals. This line of investigation is actively developed in current ionomics and ecological plant physiology and is promising for future investigations.
REFERENCES
Brooks, R.R., Lee, J., Reeves, R.D., and Jaffré, T., Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants, J. Geochem. Explor., 1977, vol. 7, p. 49.
Reeves, R.D. and Baker, A.J.M., Metal-accumulating plants, in Phytoremediation of Toxic Metals Using Plants to Clean Up the Environment, Raskin, I. and Ensley, B.D., Eds., New York: John Wiley and Sons, 2000, p. 193.
Verbruggen, N., Hermans, C., and Schat, H., Molecular mechanisms of metal hyperaccumulation in plants, New Phytol., 2009, vol. 181, p. 759.
Krämer, U., Metal hyperaccumulation in plants, Annu. Rev. Plant Biol., 2010, vol. 61, p. 517.
Hanikenne, M., Talke, I.N., Haydon, M.J., Lanz, C., Nolte, A., Motte, P., Kroymann, J., Weigel, D., and Krämer, U., Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4,Nature, 2008, vol. 453, p. 391.
Sterckeman, T., Cazes, Y., Gonneau, C., and Sirguey, C., Phenotyping 60 populations of Noccaea caerulescens provides a broader knowledge of variation in traits of interest for phytoextraction, Plant Soil, 2017, vol. 418, p. 523.
Lin, Y.F. and Aarts, M.G.M., The molecular mechanism of zinc and cadmium stress response in plants, Cell. Mol. Life Sci., 2012, vol. 69, p. 3187.
Ernst, W.H.O., Evolution of metal hyperaccumulation and phytoremediation hype, New Phytol., 2000, vol. 146, p. 357.
Assunção, A.G.L., Schat, H., and Aarts, M.G.M., Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants, New Phytol., 2003, vol. 159, p. 351.
Macnair, M.R., The hyperaccumulation of metals by plants, Adv. Bot. Res., 2003, vol. 40, p. 63.
Deng, T.H.B., Tang, Y.T., Sterckeman, T., Echevarria, G., Morel, J.L., and Qiu, R.L., Effects of the interactions between nickel and other trace metals on their accumulation in the hyperaccumulator Noccaea caerulescens,Environ. Exp. Bot., 2019, vol. 158, p. 73.
Sinclair, S.A. and Krämer, U., The zinc homeostasis network of land plants, Biochem. Biophys. Acta, 2012, vol. 1823, p. 1553.
Assunção, A.G.L., da Costa Martins, P., de Folter, S., Voojis, R., Schat, H., and Aarts, M.G.M., Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens,Plant Cell Environ., 2001, vol. 24, p. 217.
Meharg, A.A., Mechanisms of plant resistance to metal and metalloid ions and potential biotechnological applic-ations, Plant Soil, 2005, vol. 274, p. 163.
Richau, K.H., Kozhevnikova, A.D., Seregin, I.V., Vooijs, R., Koevoets, P.L.M., Smith, J.A.C., Ivanov, V.B., and Schat, H., Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator, Thlaspi caerulescens,New Phytol., 2009, vol. 183, p. 106.
Kozhevnikova, A.D., Seregin, I.V., Erlikh, N.T., Shevyreva, T.A., Andreev, I.M., Verweij, R., and Schat, H., Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens,New Phytol., 2014, vol. 203, p. 508.
Visioli, G., Gullì, M., and Marmiroli, N., Noccaea caerulescens populations adapted to grow in metalliferous and non-metalliferous soils: Ni tolerance, accumulation and expression analysis of genes involved in metal homeostasis, Environ. Exp. Bot., 2014, vol. 105, p. 10.
Seregin, I.V., Erlikh, N.T., and Kozhevnikova, A.D., Nickel and zinc accumulation capacities and tolerance to these metals in the excluder Thlaspi arvense and the hyperaccumulator Noccaea caerulescens,Russ. J. Plant Physiol., 2014, vol. 61, p. 204.
Seregin, I.V., Kozhevnikova, A.D., Zhukovskaya, N.V., and Schat, H., Cadmium tolerance and accumulation in excluder Thlaspi arvense and various accessions of hyperaccumulator Noccaea caerulescens,Russ. J. Plant Physiol., 2015, vol. 62, p. 837.
Callahan, D.L., Baker, A.J., Kolev, S.D., and Wedd, A.G., Metal ion ligands in hyperaccumulating plants, J. Biol. Inor-g. Chem., 2006, vol. 11, p. 2.
Richau, K.H. and Schat, H., Intraspecific variation of nickel and zinc accumulation and tolerance in the hyperaccumulator Thlaspi caerulescens,Plant Soil, 2009, vol. 314, p. 253.
Seregin, I.V. and Kozhevnikova, A.D., Physiological role of nickel and its toxic effects on higher plants, Russ. J. Plant Physiol., 2006, vol. 53, p. 285.
Leitenmaier, B. and Küpper, H., Compartmentation and complexation of metals in hyperaccumulator plants, Front. Plant Sci., 2013, vol. 4, p. 374.
Deng, T.H.B., van der Ent, A., Tang, Y.T., Sterckeman, T., Echevarria, G., Morel, J.L., and Qiu, R.L., Nickel hyperaccumulation mechanisms: a review on the current state of knowledge, Plant Soil, 2018, vol. 423, p. 1.
Lombi, E., Zhao, F.J., Dunham, S.J., and McGrath, S.P., Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense,New Phytol., 2000, vol. 145, p. 11.
Kozhevnikova, A.D., Seregin, I.V., Gosti, F., and Schat, H., Zinc accumulation and distribution over tissues in Noccaea caerulescens in nature and in hydroponics: a comparison, Plant Soil, 2017, vol. 411, p. 5.
Hussain, D., Haydon, M.J., Wang, Y., Wong, E., Sherson, S.M., Young, J., Camakaris, J., Harper, J.F., and Cobbett, C.S., P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis,Plant Cell, 2004, vol. 16, p. 1327.
Bernard, C., Roosens, N., Czernic, P., Lebrun, M., and Verbruggen, N., A novel CPx-ATPase from the cadmium hyperaccumulator Thlaspi caerulescens,FEBS Lett., 2004, vol. 569, p. 140.
Drager, D.B., Desbrosses-Fonrouge, A.G., Krach, C., Chardonnens, A.N., Meyer, R.C., Saumitou-Laprade, P., and Krämer, U., Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels, Plant J., 2004, vol. 39, p. 425.
Craciun, A.R., Meyer, C.-L., Chen, J., Roosens, N., Groodt, R.D., Hilson, P., and Verbruggen, N., Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation, J. Exp. Bot., 2012, vol. 63, p. 4179.
ACKNOWLEDGMENTS
We are grateful to V.B. Ivanov for critical discussion of the obtained results.
Funding
This work was partially supported by the Russian Foundation for Basic Research, project no. 19-04-00369, and the LOCOMET International Research Program.
Author information
Authors and Affiliations
Contributions
A. D. Kozhevnikova and I. V. Seregin made equal contribution to this work.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Additional information
Translated by N. Balakshina
Abbreviations: LC—La Calamine; LE—Lellingen; MP—Monte Prinzera; SF—Saint Félix de Palliéres; PB—Puente Basadre; Pr—Prayon; SLM—Saint Laurent le Minier (formerly Ganges) (accessions of hyperaccumulator Noccaea сaerulescens).
Rights and permissions
About this article
Cite this article
Kozhevnikova, A.D., Seregin, I.V. & Schat, H. Accumulation of Nickel by Excluder Thlaspi arvense and Hyperaccumulator Noccaea caerulescens upon Short-Term and Long-Term Exposure. Russ J Plant Physiol 67, 303–311 (2020). https://doi.org/10.1134/S1021443720020089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720020089