Abstract
In this work, the effect of exogenous histidine supply on zinc (Zn) and nickel (Ni) translocation to the shoots in intact plants of the hyperaccumulator Noccaea caerulescens F.K. Mey was studied. Three series of experiments were carried out. (1) Intact N. caerulescens plants (St-Félix-de-Pallières population) were pretreated for 4 h (12:00 till 16:00) with a MES/KOH-buffered 1 mM L-histidine solution or demineralized water, then exposed overnight (20 h) to 5, 25 or 250 µM Ni or Zn and harvested. (2) Intact N. caerulescens plants of the same population were pretreated with 1 mM L-histidine solution or demineralized water overnight (20 h) and then exposed to 250 µM Ni or Zn for 8 h during the day (10:00 till 18:00) and harvested. (3) Intact N. caerulescens plants (the calamine populations St-Félix-de-Pallières (SF) and La Calamine (LC), and the ultramafic population Monte Prinzera (MP)) were exposed for 8 h (10:00 till 18:00) to 250 µM Ni or Zn and then to 1 mM L-histidine solution or demineralized water overnight (20 h) and harvested. The Ni and Zn concentrations in the roots and shoots were determined by atomic absorption spectrophotometry. The translocation factor (TF), expressed as the shoot to root metal concentration ratio, the total plant Ni or Zn content, and the percentage of the total Ni or Zn content present in the shoot (% translocated) were calculated. A 4 h pretreatment with L-histidine during the afternoon (before metal exposure overnight) significantly decreased the Ni and Zn concentrations in the root and increased the concentration of Ni, but not of Zn, in the shoot, significantly increased both TF and the % translocated for both metals, albeit much more strongly for Ni, and also slightly, but significantly, increased the total plant content of Ni, but not of Zn. Overnight pretreatment with L-histidine (followed by metal exposure during the day) of the same population (SF) had basically similar effects on Ni translocation, but significantly decreased the plant total Ni content, and was without significant effects on Zn translocation, but considerably decreased the root Zn concentration. The different populations under study (SF, MP, LC) showed significant differences in their Ni and Zn uptake and translocation capacities, but in general showed qualitatively similar responses to post-treatment with L‑histidine that strongly increased the TF and the % translocated for both metals in SF and MP, whereas in LC the effect was prominent only for Ni. Significant population × histidine treatment effect interactions were obtained for the root Zn concentration, and the TF and % translocated for Ni, which were largely explained by a relatively low responsiveness to the L-histidine treatment in LC, compared to SF and/or MP. It is concluded that the high endogenous L-histidine concentrations in N. caerulescens are probably functional in the hyperaccumulation of both Ni and Zn. The overall stronger effect of exogenous L-histidine supply on the translocation of Ni, compared to Zn, seems to result, at least in part, from the high Zn burdens at the start of the treatments, particularly in the shoots, which largely mask the apparent effects of exogenous L-histidine supply on the shoot Zn concentration and, to a lower degree, the % Zn translocated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, about 720 plant species have been described as hyperaccumulators, which accumulate metals to extremely high concentrations in their aboveground organs. The threshold concentrations for nickel (Ni) and zinc (Zn) hyperaccumulation have been set at 1000 and 3000 μg/g dry weight, respectively [1, 2], which is >10-fold higher than in ‘excluders’, which accumulate metals predominantly in their roots [3–7]. Hyperaccumulators are obligate or facultative metallophytes, most of which hyperaccumulating Ni from ultramafic (serpentine) soils with high concentrations of Ni, Co, Mg, Mn, Fe and Cr, or Zn from calamine soils rich in Zn, Cd and Pb [5, 6, 8].
The hyperaccumulator Noccaea caerulescens (formerly Thlaspi caerulescens) is widely used as one of the model species in the study of the hyperaccumulation phenomenon [9, 10]. Different populations of N. caerulescens vary in their Zn, Cd and Ni accumulation capacities [3–7, 11].
Plant hyperaccumulation capacity can potentially be controlled at different levels: (1) the rate of metal uptake from the soil by plant root systems, (2) the rate of metal translocation to aboveground organs via the xylem, and (3) the rate of metal sequestration in leaves [12–16]. Next to transporters that mediate metal transport across cellular membranes [16–18], low-molecular-weight ligands, including the amino acid histidine, appear to play key roles in metal detoxification, transport and the maintenance of proper metal homeostasis [16, 19].
Among all proteinogenic amino acids, histidine has the highest binding affinity toward Ni, both at xylem pH and cytosolic pH [20], which explains the important role of histidine in Ni transport [16]. Despite the fact that the stability of histidine complexes with Zn is lower than that of its complexes with Ni [21], free histidine can bind up to 55–70% Zn in the roots of N. caerulescens, and significant amounts of Zn bound to histidine can be found in the cytoplasm of cells of both young and old root tissues [22, 23]. Histidine complexes with Ni or Zn are characterized by a fairly high stability at cytosolic pH (≈7.2–7.5) due to its deprotonated imidazole nitrogen at alkaline pH [16, 20, 22]. Based on this, it can be assumed that histidine complexes with metals are formed upon the entry of metals into the cytosol [16, 23, 24]. The binding of metals by histidine in the cytosol limits their entry into the vacuoles of the root cells and determines the effective radial transport of metals towards the central cylinder and, consequently, to the vascular tissues of the root [16, 24, 25].
The hyperaccumulator N. caerulescens is characterized by a constitutively enhanced level of free histidine (± 10-fold) in its roots, but not in its shoots, compared to the related non-hyperaccumulator, Thlaspi arvense [24]. Exogenous L-histidine can be equally well absorbed by the root systems of T. arvense and all studied populations of N. caerulescens [24].
Pretreatment of N. caerulescens with exogenous L‑histidine in most cases led to an increase in the Ni and Zn concentrations in the xylem exudates of shoot-excised root systems [24, 25]. Considering that upon the pretreatment of plants with L-histidine the volume of the collected xylem exudate in most cases did not change significantly or increased, whereas the concentrations of Ni and Zn in the xylem sap of N. caerulescens increased, it was suggested that pretreatment with histidine stimulated the loading of metals into the xylem vessels [24, 25]. However, these experiments were carried out on shoot-excised root systems. Therefore, an important and intriguing question remained: to what extent will the effect of histidine be manifested in intact plants? In order to solve this problem, in this work, we studied the effect of pretreatment and post-treatment with L-histidine on Ni and Zn accumulation and translocation in intact plants of N. caerulescens.
MATERIALS AND METHODS
Plant culture. Seeds of Noccaea caerulescens F.K. Mey (populations St-Félix-de-Pallières (SF, France, 44°02′ N, 03°56′ E) and La Calamine (LC, Belgium, 50°42′ N, 06°00′ E), both from calamine soils, and Monte Prinzera (MP, Italy, 44°38′ N, 10°05′ E) from ultramafic (serpentine) soil), were sown on moist vermiculite. Seed germination and experiments were performed in a climate chamber (20/15°C day/night; 250 μmol (m2 s) at plant level, 14 h/d; 70% RH). Two-week-old seedlings were transferred to a hydroponics system, consisting of 1 L PVC pots (2 plants per pot), filled with a modified half-strength Hoagland’s solution composed of 3 mM KNO3, 2 mM Ca(NO3)2, 1 mM NH4H2PO4, 0.5 mM MgSO4, 1 µM KCl, 25 µM H3BO3, 2 µM ZnSO4, 2 µM MnSO4, 0.1 µM CuSO4, 0.1 µM (NH4)6Mo7O24, 20 µM FeEDDHA, and 2 mM of the pH buffer MES, in demineralized water. The pH was set at 5.5, using KOH [25]. The nutrient solution was replaced once a week.
Experimental procedures. Four experiments were carried out after a 7-week pre-culture. To study the effect of exogenous pretreatment with histidine on Ni and Zn uptake and translocation in intact plants, N. caerulescens (SF) was pretreated for 4 h (12:00 till 16:00) on a 1 mM L-histidine solution in a 2 mM MES/KOH buffer (pH 5.5) or MES/KOH-buffered demineralized water (pH 5.5), and then overnight (20 h) exposed to fresh half-strength Hoagland`s solution amended with 5, 25 or 250 µM Zn or Ni (following the scheme of the experiment with shoot-excised root systems described in [24, 25]). Then the plants were harvested as described below (experiment 1).
To check the potential effect of the day-night cycle on the His effect, intact N. caerulescens (SF) was exposed overnight (14.00 till 10.00) to 1 mM L-histidine in a 2 mM MES/KOH buffer (pH 5.5) or MES/KOH-buffered demineralized water (pH 5.5) and then exposed to half-strength Hoagland`s solution amended with 250 µM Zn or 250 µM Ni for 8 h during the day (10:00 till 18:00) and harvested (experiment 2).
To check whether the histidine effect depends on the order of the metal and the histidine treatments, a reverse type of experiment (experiment 3) was carried out as well: intact N. caerulescens plants (LC, MP, and SF) were exposed for 8 h (10:00 till 18:00) to half-strength Hoagland`s solution amended with 250 µM Zn or 250 µM Ni, and then exposed to 1 mM L-histidine in a 2 mM MES/KOH buffer (pH 5.5) or MES/KOH-buffered demineralized water (pH 5.5) overnight (20 h). Then the plants were harvested as described below. To estimate the potential effect of metal burdens at the start of the treatments, this experiment was repeated with MP plants that had been grown for 1 week at 25 µM Ni, prior to the start of the 250 µM Ni exposure (experiment 4).
Determination of nickel and zinc concentrations. At harvest, the roots were desorbed with 20 mM Na2EDTA for 10 min at room temperature, and then rinsed with demineralized water. The shoots were washed with demineralized water. Plant material was blotted dry on filter paper and then dried to a constant weight at 80°C for 48 hours in an oven, and then weighed. Dry roots and shoots were powdered and samples (50–100 mg) were digested in 2 mL of a 4 : 1 mixture of concentrated HNO3 (65%) and HCl (37%), in Teflon bombs (7 h at 140°C). After addition of 3 mL of demineralized water, the digests were analyzed for Ni or Zn, after appropriate dilution with demineralized water, using flame atomic absorption spectrophotometry (AAnalist 100, Perkin Elmer, the Netherlands). The translocation factor was calculated as the shoot to root Ni or Zn concentration ratio. “Total Ni or Zn uptake” was calculated as the sum of the total amount of Zn or Ni present in the roots and shoots, expressed on a plant dry weight basis.
Statistical data processing. Six to ten plants were examined per treatment variant. The data were analyzed using one-way or two-way ANOVA. The minimum significant range (MSR) was used as a statistic for a posteriori comparison of individual means [26].
RESULTS
Experiment 1
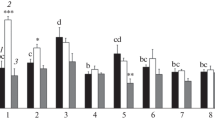
Pretreatment (4 h) with L-histidine during the afternoon significantly decreased the Ni concentration in the roots at all Ni concentrations, but significantly increased the Ni concentration in the shoots at 250 µM Ni, as well as the % Ni translocated and the Ni TF at all Ni concentrations (Fig. 1, Table 1). The overall analysis of the data by two-way ANOVA revealed a significant increase in the total uptake of Ni in histidine-treated plants (Table 1), although there were no significant effects within the individual Ni treatment levels (Fig. 1). For the root Ni concentration and the % Ni translocated, the histidine effect was significantly dependent on the Ni exposure concentration (Table 1), i.e., in both cases decreasing with increasing Ni exposure level (Fig. 1). The histidine pretreatment also in most cases considerably decreased the root Zn concentration, and significantly increased the Zn TF, but, in contrast to the case for Ni, the shoot Zn concentration and the total Zn uptake were not significantly affected (Fig. 2, Table 1). The ANOVA also revealed a significant increase in the % Zn translocated in histidine-treated plants (Table 1), although there were no significant effects within the individual Zn treatment levels (Fig. 2). There were no significant interactions between the histidine pretreatment effect and the Zn exposure level (Table 1).
Ni concentration in the roots (a) and shoots (b), Ni translocation factor (c), total Ni uptake (d), and the amount of Ni translocated given as a percentage of total uptake (e) (means + SE) in Noccaea сaerulescens (SF population) after pretreatment with MES/KOH-buffered demineralized water (1) or 1 mM L-histidine solution (2) for 4 h followed by overnight (20 h) exposure to 5, 25 or 250 µM Ni. Values assigned with different letters indicate a significant difference between the means (P < 0.05, two-way ANOVA followed by post-hoc MSR test).
Zn concentration in the roots (a) and shoots (b), Zn translocation factor (c), total Zn uptake (d), and the amount of Zn translocated given as a percentage of total uptake (e) (means + SE) in Noccaea сaerulescens (SF population) after pretreatment with MES/KOH-buffered demineralized water (1) or 1 mM L-histidine solution (2) for 4 h followed by overnight (20 h) exposure to 5, 25 or 250 µM Zn. Values assigned with different letters indicate a significant difference between the means (P < 0.05, two-way ANOVA followed by post-hoc MSR test).
Experiment 2
Overnight pretreatment with L-histidine, followed by metal exposure (250 µM) during the light period, markedly decreased the root Ni concentration, and considerably increased the Ni TF and the % Ni translocated (Fig. 3), just like in experiment 1, but significantly decreased the shoot Ni concentration and the total Ni uptake, in contrast with the effect of the 4 h histidine pretreatment in daylight in experiment 1 (Fig. 1). The root Zn concentration was significantly decreased, and the shoot Zn concentration and the Zn TF slightly, but not significantly, increased (Fig. 3), while there were no significant effects on the % Zn translocated or the total Zn uptake (Fig. 3). The latter effects were neither significant in experiment 1, specifically at the 250 µM Zn exposure level (compare Figs. 2 and 3).
Ni and Zn concentrations in the roots (a, f) and shoots (b, g), Ni (c) and Zn (h) translocation factor, Ni (d) and Zn (i) total uptake, and the amount of Ni (e) and Zn (j) translocated given as a percentage of total uptake (means + SE) in Noccaea сaerulescens (SF population) after overnight (20 h) pretreatment with MES/KOH-buffered demineralized water (1) or 1 mM L-histidine solution (2) followed by 8 h exposure to 250 µM Ni (a–e) or Zn (f–j). Values assigned with asterisks indicate a significant difference between the means (*P < 0.05, ** P < 0.01, NS—not significant, one-way ANOVA).
Experiment 3
Post-hoc overnight (20 h) treatment with L-histidine, after an 8 h 250 µM metal exposure during the day, strongly and significantly decreased the root Ni concentrations, and strongly and significantly enhanced the Ni TF and the % Ni translocated in plants from all populations under study (Fig. 4, Table 2). These effects were much stronger than those in experiments 1 and 2 in SF at 250 µM Ni (compare Figs. 1, 3 and 4). In contrast to experiments 1 and 2, there were neither significant effects on the shoot Ni concentration, nor the total Ni uptake (Fig. 4, Table 2). Also for Zn, the root concentration was markedly decreased, and the TF and the % translocated significantly increased (Fig. 5, Table 2) in SF much more strongly than in experiments 1 or 2 at 250 µM Zn (compare Figs. 2, 3 and 5). A considerable increase in the Zn TF and the % translocated was also observed in histidine-treated MP plants, but the effect was negligible in LC plants (Fig. 5). The shoot Zn concentrations, however, were not significantly affected, as in experiments 1 and 2, but the total Zn uptake was slightly, but overall significantly decreased (Table 2), although there were no significant effects within individual populations (Fig. 5).
Ni concentration in the roots (a) and shoots (b), Ni translocation factor (c), total Ni uptake (d), and the amount of Ni translocated given as a percentage of total uptake (e) (means + SE) in Noccaea сaerulescens from calamine (LC, SF) and ultramafic (MP) populations after exposure for 8 h to 250 µM Ni followed by overnight (20 h) post-treatment with MES/KOH buffered demineralized water (1) or 1 mM L-histidine solution (2). Values assigned with different letters indicate a significant difference between the means (P < 0.05, two-way ANOVA followed by post-hoc MSR test).
Zn concentration in the roots (a) and shoots (b), Zn translocation factor (c), total Zn uptake (d), and the amount of Zn translocated given as a percentage of total uptake (e) (means + SE) in Noccaea сaerulescens from calamine (LC, SF) and ultramafic (MP) populations after exposure for 8 h to 250 µM Zn followed by overnight (20 h) post-treatment with MES/KOH buffered demineralized water (1) or 1 mM L-histidine solution (2). Values assigned with different letters indicate a significant difference between the means (P < 0.05, two-way ANOVA followed by post-hoc MSR test).
Both for Ni and Zn, and for all the parameters (root and shoot concentrations, total uptake, % translocated and TF) the main effects of the factor 'population' were consistently significant (Table 2). The histidine treatment effect × population interactions were mostly insignificant, except for the Zn concentration in the roots, and the % translocated and the TF for Ni. In all these cases, the interaction is largely explained by a relatively low responsiveness to post-hoc histidine treatment in LC, compared with SF and/or MP (Figs. 4, 5).
Experiment 4
Pre-culture in Ni-amended nutrient solution (one week at 25 µM Ni, prior to the start of the 250 µM Ni treatment) did not abolish the significant effects of the post-hoc histidine treatment but, as expected, decreased its effects, at least on the root Ni concentration and the Ni TF, but not on the % Ni translocated (compare Figs. 4 and 6).
Ni concentration in the roots (a), shoots (b), Ni translocation factor (c), total Ni uptake (d), and the amount of Ni translocated given as a percentage of total uptake (e) (means + SE) in the intact MP plants pre-grown for 1 week at 25 µM Ni and then exposed for 8 h to 250 µM Ni with subsequent 20 h post-treatment with MES/KOH buffered demineralized water (1) or 1 mM L-histidine solution (2). Values assigned with asterisks indicate a significant difference between the means (**P < 0.01, ***P < 0.001, one-way ANOVA).
DISCUSSION
Zn hyperaccumulation capacity seems to be species-wide in N. caerulescens, yet there is intra-specific variation in degree [5, 6], although the threshold foliar Zn concentration defined for Zn hyperaccumulation, i.e. 3000 mg/kg dry weight [1], is not always surpassed in nature, e.g., in some ultramafic populations, such as MP [11, 27]. Nevertheless, so far known, all the populations tested thus far have been shown to be able to hyperaccumulate Zn under experimental conditions [3, 5, 6]. Although Ni hyperaccumulation is in nature confined to its ultramafic populations, the capacity to hyperaccumulate Ni under experimental conditions seems to be widespread in N. caerulescens, or at least not confined to its ultramafic populations, although some calamine populations seem to have lost their Ni hyperaccumulation capacity, possibly as a by-product of an exclusion strategy for Cd hypertolerance [6, 11, 28, 29].
High concentrations of free L-histidine in hyperaccumulator roots, either constitutive or induced upon Ni exposure, have been previously associated with Ni hyperaccumulation [20, 24, 30, 31], but might also be involved in Zn hyperaccumulation, at least in N. caerulescens [25]. The proposed role for histidine in hyperaccumulation, both of Ni and Zn, is supposed to lie in the prevention of the vacuolar sequestration of these metals in the root cortex, through complex formation in the root cytoplasm, thus keeping them available for radial symplastic transport across the root into the stele, and thus eventually, for loading into the xylem [16, 24, 25].
In conformity with the above, exogenous L-histidine supply, via the nutrient solution, has been shown to enhance the Ni concentration in the root pressure exudates after shoot excision in several Odontarrhena (previously, Alyssum) Ni hyperaccumulators and/or their close relatives [20, 32], and in N. caerulescens [24, 25]. However, at least in N. caerulescens, exogenous histidine supply also strongly enhanced the Zn concentrations in xylem exudates from shoot-excised root systems [25]. Still, significant effects of exogenous histidine on the Zn translocation in intact plants have not been reported thus far.
Exogenous histidine supply usually significantly promotes the translocation of both Ni and Zn in intact plants, as shown by the results of the present study. Moreover, in the same experimental settings (4 h pretreatment with L-histidine, followed by overnight 250 µM metal exposure) the histidine-induced decreases of the Ni and Zn concentrations in the roots were quite similar in intact plants and shoot-excised root systems (A.D. Kozhevnikova, I.V. Seregin and H. Schat, unpublished data). Thus, the histidine effect seems to be independent of the presence of the shoot, both for Ni and for Zn.
In general, in all our experimental settings, exogenous histidine more strongly affected the translocation of Ni than that of Zn in intact plants. However, this may be largely attributable to ‘artifacts’. First, we grew the plants, prior to the treatments, in solution with Zn (2 µM), but without added Ni. This is unavoidable, because N. caerulescens has a relatively high requirement for Zn for normal growth [3, 33, 34], but performs well without added Ni (H. Schat, A.D. Kozhevnikova and I.V. Seregin, unpublished results), maybe except for (some of) its ultramafic populations [3, 35]. This means that there must have been a significant Zn burden at the start of the treatments. We have no information for all the populations, but SF in experiments 1 and 2, for example, already had Zn concentrations of ~138 and 816 mg/kg dry weight in its roots and shoots, respectively, at the start of the histidine pretreatment. This corresponds with a % Zn translocated of ± 97%, a Zn TF of about 6.6, and a total Zn uptake of ± 750 mg/kg root dry weight. It cannot be expected, therefore, that a 20 h exposure to higher Zn concentrations would greatly increase these values. This is exactly what has been found: at the 5 and 25 µM Zn treatment levels, the mean total uptake values are not significantly different from the starting values and the % Zn translocated is even lower (Fig. 2). The root Zn concentrations, however, are much higher, and the Zn TF values much lower, than the starting values (Fig. 2), suggesting that a large part of the Zn taken up during the Zn treatments after the histidine pretreatments must still be in the roots at harvest. The latter is apparently also the case in the 250 µM Zn treatment, which significantly increased the total Zn uptake and the Zn concentration in the shoot, but particularly that in the root (Fig. 2). Anyway, there is a clear and significant effect of the histidine pretreatment on the root Zn concentrations and, as a consequence, on the Zn TF, showing that exogenous histidine does promote the removal of Zn from the roots, most probably through enhancing its translocation to the shoot. The alternative explanation, i.e., histidine-promoted Zn efflux from the roots into the nutrient solution, is avowedly unlikely, since there was no decrease in the Zn total uptake values (Fig. 2). The absence of a significant histidine effect on the shoot Zn concentration, and the quantitatively small, but overall just significant effect on the % Zn translocated, can be satisfactorily explained by the high initial Zn burden of the shoot, compared to that of the root, and the high shoot-to-root dry weight ratio (both >5). Of course, the initial root and shoot Ni burdens, at the start of the treatments, must have been much lower (<10 mg/kg dry weight (unpublished data), which could explain the stronger and more significant effects of the histidine pretreatment on the root and shoot concentrations, the TF, and the % translocated of Ni, compared to Zn.
The potential importance of the metal burden at the start of the treatment is further demonstrated by comparing the results obtained with MP in experiments 3 and 4 (Figs. 4 and 6). It is obvious that ‘pre-culturing’ MP for one week with 25 µM Ni in the nutrient solution, prior to the treatments, strongly decreased the effects of the histidine treatment on the root Ni concentration, and the Ni TF.
Another factor that might have counteracted the visibility or the magnitude of the effect of the histidine treatment on Ni and Zn translocation is continuous uptake during the period of metal treatment after a histidine pretreatment. In this respect, it is interesting to compare experiments 1 and 2 (first histidine, then metal) with experiment 3 (first metal, then histidine). The effects of a histidine treatment on the root Ni concentration, the Ni TF, and the % Ni translocated are much stronger when the histidine is supplied after, rather than before the metal treatment (compare SF at 250 µM in Figs. 1, 3 and 4). For example, the Ni TF is increased by 1.6 and 11 times in histidine-treated SF plants at 250 µM Ni in experiments 2 and 3, respectively (compare Figs. 3 and 4). The same also seems to apply to Zn, but to a lower degree (compare SF at 250 µM in Figs. 2, 3 and 5): for example, the Zn TF is increased by the histidine treatment by 1.4 and 2.2 times in experiments 2 and 3, respectively. This suggests that exogenously supplied histidine is more effective in translocating ‘pre-existent’ root Ni burdens than root Zn burdens. However, the pre-existent Ni root burdens will also be smaller and ‘younger’, at least, largely accumulated after the start of the metal treatment, so these results do not conclusively show that histidine is less important for Zn translocation than for Ni translocation under natural conditions. In any case, they do suggest that continuous metal uptake after the histidine pretreatment may have significantly decreased the visibility of the effects of this histidine pretreatment on translocation, particularly in case of Zn, because Zn is taken up many times faster than Ni.
Our results (experiment 3) clearly confirm that N. caerulescens displays a considerable, largely independent variation in its capacity to accumulate and translocate Ni and Zn. However, the population × histidine interactions are not significant for Zn translocation (TF and % translocated), but highly significant for Ni translocation (Table 2), which may be taken to confirm that histidine is (also) generally essential for Zn translocation in N. caerulescens.
Finally, concerning the question whether metal uptake in hyperaccumulators is regulated by translocation, such as suggested for Zn hyperaccumulation in A. halleri [36], our results (experiment 1) indicate that the uptake of Ni, rather than that of Zn, is stimulated by exogenous histidine (Table 1), and thus potentially is ‘translocation-driven’. Zn uptake, on the other hand, is unaffected by translocation in experiment 1. The small, but overall significant decrease in Zn uptake in experiment 3 (Table 2) is remarkable, because in this experiment the metal treatment preceded the histidine treatment, which would be expected to prevent any significant effects on total metal uptake, such as enhanced Ni uptake in experiment 1. The only conceivable explanation for this phenomenon is desorption of Zn from the root free space, during the post-hoc histidine treatment.
In conclusion, the high endogenous L-histidine concentrations in N. caerulescens are probably functional in the hyperaccumulation of both Ni and Zn. The overall stronger effect of exogenous L-histidine supply on the translocation of Ni, compared to Zn, seems to result, at least in part, from the high Zn burdens at the start of the treatments, particularly in the shoots, which largely mask the apparent effects of exogenous L-histidine supply on the shoot Zn concentration and, to a lower degree, the % Zn translocated.
REFERENCES
van der Ent, A., Baker, A.J.M., Reeves, R.D., Pollard, A.J., and Schat, H., Hyperaccumulators of metal and metalloid trace elements: facts and fiction, Plant Soil, 2013, vol. 362, p. 319.
Reeves, R.D., Baker, A.J., Jaffré, T., Erskine, P.D., Echevarria, G., and van der Ent, A., A global database for plants that hyperaccumulate metal and metalloid trace elements, New Phytol., 2018, vol. 218, p. 407.
Seregin, I.V., Erlikh, N.T., and Kozhevnikova, A.D., Nickel and zinc accumulation capacities and tolerance to these metals in the excluder Thlaspi arvense and the hyperaccumulator Noccaea caerulescens, Russ. J. Plant Physiol., 2014, vol. 61, p. 204.
Seregin, I.V., Kozhevnikova, A.D., Zhukovskaya, N.V., and Schat, H., Cadmium tolerance and accumulation in excluder Thlaspi arvense and various accessions of hyperaccumulator Noccaea caerulescens, Russ. J. Plant Physiol., 2015, vol. 62, p. 837.
Sterckeman, T., Cazes, Y., Gonneau, C., and Sirguey, C., Phenotyping 60 populations of Noccaea caerulescens provides a broader knowledge of variation in traits of interest for phytoextraction, Plant Soil, 2017, vol. 418, p. 523.
Kozhevnikova, A.D., Seregin, I.V., Aarts, M.G., and Schat, H., Intra-specific variation in zinc, cadmium and nickel hypertolerance and hyperaccumulation capacities in Noccaea caerulescens, Plant Soil, 2020, vol. 452, p. 479.
Kozhevnikova, A.D., Seregin, I.V., and Schat, H., Accumulation of nickel by excluder Thlaspi arvense and hyperaccumulator Noccaea caerulescens upon short-term and long-term exposure, Russ. J. Plant Physiol., 2020, vol. 67, p. 303.
van der Ent, A., Vinya, R., Erskine, P.D., Malaisse, F., Przybyiowicz, W.J., Barnabas, A.D., Harris, H.H., and Mesjasz-Przybyiowicz, J., Elemental distribution and chemical speciation of copper and cobalt in three metallophytes from the copper–cobalt belt in Northern Zambia, Metallomics, 2020, vol. 12, p. 682.
Assunção, A.G., Schat, H., and Aarts, M.G., Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants, New Phytol., 2003, vol. 159, p. 351.
Milner, M.J. and Kochian, L.V., Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system, Ann. Bot., 2008, vol. 102, p. 3.
Assunção, A.G., Bookum, W.M., Nelissen, H.J., Vooijs, R., Schat, H., and Ernst, W.H., Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types, New Phytol., 2003, vol. 159, p. 411.
Verbruggen, N., Hermans, C., and Schat, H., Molecular mechanisms of metal hyperaccumulation in plants, New Phytol., 2009, vol. 181, p. 759.
Krämer, U., Metal hyperaccumulation in plants, Ann. Rev. Plant Biol., 2010, vol. 61, p. 517.
Merlot, S., de la Torre, V.S., and Hanikenne, M., Physiology and molecular biology of trace element hyperaccumulation, in Agromining: Farming for Metals: Extracting Unconventional Resources Using Plants, van der Ent, A., et al., Eds., Cham: Springer-Verlag, 2018, p. 93.
Corso, M. and de la Torre, V.S.G., Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants, Metallomics, 2020, vol. 12, p. 840.
Seregin, I.V. and Kozhevnikova, A.D., Low-molecular-weight ligands in plants: role in metal homeostasis and hyperaccumulation, Photosynth. Res., 2020. https://doi.org/10.1007/s11120-020-00768-1
Krämer, U., Talke, I.N., and Hanikenne, M., Transition metal transport, FEBS Lett., 2007, vol. 581, p. 2263.
Andresen, E., Peiter, E., and Küpper, H., Trace metal metabolism in plants, J. Exp. Bot., 2018, vol. 69, p. 909.
Clemens, S., Metal ligands in micronutrient acquisition and homeostasis, Plant Cell Environ., 2019, vol. 42, p. 2902.
Krämer, U., Cotter-Howells, J.D., Charnock, J.M., Baker, A.J., and Smith, J.A., Free histidine as a metal chelator in plants that accumulate nickel, Nature, 1996, vol. 379, p. 635.
Blindauer, C.A. and Schmid, R., Cytosolic metal handling in plants: determinants for zinc specificity in metal transporters and metallothioneins, Metallomics, 2010, vol. 2, p. 510.
Salt, D.E., Prince, R.C., Baker, A.J.M., Raskin, I., and Pickering, I.J., Zinc ligands in the metal hyperaccumulator Thlaspi caerulescens as determined using X-ray absorption spectroscopy, Environ. Sci. Technol., 1999, vol. 33, p. 713.
Monsant, A.C., Kappen, P., Wang, Y., Pigram, P.J., Baker, A.J.M., and Tang, C., In vivo speciation of zinc in Noccaea caerulescens in response to nitrogen form and zinc exposure, Plant Soil, 2011, vol. 348, p. 167.
Richau, K.H., Kozhevnikova, A.D., Seregin, I.V., Vooijs, R., Koevoets, P.L., Smith, J.A., Ivanov, V.B., and Schat, H., Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator Thlaspi caerulescens, New Phytol., 2009, vol. 183, p. 106.
Kozhevnikova, A.D., Seregin, I.V., Erlikh, N.T., Shevyreva, T.A., Andreev, I.M., Verweij, R., and Schat, H., Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens, New Phytol., 2014, vol. 203, p. 508.
Sokal, R.R. and Rohlf, F.J., Biometry, San Francisco: W.H. Freeman, 1981, 2nd ed.
Reeves, R.D., Schwartz, C., Morel, J.L., and Edmondson, J., Distribution and metal-accumulating behavior of Thlaspi caerulescens and associated metallophytes in France, Int. J. Phytorem., 2001, vol. 3, p. 145.
Assunção, A.G., Martins, P.D., De Folter, S., Vooijs, R., Schat, H., and Aarts, M.G.M., Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens, Plant Cell Environ., 2001, vol. 24, p. 217.
Assunção, A.G.L., Bleeker, P., Ten Bookum, W.M., Vooijs, R., and Schat, H., Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: evidence from binary exposures, Plant Soil, 2008, vol. 303, p. 289.
Kerkeb, L. and Krämer, U., The role of free histidine in xylem loading of nickel in Alyssum lesbiacum and Brassica juncea, Plant Physiol., 2003, vol. 131, p. 716.
Ingle, R.A., Mugford, S.T., Rees, J.D., Campbell, M.M., and Smith, J.A.C., Constitutively high expression of the histidine biosynthetic pathway contributes to nickel tolerance in hyperaccumulator plants, Plant Cell, 2005, vol. 17, p. 2089.
Seregin, I.V., Kozhevnikova, A.D., and Schat, H., Comparison of L-histidine effects on nickel translocation into the shoots of different species of the genus Alyssum, Russ. J. Plant Physiol., 2019, vol. 66, p. 340.
Haines, B.J., Zincophilic root foraging in Thlaspi caerulescens, New Phytol., 2002, vol. 155, p. 363.
Klein, M.A., Sekimoto, H., Milner, M.J., and Kochian, L.V., Investigation of heavy metal hyperaccumulation at the cellular level: development and characterization of Thlaspi caerulescens suspension cell lines, Plant Physiol., 2008, vol. 147, p. 2006.
Tognacchini, A., Salinitro, M., Puschenreiter, M., and van der Ent, A., Root foraging and avoidance in hyperaccumulator and excluder plants: a rhizotron experiment, Plant Soil, 2020, vol. 450, p. 287.
Hanikenne, M., Talke, I.N., Haydon, M.J., Lanz, C., Nolte, A., Motte, P., Kroymann, J., Weigel, D., and Krämer, U., Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4, Nature, 2008, vol. 453, p. 391.
ACKNOWLEDGMENTS
The authors wish to thank Rudo Verweij, Riet Vooijs, Richard van Logtestijn and Rob Broekman for technical assistance, and the Ministry of Science and Higher Education of the Russian Federation (project 121040800153-1) for the maintenance of growth and research facilities at the Timiryazev Institute of Plant Physiology, Russian Academy of Sciences.
Funding
This work was supported by the Russian Science Foundation (grant no. 21-14-00028) (studies of SF and MP populations of N. caerulescens, experiments 1–4) and partially (studies of LC population, experiment 3) by the Russian Foundation for Basic Research (project no. 19-04-00369).
Author information
Authors and Affiliations
Contributions
Authors I.V. Seregin and A.D. Kozhevnikova contributed equally to the work.
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of humans or animals. This article does not contain any studies involving animals performed by any of the authors.
Additional information
Abbreviations: LC—La Calamine, MP—Monte Prinzera, SF— St-Félix-de-Pallières, (populations of the hyperaccumulator Noccaea caerulescens); TF—translocation factor.
Rights and permissions
About this article
Cite this article
Kozhevnikova, A.D., Seregin, I.V., Zhukovskaya, N.V. et al. Histidine-Mediated Nickel and Zinc Translocation in Intact Plants of the Hyperaccumulator Noccaea caerulescens . Russ J Plant Physiol 68 (Suppl 1), S37–S50 (2021). https://doi.org/10.1134/S1021443721070074
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443721070074