Abstract
Phosphorus (P) deficiency in soil is becoming more common, and low P efficiency restrains soybean growth and production. To explore critical factors determining efficient P use, root growth and physiological characteristics were investigated in the P-efficient soybean (Glycine max (L.) Merr.) L13 and P-inefficient soybean T3 in a sand culture experiment with two P deficient treatments (0.25 and 0.0 mM P) and a control-standard P (0.5 mM P) treatment. Results showed a decrease in soybean yield under P deficiency, but the yield reduction of genotype L13 was much less than that of T3. The grain yield of L13 was 40.5, 64.2 and 141.6% higher than that of T3, under 0.5, 0.25 and 0.0 mM P treatments, respectively. Genotype L13 activated sugar metabolism in roots and released H+ into the root hair zone to acidify the rhizosphere to resist P deficiency. P deficiency induced an increase in aspartic acid concentrations in root, the increase being substantially greater in the P efficient genotype. These findings suggested that the P-efficient genotype L13 has established more responsive mechanisms and better countermeasures against P deficiency. The findings of this study will be useful in further research aimed at optimizing soybean responses to P deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Phosphorus (P) is an essential macro-element for plant growth, but it is highly immobile in soil as it reacts with many chemical and biological soil constituents. Low P availability is a primary constraint to plant productivity [1]. Soybean (Glycine max (L.) Merr.) is one of the important legume crops, mostly planted in tropical, subtropical and temperate areas, where the soils are often deficient in P [2]. Different P levels are known to induce numerous changes in plant architecture and metabolism [3]. For root, under P-deficient conditions, there is a high degree of plasticity in terms of root architecture during root growth. Many studies have confirmed that primary root growth is suppressed under low P conditions, while the growth of lateral roots and root hairs is promoted, ensured much higher P uptake ability of plants [1]. Under low P stress, root cells become shortened and epidermal cells are formed, while the growth and elongation of root hairs is enhanced, resulting in a long root hair system with increasing number of root hairs per unit root length [4]. Furthermore, studies have revealed that low P stress-induced changes in root architecture are accompanied by a decrease in shoot biomass and P accumulation in plants [5].
P availability in soils also induces numerous changes in plant physiology. In common beans, root-carbohydrate content and activities of enzymes hydrolyzing sucrose in roots were found to increase with increased environmental P concentration [6], while root-ATP concentration was found to decline [7]. Low P availability in soil greatly limits soybean nodule growth and nitrogenase activity, since nodules responsible for N fixation have a high requirement for ATP for energy transfer [8, 9]. P limitation could further restrict ureide transport from nodules to plants [10] and affect the subsequent transformation of ureide to amino acids [9]. In support of this, a higher amino-acid (asparagine) concentration in the root tissue of white clover under P limitation has been observed [11]. Other physiological processes that may be affected by P limitation include root osmotic adjustment [12, 13], and regulation of enzymatic activities including those of antioxidant enzymes [14]. The root cap is the sensing site of exogenous P concentration and P supply has been found to regulate the osmotic potential of cell sap in the root cap of African violet (Saintpaulia ionantha) [12]. P deficiency increases H+-flow activity, promoting acidification of the rhizosphere soil as well as nutrient availability [13].
Crops differ in nutrient use efficiency, and these differences are profoundly influenced by genetic and physiological components. Nutrient efficient plants are defined as plants that can produce higher yields per unit of nutrient applied or absorbed, compared with other plants grown under similar agroecological conditions [15]. In previous studies, our laboratory has screened P-efficient soybean genotypes with capability of producing higher yields per unit of nutrient applied than P-inefficient genotypes did under P-deficient conditions [16]. P-efficient genotypes displayed greater root length, root volume, root surface area and root vitality than P-inefficient genotypes under low P concentrations [17]. The P agronomic efficiency and P harvest index were also significantly greater in P-efficient than P-inefficient genotypes [18]. However, the importance of physiological processes in determining P efficiency in soybean genotypes has not been examined.
The object of the present study was to compare plant growth, grain yield, cell metabolite concentration, ion flux, and antioxidant enzymes activities of P efficient and inefficient soybean genotypes under P limiting and no-limiting conditions.
MATERIALS AND METHODS
Plant materials and growth conditions. The experiment was carried out in a transparent canopy facility with sand culture in Shenyang, Liaoning province, China, in 2012 and 2013. The sowing date was 15th June in both years. Two soybean (Glycine max (L.) Merr.) genotypes, namely P-efficient genotype L13 and P-inefficient genotype T3, were used [16]. Before sowing, healthy seeds were surface sterilized with H2O2 for 10 min, then rinsed with distilled water. The sterilized seeds were soaked in distilled water for 72 h and then transplanted in black polyvinylchloride pots (45 cm length, 20 cm diameter) filled with clean quartz sand (particle diameter ≤2mm). Plants were thinned to two per pot at 7 days after transplanting (DAT). During this period, the seedlings were watered daily with 1/2 strength nutrient solution. Experimental treatments were conducted with full nutrient solution 7 DAT. The full nutrient solution was composed of the following: 3.6 mM CaSO4 × 2H2O, 0.5 mM KH2PO4, 2 mM KNO3, 18 μM FeSO4 × 7H2O, 18.9 μM KCl, 9.3 μM H3BO3, 0.9 μM MnSO4 × H2O, 0.9 μM ZnSO4 × 7H2O, 0.18 μM CuSO4 × 5H2O, 0.18 μM (NH4)6Mo7O24 and 250 μM MgSO4 × 7H2O [19]. KH2PO4 was used as the source of P in the following three phosphate treatments: 0, 0.25 and 0.5 mM, in which the plants grown in 0.5 mM P were used as the control. KCl was used to substitute for KH2PO4 to ensure equal concentrations of K were maintained in the solutions. The pH of the solution was adjusted to 6.0 by continuous monitoring and automatic additions of 0.025 M H2SO4. The plants in all treatments were watered at 08:00 h with 1 L deionized water (adjusted to pH 4.5) to leach excess salts accumulated in the sand media. After draining for approximately 30 min, each pot received 500 mL of fresh nutrient solution according to the experimental design. The pots were flushed again with 500 mL deionized water (pH 4.5) at 16:00 h daily. There were three replications (pots) of each treatment and the pots were laid out in a completely random arrangement.
Plant sampling. Plants were sampled at six different growth stages [20]: V4 (Fourth-node: June 29 in 2012, and June 30 in 2013), V7 (Seventh-node: July 11 in 2012, and July 12 in 2013), R2 (Full bloom: July 21 in 2012 and July 23 in 2013), R4 (Full pod: August 7 in 2012 and 2013), R6 (Full seed: August 29 in 2012, and August 30 in 2013) and R8 (Full maturity: September 30 in 2012 and 2013). The V stages represent the vegetative growth stages and R stages represent reproductive growth stages. The plants were carefully removed from the sand by washing gently with running water, then rinsed with a compact agricultural spray (SX-CSSC, Seesa, China). The samples were separated into shoots and roots at the cotyledon node prior to measurements. Fresh root samples were surface-dried with a paper towel and immediately cooled in liquid nitrogen then stored at –80°C in an ultra-low temperature refrigerator before measurement of soluble protein and related enzymes. The cleaned root and shoot samples were first dried at 105°C for 30 min and then at 70°C to constant weight. Dry root samples were ground for determination of P concentration and amino acid measurements. Soybean plants were harvested at maturity (R8) to determine the grain yield.
P concentration. Dry root samples were digested with H2SO4–H2O2, then the P concentration of roots was determined by vanadium molybdenum yellow colorimetry [21].
Soluble protein concentration, antioxidant enzyme activity and malondialdehyde concentration. Frozen fresh root samples were used to determine soluble protein and soluble sugar concentration, antioxidant enzyme activity and malondialdehyde (MDA) concentration. The soluble protein and soluble sugar concentrations were determined with the Coomassie brilliant blue method and the anthrone reagent method, respectively [22]. The activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) were determined with nitro blue tetrazolium (NBT), potassium permanganate titration, and the guaiacol method, respectively, and MDA concentration was determined with thiobarbituric acid colorimetry [23, 24].
Amino acid assay. Amino acids were extracted using a modification of the procedure [25, 26]: dry root samples (0.15 g) were hydrolysed with 6 M HCl for 24 h at 110°C, followed by centrifugation at 10 000 g for 20 min. The supernatant was used for amino acid assay. For the assay, 2 mL of 5% sulfosalicylic acid was added to 2 mL of the hydrolysed root samples then centrifuged at 10 000 g, decolorized and filtered using a 0.22 μm filter before pouring into a vial for measurement. Amino acid contents were determined with an automatic Amino Acid Analyzer (Hitachi L-8800, Japan). Chromatography conditions were as follows. Analytical Column: model 2622 SC, 4.6 × 60 mm (temperature: 50°C); reaction column: 4.6 × 40 mm (temperature: 135°C); mobile phase flow rate: 0.40 mL/min; analytical column pressure: 90 kg/cm2; ninhydrin flow rate: 0.35 mL/min; reaction pressure: 9 kg/cm2; injection volume: 20 μL; wavelength range: proline 470 nm, all others 570 nm.
Proton pump (H+ flow) and indole-3-acetic acid (IAA) flow. Non-Damage Micro Test technology uses a specific ion/molecule selection electrode under automatic control of a computer to determine the concentration, velocity of flow and direction of motion of various ions and molecules in a sample. Changes of proton flux (H+) and IAA flow in the root hair zone of root tips were determined using the Scanning Ion-selective Electrode Technique (SIET) at the soybean V4 stage [27].
Statistical analysis. All data were statistically analyzed using SPSS software v. 18.0 (SPSS Inc., United States). Two-way analysis of variance (ANOVA) was used to compare treatment means. Where the F test indicated there were significant differences between means. Tukey’s test at significance level of P < 0.05 was used to determine which means were significantly different.
RESULTS
Grain Yield
Significant reductions in grain yields were observed in P deficient conditions compared with the control (Table 1). Compared with the control, the grain yield (mean of both years) decreased by 89.8 and 94.5% with 0 mM P, and by 30.2 and 40.5% with 0.25 mM P, for L13 and T3, respectively. The mean grain yield of L13 was 40.5, 64.2 and 141.6% (P < 0.05) higher than that of T3, under 0.5, 0.25, and 0 mM P treatments, respectively.
Plant Biomass, Root Dry Weight and Root/Shoot Ratio
At the individual growth stages, whole plant biomass was similar in the 0.5 and 0.25 mM P treatments (Fig. 1). Plant biomass decreased significantly (P < 0.05) in the 0 mM P treatment, being reduced by 84.2 and 91.8% at R8 for L13 and T3, respectively, compared to control biomass. However, at R8 stage, the biomass of L13 was much greater than that of T3 and the differences were intensified under P deficient conditions (41.2 and 83.6% greater in L13 than in T3, under 0.25 and 0 mM P treatments, respectively).
Consistent with plant biomass, soybean root dry weight was significantly lower (P < 0.05) in the 0 mM P treatment compared with the 0.25 and 0.5 mM P treatments (Fig. 1). Root biomass of L13 tended to be significantly greater than T3 in the latter growth stages (R4-R8) in all P treatments, however the difference was relatively greater in the 0 and 0.25 mM P treatments. Root/shoot ratio was slightly decreased under 0.25 mM P, but was increased under 0 mM P compared with the control. At 0 mM P, root/shoot ratio displayed a peak at growth stage R2, before declining, which differed from the continued declining trends observed in the 0.5 and 0.25 mM P treatments. With one exception, L13 displayed a significantly higher root/shoot ratio than T3 in the 0 and 0.25 mM P treatments, whereas ratios in the two genotypes were mostly similar in the 0.5 mM P treatment.
Root Phosphorus, Soluble Protein and Soluble Sugar Concentrations
Little difference in root P concentration was found between the 0.5 and 0.25 mM P treatments in either soybean genotype, while root P concentration significantly decreased in the 0 mM P treatment compared to the control (Fig. 2). In the 0 mM P treatment, L13 had a 39.9% higher root P concentration than T3 at R6 stage, and had 80.3 and 18.4% higher root P concentration than T3, in the 0.5 and 0.25 mM P treatments, respectively at the R8 stage.
Root soluble protein concentrations increased in both genotypes with growth stage, with peaks at the R2 (0 mM P) and R4 (0.25 mM P) stages (Fig. 3). At the later growth stages, root soluble protein content declined. At the R2 and R4 stages, root soluble protein concentrations significantly decreased for L13 but not for T3 under the 0.25 and 0 mM P treatments, compared to the 0.5 mM P treatment.
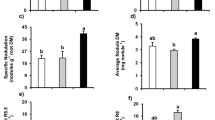
The genotypes differed in the influence of P treatment on root soluble sugar concentration. Compared to the control treatment, at the V4 growth stage, root soluble sugar concentrations in genotype L13 were significantly (P < 0.05) increased in the P deficient treatments (by 38.3 and 89.1% in the 0.25 and 0 mM P treatments, respectively) (Fig. 4). In contrast, in genotype T3, root soluble sugar concentration at this growth stage was significantly decreased (by 35.2%, P < 0.05) under 0.25 mM P compared to the control. In the 0 mM P treatment, at growth stages V4, V7 and R2, root soluble sugar concentrations were significantly higher (by 34.5, 11.4 and 43.7% respectively) in genotype L13 than in T3. At later growth stages, these differences were lost. At the higher P levels, there were also significant differences between genotypes, but they were not consistent across growth stages.
Antioxidant Enzyme Activity and MDA Content in Root
Genotype L13 displayed significantly higher SOD activity in roots than genotype T3 (Fig. 5a). The effects of P deficiency on SOD activity in roots were not consistent among the different growth stages and soybean genotypes. Compared to the 0.5 mM P condition, the SOD activity in L13 roots significantly decreased at most growth stages except R4 in 0.25 mM P treatment, while it increased in the R2 to R4 stages in the 0 mM P treatment. For genotype T3, the SOD activity in roots was significantly greater at the V7 and R2 stages in the 0 mM P treatment than in higher P levels. Few differences were found in POD activity between soybean genotypes or among P treatments (Fig. 5b). For both soybean genotypes, CAT activity significantly decreased at growth stages R4 and R6 in the 0 mM P treatment compared to the control. The decrease amounted to 23.5 and 13.9% at R4, and 50.8 and 68.2% at R6, for genotypes L13 and T3, respectively (Fig. 5c). There was no significant difference in root MDA concentration between the different soybean genotypes at the 0.5 mM P level (Fig. 5d). However, at 0.25 mM P, the MDA concentration was higher in T3 at most growth stages and it was also higher at the R6 stage under the 0 mM P level.
Root Amino Acid Content
Sixteen amino acids were determined in plant roots. Significant differences among P treatments were found in aspartic acid and total amino acid concentrations (Fig. 6). Compared to 0.5 mM P, the total amino acid concentration of L13 significantly increased at R2 and R6 stages, while that of T3 severely decreased at reproductive stages (R4, R6 and R8), under 0.25 mM P treatment (Figs. 6a, 6b). In the 0 mM P treatment total amino acid concentration for both soybean cultivars was higher at the R4 stage than other stages. Total amino acid concentrations were significantly higher in genotype L 13 than in T3 at three growth stages in the 0.25 mM P treatment (Fig. 6b), however there were no significant differences between genotypes in the other two P treatments (Figs. 6a, 6c).
The aspartic acid (Asp) concentrations of both soybeans were substantially higher in the 0 mM P treatment than in the 0.25 and 0.5 mM P treatments (compare Fig. 6f with Figs. 6d, 6e). The accordingly increment for L13 amounted to 61.3, 73.6, 294.8, 392.4 and 239.8% for each growth stage from V4 to R6, respectively; and was 54.1, 12.2, 335.9, 111.6, 6.3 and 98.7% for T3 at each growth stage from V4 to R8, respectively. Asp concentrations were significantly higher in genotype L13 than in T3 at the R8 stage in the high P treatment, at the R6 and R8 stages in the 0.25 mM P treatment and at the V7, R4 and R6 growth stages in the 0 mM P treatment (Figs. 6d–6f).
Root Tip H+ Flow and IAA Flow
An influx of H+ (Fig. 7a) and IAA (Fig. 7b) was observed in root hair regions of root tips in both soybean cultivars under the 0.5 mM P treatment at the V4 growth stage. In the 0.5 mM P treatment, the H+ transport rate in the root hair region of L13 was significantly higher than in T3. In P deficient conditions, significant genotypic differences were observed. A release of H+ occurred in genotype L13, but not in T3. In both genotypes, the transport rate of H+ was faster in the 0 mM P treatment than in the 0.25 mM P treatment. A release of IAA in the root hair region of genotype T3 was found in P deficient treatments, while an influx occurred in L13.
DISCUSSION
P deficiency limited nutrient absorption and growth of soybean plants. Different degrees of P deficiency induced different plant responses. When P supply was slightly reduced (0.25 mM P level), even though soybean whole plant biomass and root P concentration changed little, both root biomass and root/shoot ratio were reduced compared with when P was in sufficient supply. However, when treated with 0 mM P, whole plant biomass, root biomass and root P concentration were significantly reduced, and the root/shoot ratio was increased, compared to when P was sufficient. The main reason for the differing results between the two P deficient treatments may be that, under the 0.25 mM P treatment, insufficient P limited root growth, and the P absorbed by the roots was maximally transported into the shoots to support shoot growth, whereas under the 0 mM P condition, no external P was available to support shoot growth which was extremely limited. These differences explain the differing trends of root/shoot ratio along the growth period between the 0 mM P treatment and the other two P conditions.
Under the same P level, the P-efficient genotype L13 had a higher root weight and root/shoot ratio than the P-inefficient genotype T3 under P deficient conditions, while the root P concentration tended to be higher in genotype L13 in all P treatments. The P concentration in the root system is an important indicator of P uptake capacity and affinity. Previous results indicate that genotype L13 can regulate root growth to adjust to low P conditions, allowing it to maintain yield better than genotype T3 under P deficient conditions [17]. Soluble sugars play a central role in plant structure and metabolism at the cellular and whole-plant levels. They are involved in the responses to a number of stresses and act as nutrient and metabolite signaling molecules [28]. The present findings revealed that the soluble sugar concentrations in L13 but not T3 roots increased when P deficient at an early growth stage (V4), and L13 displayed significantly higher root soluble sugar concentrations than T3 from growth stages V4 to R2 when treated with 0 P. An increase in soluble sugar content in response to stress is conducive to plant resistance, enhancing adaptability to the stress environment [29]. Under 0 mM P treatment, at growth stages V4 and R2, the very high root soluble sugar concentrations observed may be caused by the severity of P deficiency in the root system, leading to possible redistribution to the roots as part of the stress response. Combined with the amino acid findings described below, the increase in soluble sugar concentration was likely a response of the soybean root system to P-deficiency stress; however, details of the actual mechanism require further study.
Amino acid accumulation can improve the adaptability of plants to abiotic stress. When plants are exposed to external stress conditions, amino acids alter physiological metabolism as well as regulate expression of related genes and key enzyme activity, thereby enhancing the adaptive responses [30]. In the present study, few genotypic differences in amino acid contents were observed under the 0.5 mM P level. It is worth noting that in the 0 mM P treatment the aspartic acid concentration in roots of the P-efficient genotype was significantly higher than the P-inefficient genotype at the V7, R4 and R6 growth stages. These findings suggest that the response mechanisms of soybean differ under low and no P stresses. Under no P stress, soybean plants could regulate the amino acid content in roots and promote carbohydrate synthesis, to increase plant resistance and photosynthesis, and thereby adjust assimilate distribution and root growth.
Tests of ion dynamic flow provide accurate information on the regulation of plant tissue and cell ion balance. P deficiency induces H+—flow activity, promoting acidification of the rhizosphere soil as well as nutrient availability [13]. The present study revealed a significant increase in the efflux of H+ in the root hair zone in the P-efficient soybean under low and no P stresses, promoting acidification in the rhizoshpere. Moreover, the root H+ flow velocity was much faster in the 0 mM P than in the 0.25 mM P treatment. This suggests that the protoplasmic pump in the root cell plasma membrane of the P-efficient soybean genotype could adjust osmosis through increasing the transmembrane H+ concentration gradient and driving a flow of H+ ions across the plasma membrane.
Auxin transport is mediated by a protein carrier in the plasma membrane. Auxin plays an important role in initiating lateral root primordia and subsequent lateral root development. Enhanced IAA flow activity acts to promote the formation of lateral roots and root hairs [23]. In contrast to H+, IAA outflow strengthened in the P-inefficient genotype under the low and no P treatments, indicating a significant genotypic difference in the response and intensity of auxin transport under P deficiency stress. This is perhaps an important factor affecting P utilization efficiency.
In summary, plant roots are actively involved in absorption as well as being the first organ to sense and transport nutrient stress signals. Under P deficiency stress in this study, growth inhibition was less and root growth was greater in the P-efficient genotype than the P-inefficient genotype. This in turn caused an increase in P absorption and soluble sugar synthesis, thereby reducing the negative effect of low P stress on growth. Thus, root growth and physiological characteristics of the two genotypes resulted in different responses to the low concentrations of P, reflecting their specific ability to adapt to P deficiency stress. Different soybean genotypes therefore respond differently to low P and no P conditions. Overall, the results of this study suggest that the different response mechanisms of the soybean genotypes to nil and low P environments largely reflect changes to sugar metabolism, the allocation of auxin and response times. Further verification of these responses is still required.
REFERENCES
Lynch, J.P., Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops, Plant Physiol., 2011, vol. 156, p. 1041.
Liang, Q., Cheng, X., Mei, M., Yan, X., and Liao, H., QTL analysis of root traits as related to phosphorus efficiency in soybean, Ann. Bot., 2010, vol. 106, p. 223.
Carroll, P.V., Claudia, U.S., and Deborah, L.A., Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource, New Phytol., 2003, vol. 157, p. 423.
Ma, Z., Bielenberg, D.G., Brown, K.M., and Lynch, J.P., Regulation of root hair density by phosphorus availability in Arabidopsis thaliana,Plant Cell Environ., 2001, vol. 24, p. 459.
Chaudhary, M.I., Adu-Gyamfi, J.J., Saneoka, H., Nt, N., Suwa, R., Kanai, S., EI-Shemy, H., Lightfoot, D.A., and Fujita, K., The effect of phosphorus deficiency on nutrient uptake, nitrogen fixation and photosynthetic rate in mashbean, mungbean and soybean, Acta Physiol. Plant., 2008, vol. 30, p. 537.
Rychter, A.M., Randall, D.D., The effect of phosphate deficiency on carbohydrate metabolism in bean roots, Physiol. Plant., 1994, vol. 91, p. 383.
Rychter, A.M., Chauveau, M., Bomsel, J.L., Lance, C., The effect of phosphate deficiency on mitochondrial activity and adenylate in bean roots, Physiol. Plant., 1992, vol. 84, p. 80.
Drevon, J.J. and Hartwig, U.A., Phosphorus deficiency increases the argon-induced decline of nodule nitrogenase activity in soybean and alfalfa, Planta, 1997, vol. 201, p. 463.
Olivera, M., Tejera, N., Iribarne, C., Ocana, A., and Lluch. C., Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): effect of phosphorus, Physiol. Plant., 2004, vol. 121, p. 498.
Sa, T.M. and Israel, D.W., Nitrogen assimilation in nitrogen-fixing soybean plants during phosphorus deficiency, Crop Sci., 1955, vol. 35, p. 814.
Almeida, J.P., Hartwig, U.A., Freshner, M., Nösberger, J., and Lüsher, A., Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.), J. Exp. Bot., 2000, vol. 51, p. 1289.
Sawwan, J., Shibli, R.A., Swaidat, I., and Tahat, M., Phosphorus regulates osmotic potential and growth of African violet under in vitro-induced water deficit, J. Plant Nutr., 2000, vol. 23, p. 759.
Zhang, R., Liu, G., Wu, N., Gu, M., Zeng, H.Q., Zhu, Y.Y., and Xu, G.H., Adaptation of plasma membrane H+ ATPase and H+ pump of P deficiency in rice roots, Plant Soil, 2011, vol. 349, p. 3.
Ahn, T., Schofield, A., and Paliyath, G., Antioxidant enzyme activities during tomato fruit development and in response to phosphorus nutrients, Proc. XXVI Int. Horticultural Congr., Toronto, Leuven: Int. Soc. Hortic. Sci., 2002.
Fageria, N.K., Baligar, V.C., and Li, Y.C., The role of nutrient efficient plants in improving crop yields in the twenty first century, J. Plant Nutr., 2008, vol. 31, p.1121.
Li, Z.G., Xie, F.T., and Song, S.H., The selection of high phosphorus using efficient soybean genotype, Chin. Agric. Sci. Bull., 2004, vol. 20, p. 126.
Ao, X., Xie, F.T., Zhang, H.J., Liu, J.Q., and Wang, H.Y., Effect of phosphorus on root traits of soybean cultivars with different phosphorus efficiencies, Soybean Sci., 2008, vol. 27, p. 787.
Ao, X., Guo, X.H., Zhu, Q., Zhang, H.J., Wang, H.Y., Han, X.R., and Xie, F.T., Effect of phosphorus fertilization to P uptake and dry matter accumulation in soybean with different P efficiencies, J. Integr. Agric., 2014, vol. 13, p. 326.
Villagarcia, M.R., Thomas, E., Carter, J., Rufty, W.T., Niewoehner, A.S., Jennette, M.W., and Arrellano, C., Genotypic rankings for aluminum tolerance of soybean roots grown in hydroponics and sand culture, Crop Sci., 2001, vol. 41, p. 1499.
Fehr, W.E., Caviness, C.E., Burmood, P.T., and Pennington, J., Stage of development description of soybean, Crop Sci., 1971, vol. 11, p. 929.
Cavell, A.J., The colorimetric determination of phosphorus in plant materials, J. Sci. Food Agric., 1955, vol. 6, p. 479.
Chen, C., Jiang, Q., Ziska, L.H., Zhu, J., Liu, G.Q., Zhang, J.S., Ni, K., Seneweera, S., and Zhu, C.W., Seed vigor of contrasting rice cultivars in response to elevated carbon dioxide, Field Crop Res., 2015, vol. 178, p. 63.
Li, H.S., Principle and Technology of Plant Physiology and Biochemistry Experiment, Beijing: High Educ. Press China, 2002.
Shah, K. and Nahakpam, S., Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars, Plant Physiol. Biochem., 2012, vol. 57, p. 106.
Ren, P., Zhu, Z., Dong, B., Zang, J., and Gong, L., Determination of energy and amino acid digestibility in growing pigs fed corn distillers' dried grains with solubles containing different lipid levels, Arch. Anim. Nutr., 2011, vol. 65, p. 303.
Jiang, T., Feng, Y.J., He, X.C., Hu, J.R., Tang, K., Li, H.Z., Liu, Y.H., and Sheng, Q., Research on localized reagent and rapid analysis method of amino acid analyzer, Chem. Res. Appl., 2012, vol. 24, p. 1159.
Ying, L.P., Shangguan, Y., and Xu, Y., Non-injured scanning ion selective electrode technique and its application in the research of higher plants, Prog. Nat. Sci., 2006, vol. 16, p. 262.
Couée, I., Sulmon, C., Gouesbet, G., and El Amrani, A., Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants, J. Exp. Bot., 2006, vol. 57, p. 449.
Wang, X.H., Li, D.Y., Xu, K.Z., Zhang, Z.A., Wu, Z.H., Chen, Z.Y., Yang, G.Y., and Yang, C.M., Comparison on some physiological characteristics of three evolution types of soybean, Plant Physiol. Commun., 2006, vol. 42, p. 191.
Song, Q.C., Cao, F.Q., Gong, Y.Y., Cheng, X.Y., Xin-Yuan, B.I., and Liu, L.H., Current research progresses of amino acids uptake, transport and their biological roles in higher plants, Plant Nutr. Fertil. Sci., 2012, vol. 18, p. 1507.
Funding
The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China (project no. 31271643) and the Key Project of Liaoning Natural Science Foundation (project no. 20170540809).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants as objects of research.
Rights and permissions
About this article
Cite this article
Zhu, Q., Wang, H., Shan, Y.Z. et al. Physiological Response of Phosphorus-Efficient and Inefficient Soybean Genotypes under Phosphorus-Deficiency. Russ J Plant Physiol 67, 175–184 (2020). https://doi.org/10.1134/S1021443720010276
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443720010276