Abstract
Understanding how phosphorus (P) deficiency during the reproductive phase of soybean [Glycine max (L.) Merril] affects nitrogen (N) acquisition via biological N fixation (BNF), and seed yield per unit of the accumulated nutrient remains incomplete. Soybean plants were fertigated with a sufficient concentration of P in the nutrient solution (500 µmol L-1 P) until flowering. Subsequently, plants were maintained under this condition or subjected to nutrient deficiencies (20 or 100 µmol L-1 P), resulting in three regimes of P supply during the reproductive phase. At the onset of maximum grain-filling rate and physiological harvest, various parameters were assessed, including nodulation traits, plant nutritional status and biomass production, accumulation, partitioning, and utilization efficiency of P and N. P deficiency after flowering negatively impacted soybean yield and dry mass production, as well as the concentration of P and N in plant organs, their total shoot content, and partitioning to grains. The poor BNF performance was associated with a reduction in the number and dry mass of nodules, triggered by a decrease in plant’s N demand. Nevertheless, low-P stress did not affect seed yield per unit of acquired nutrient, which was related to the fact that the decline in N partitioning to grains was accompanied by a proportional decreasing in their N concentration. The down-regulation of BNF, rather than an impaired N utilization efficiency, contributes to explaining reduced yield of soybean plants facing post-flowering P deficiency. Therefore, the development of precise P fertilization management approaches to maximize BNF and crop yield should prioritize strategies that ensure adequate P supply across the reproductive phase of soybean.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The sustainability of soybean [Glycine max (L.) Merril] production relies heavily on efficient biological nitrogen fixation (BNF) to meet high plant’s nitrogen (N) requirements. This process of N acquisition reduces environmental risks associated with the overapplication of synthetic N fertilizers (Ciampitti and Salvagiotti 2018; Telles et al. 2023). Thus, addressing detrimental consequences of nutritional stresses on BNF is a crucial step toward precise nutrient management of soybean fields, aiming for long-term sustainable gains in resource use efficiency (O’hara et al. 1988; Santachiara et al. 2019; Latifinia and Eisvand 2022). In this context, ameliorating the effects of phosphorus (P) deficiency deserves special attention, as it is a widespread nutritional disorder in weathered tropical soils (Vance et al. 2003) impairing growth, yield, and BNF performance of soybean (Qin et al. 2012; Medina et al. 2022; Mirriam et al. 2022). As a response to this adverse condition, the application of the entire recommended rate of P at sowing has been a commonly adopted fertilization strategy in fields cultivated with this grain legume (Li et al. 2011; Farmaha et al. 2012). While proper P supply at sowing is crucial for maximizing seedling vigor and crop harvests (Grant et al. 2001), it also contributes to N acquisition by stimulating formation and functioning of nodules at early plant developmental stages (Kouas et al. 2005; Bulgarelli et al. 2017; Qiao et al. 2017).
Nevertheless, it is noteworthy that the demand and uptake rates of P by soybean are relatively low during the vegetative phase, with the majority of this nutrient accumulating after flowering (Bender et al. 2015; Gaspar et al. 2017a). Such a phenomenon, coupled with a simultaneous decline in the availability of P and its uptake capacity by the roots (Howard et al. 1999; Vengavasi and Pandey 2018; Yang et al. 2023), leads to the risks of late P deficiency. This might have further implications for N acquisition via BNF (Li et al. 2022), given that N2 fixation continues at high rates until the end of grain-filling phase, unless the plants experience events of abiotic stresses (Mastrodenico and Purcell 2012; Mastrodenico et al. 2013; Van Roekel et al. 2015). Indeed, the peak of N demand (Bender et al. 2015; Gaspar et al. 2017b) and the stabilization of nodules production in soybean occur during the reproductive phase (Gray et al. 2012). Despite this and the vulnerability of N2 fixation to unfavorable conditions across the post-flowering period, the specific effect of P deficiency on BNF performance has not been widely explored in soybean.
Moreover, it has been proposed that a disturbed plant P nutritional status contributes to hampering overall crop performance by constraining the conversion of the acquired N into biomass production (N utilization efficiency, NUtE). The decline in NUtE is associated with impaired reduction and assimilation of the inorganic N acquired by the roots (Rufty et al. 1990; De Groot et al. 2003; Gan et al. 2016). In addition to this, the conversion of the accumulated N into yield formation depends directly also on the amount of the nutrient that is partitioned to grains to sustain their proper development (The et al. 2021; Liang et al. 2023). Accordingly, NUtE at crop maturity can be negatively impacted by adverse conditions hindering remobilization and allocation of N to the harvestable organs during the post-flowering period (Marmagne et al. 2020, 2022). However, in the case of nodulated soybean plants, the incorporation of soluble-reduced N into organic compounds has been shown to be less sensitive to the negative effects of low-P stress compared to N2 fixation (Israel and Rufty 1988; Sa and Israel 1995). In this sense, whether a restricted P availability after flowering affects the conversion of the fixed N2 into grain yield at physiological harvest in nodulated soybean plants remains to be clarified.
Herein, we tested the hypothesis that detrimental effects on soybean yield resulting from a constrained N2 fixation capacity under post-flowering P deficiency stress would be exacerbated by a less efficient conversion of the acquired N into yield formation. Therefore, we conducted a study examining the effects of varying levels of post-flowering P supply (sufficiency, mild and severe deficiencies) on nodulation parameters, plant growth and nutritional status, yield performance, as well as the accumulation and partitioning of P and N. The P nutrition regimes were specifically implemented during the reproductive phase to replicate field conditions where a lack of synchrony between availability and demand of P could affect soybean nutritional status during grain-filling (Duarte et al. 2022; Laira et al. 2023).
2 Materials and Methods
2.1 Plant Material, Nutritional Management until Flowering, and P Treatments during the Reproductive Phase
The experiment was conducted in a greenhouse at the Agronomic Institute’s experimental area from October 2019 to February 2020. We utilized the soybean variety NA 5909RG, known for its indeterminate growth habit, relative maturation group of 6.2, and widespread cultivation in major producing regions (Matei et al. 2017). Throughout the experimental period, the average air temperature and relative humidity were 38 °C and 85%, respectively. Soybean seeds were inoculated with Bradrhizobium japonicum (Biovita Turfa Soja®) at a rate of 1.2 g kg− 1 of seeds. Subsequently, eight seeds were sown in pots containing 7.5 dm³ of fine-textured vermiculite (Global Minérios®).
Thinning was performed five days after emergence (DAE), retaining the three most vigorous plants. Concurrently, we initiated fertigation with a nutrient solution diluted at 50% of its final concentration (details provided below). At ten DAE, we transitioned to a solution with 100% nutrient concentration, which was used until the end of the experiment. The nutrient solution, devoid of N source, contained the following concentrations (µmol L− 1): 5,000 Ca; 3,000 K; 500 P; 1,300 Mg; 1,300 S; 41.6 B; 0.3 Co; 1.0 Cu; 46.7 Fe; 10.0 Mn; 1.3 Mo; 0.01 Ni and 3.5 Zn (Duarte et al. 2022). Fertigation was performed every other day throughout the experimental period, with the volume of applied solution tailored to ensure leaching, ranging from 450 to 900 mL per pot depending on the plant’s phenological phase.
Soybean plants were fertigated with the aforementioned nutrient solution until they reached the R1 phenological phase, characterized by the emergence of the first flower on the main stem (Fehr and Caviness 1977). To simulate varying P availabilities in the rooting medium during the reproductive phase, different concentrations of P were applied from R1 until physiological harvest (R8) (Fehr and Caviness 1977). Treatments included severe deficiency (20 µmol L− 1 P, P20), mild deficiency (100 µmol L− 1 P, P100), and sufficiency (500 µmol L− 1 P, P500), defined according to previous studies (Singh et al. 2018; Duarte et al. 2022). The experiment followed a randomized block design with four replicates for each regime of post-flowering P supply. Each replicate consisted of two pots with three plants, totaling 24 pots.
2.2 Assessment of Soybean Responses to Post-flowering P Treatments: Nodulation Traits, Biomass Production, and Nutrient Efficiency
During the reproductive phase, in addition to the evaluation at physiological harvest (R8), an earlier assessment was performed at the R5.5 phenological phase, which corresponds to the beginning of the maximum grain-filling rate (Fehr and Caviness 1977). This evaluation was chosen because R5.5 represents also the period of maximum P and N accumulation rates in soybean plants (Bender et al. 2015; Gaspar et al. 2017a, b) and potentially the point when nodule production by this legume species is at its highest (Gray et al. 2012).
At R5.5, half of the experimental units (12 pots) were destructively sampled. The plants were separated into leaves, stems with petioles, reproductive structures (pods with grains), roots, and nodules. These parts were washed in tap water, rinsed with deionized water and dried at 60 ⁰C for three days to measure dry mass (DM) production. Nodules were counted to obtain nodule number (nodules pot− 1) and to estimate specific nodulation (nodules g− 1 root DM). Furthermore, average nodule DM (mg nodule− 1) was calculated as the ratio between nodule DM (mg pot− 1) and nodule number.
At R5.5, shoot DM production (g pot− 1) was determined by summing DM of leaves, stems with petioles, and reproductive structures. Harvest index (HI, %) was calculated as the ratio between DM of reproductive structures and shoot DM. At R8, the assessment of DM production followed the same procedures, except for nodules, which were senescent and could not be separated from roots. In addition, pods and grains were individually evaluated, providing pod-shell DM (g pot− 1) and grain DM (g pot− 1). Then, HI at R8 was the ratio between grain DM and shoot DM.
The nodules collected at R5.5 and all plant shoot organs sampled at R5.5 and R8 were ground and P concentration (g kg− 1) was determined using inductively coupled plasma optical emission spectrometry (ICP-OES) after wet digestion with nitric and perchloric acids. The concentration of N (g kg− 1) in the same organs was measured through distillation after sulfuric acid digestion. Shoot accumulation (g pot− 1) of P and N was estimated as the sum of the content of P and N in each organ, which was calculated as the product of their respective nutrient concentration and DM production. Given absence of N supply through nutrient solution, the amount of N accumulated (g pot− 1) in soybean shoots at R5.5 and R8 was assumed to originate from BNF (Prudent et al. 2016). Based on this, nodule activity (mg N nodule− 1) at R5.5 and R8 was the ratio of shoot N content at each sampling time and nodule number at R5.5.
Furthermore, utilization efficiency (g g− 1) of P (PUtE) and N (NUtE) was estimated as the ratio between DM of reproductive structures (at R5.5) or grain (at R8) and shoot content of P or N at each phenological phase. P harvest index (PHI, %) and N harvest index (NHI, %) were the ratio between the content (g pot− 1) of P and N in the reproductive structures (at R5.5) or grains (at R8) relative to their respective shoot content in each evaluation period.
2.3 Statistical Analysis
The obtained data were subjected to one-way analysis of variance (ANOVA) using Sisvar software (Ferreira 2011). Tukey’s multiple range test (p < 0.05) was then employed to compare the means of the treatments, the concentration of P in the nutrient solution throughout the reproductive phase.
3 Results
3.1 Nodulation Traits
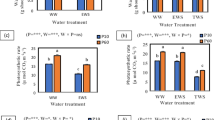
At R5.5, plants subjected to post-flowering P deficiency exhibited reduction in several nodulation traits compared to those under sufficiency. Specifically, for the average of P20 and P100 plants, nodule number decreased by 47%, nodule DM by 53%, and specific nodulation by 47% (Fig. 1a-c). Only P100 plants showed a lower average nodule DM compared to P500 plants and with a less pronounced effect size relative to the other nodulation traits (Fig. 1d). Nodule activity at R5.5 and R8 of P-limited plants was either higher or similar compared to those found in well-nourished plants (Fig. 1e, f).
Nodule number (in a), nodule dry mass (DM) (in b), specific nodulation (in c), average nodule DM (in d) of soybean plants at the beginning of maximum grain-filling rate (R5.5) and nodule activity at R5.5 (in e) and physiological harvest (R8) (in f). Supply of phosphorus (P) during the reproductive phase corresponded to fertigation with 20 µmol L− 1 P (P20, severe deficiency), 100 µmol L− 1 P (P100, mild deficiency) and 500 µmol L− 1 P (P500, sufficiency). Columns followed by different letters indicate significant differences based on Tukey’s multiple range test (p < 0.05). Bars indicate the standard error (n = 4)
3.2 Concentration of P and N in Shoot Organs and Nodules
At R5.5, the concentration of P in the shoot organs of plants grown under P500 condition was higher than those subjected to P20 and P100 conditions, with differences ranging from 21 to 90% (Fig. 2a, c, e). However, in the case of nodules, no response to varying P supply was found (Fig. 2g). P limitation decreased also N concentration in leaves and stems of soybean, but there was a lack of effect on reproductive structures and nodules (Fig. 2b, d, f, h).
Concentration of phosphorus (P) or nitrogen (N) in the leaves (in a and b), stems (in c and d), reproductive structures (pods with grains, in e and f) and nodules (in g and h) of soybean plants at the beginning of maximum grain-filling rate (R5.5). Supply of P during the reproductive phase corresponded to fertigation with 20 µmol L− 1 P (P20, severe deficiency), 100 µmol L− 1 P (P100, mild deficiency), and 500 µmol L− 1 P (P500, sufficiency). Different letters indicate significant differences based on Tukey’s multiple range test (p < 0.05). Bars indicate the standard error (n = 4)
By R8, post-flowering P supply did not affect the concentration of P and N in the leaves, while the concentration of these nutrients in the stems decreased under P limitation (Fig. 3a-d). Low-P stress reduced the concentration of P in pod-shells, but the opposite occurred in the case of N (Fig. 3e, f). In the grains, concentration of both P and N were lower under P deficiency than P sufficiency; however, the magnitude of the decreasing was greater for P, a reduction of 32% in P20 and P100 plants compared to P500 plants (Fig. 3g, h).
Concentration of phosphorus (P) or nitrogen (N) in the leaves (in a and b), stems (in c and d), pod-shells (in e and f) and grains (in g and h) of soybean plants at the physiological harvest (R8). Supply of P during the reproductive phase corresponded to fertigation with 20 µmol L− 1 P (P20, severe deficiency), 100 µmol L− 1 P (P100, mild deficiency), and 500 µmol L− 1 P (P500, sufficiency). Different letters indicate significant differences based on Tukey’s multiple range test (p < 0.05). Bars indicate the standard error (n = 4)
3.3 Plant Biomass Production and Partitioning
At R5.5, DM production of reproductive structures was 17% lower in P20 and 10% lower in P100 plants compared to P500 ones (Fig. 4a). By R8, the imposition of P limitation during the reproductive phase also resulted in compromised yield performance: the relative grain DM of P20 and P100 plants, compared to P500 plants, corresponded to 58% and 67%, respectively. Additionally, grain DM of plants under P100 was 15% higher than that in P20 plants (Fig. 4b). DM production of vegetative organs (leaves combined with stems and petioles) at R5.5 and R8 as well as shoot DM at R5.5 did not vary as a function of P supply (Fig. 4c, d, e). However, shoot DM at R8 was 27% lower in P20 plants and 16% lower in P100 plants compared to those grown under sufficiency (Fig. 4f). Regarding partitioning of DM, HI of P-limited plants decreased by 9% at R5.5 and by 22% at R8 compared to P500 plants (Fig. 4g, h).
Dry mass (DM) production of reproductive structures (pods with grains) at the beginning of maximum grain-filling rate (R5.5) (in a), grains at physiological harvest (R8) (in b), vegetative organs (leaves combined with stems and petioles) at R5.5 (in c) and R8 (in d), total shoot at R5.5 (in e) and R8 (in f), and harvest index at R5.5 (in g) and R8 (in h) of soybean plants. Supply of phosphorus (P) during the reproductive phase corresponded to fertigation with 20 µmol L− 1 P (P20, severe deficiency), 100 µmol L− 1 P (P100, mild deficiency), and 500 µmol L− 1 P (P500, sufficiency). Different letters indicate significant differences based on Tukey’s multiple range test (p < 0.05). Bars indicate the standard error (n = 4)
3.4 Accumulation, Partitioning and Utilization Efficiency of P and N
At the earlier sampling time (R5.5), the content of P in the reproductive structures and in the whole shoot of P-limited plants was on average 41% and 43% lower, respectively, than under P sufficiency; and, in the case of N, these differences corresponded to 20% and 22%, respectively (Fig. 5a-d). By R8, content of P and N in the grains was negatively impacted by P deficiency, with reductions of 57% and 45%, respectively (Fig. 5e, f). At R8, shoot P content was 2.3- and 2.0-fold higher in P500 plants than in P20 and P100 ones, respectively; also, shoot P content under P100 condition was 14% higher than under P20 (Fig. 5g). Compared to P500 plants, shoot N content decreased by 41% and 33% in P20 and P100 plants, respectively (Fig. 5h).
Content of phosphorus (P) or nitrogen (N) in the reproductive structures (pods with grains) at the beginning of maximum grain-filling rate (R5.5) (in a and b), in the whole shoot at R5.5 (in c and d), in the grains at physiological harvest (R8) (in e and f) and in the whole shoot at R8 (in g and h) of soybean plants. Supply of P during the reproductive phase corresponded to fertigation with 20 µmol L− 1 P (P20, severe deficiency), 100 µmol L− 1 P (P100, mild deficiency), and 500 µmol L− 1 P (P500, sufficiency). Different letters indicate significant differences based on Tukey’s multiple range test (p < 0.05). Bars indicate the standard error (n = 4)
At R5.5, post-flowering P supply did not influence the partitioning of P and N to the reproductive structures and NUtE, but PUtE was 37% lower under P500 than other P supplies (Fig. 6a-d). At R8, while P deficiency had a negative effect on PHI, PUtE was higher in P limited plants than well-nourished ones (Fig. 6e, g). Restricted P availability posed a negative impact on NHI, with a decrease of 14% on this parameter compared to P500 plants (Fig. 6f). However, P deficiency did not influence NUtE at R8, and the average value across nutrition treatments corresponded to 14.9 g g− 1 (Fig. 6h).
Shoot harvest index of phosphorus (P) or nitrogen (N) at the beginning of maximum grain-filling rate (R5.5) (in a and b) and physiological harvest (R8) (in e and f) and utilization efficiency of P or N at R5.5 (in c and d) and R8 (in g and h) of soybean plants. Supply of P during the reproductive phase corresponded to fertigation with 20 µmol L− 1 P (P20, severe deficiency), 100 µmol L− 1 P (P100, mild deficiency), and 500 µmol L− 1 P (P500, sufficiency). Different letters indicate significant differences based on Tukey’s multiple range test (p < 0.05). Bars indicate the standard-error (n = 4)
4 Discussion
The productivity of soybean can be described as the product of plant N content and grain DM produced per unit of the nutrient accumulated throughout the crop cycle (Rotundo et al. 2014; Ortez et al. 2019). Accordingly, examining the impact of nutritional disorders on these yield determinants would be useful to understand how adverse growing conditions affect soybean performance and to propose avenues for crop improvement. In this respect, our results revealed that shoot N content of P20 and P100 plants was lower than that of P500 plants at both R5.5 and R8, with the difference becoming more pronounced at the latter phenological phase. Nevertheless, the efficiency of soybean in converting the acquired N into DM production of reproductive structures at R5.5 and grains at R8 was not influenced by P supply regime. As a result, the poor crop performance under post-flowering P deficiency condition was a primary consequence of impairments in the plant’s capability of accumulating N in their shoots over the reproductive phase. Hence, while a sufficient P availability since early phenological phases has proved to be essential for nodule formation and functioning (Qin et al. 2012; Bulgarelli et al. 2017; Qiao et al. 2017), it is also evident that BNF is sensitive to post-flowering P deficiency, as already observed for other abiotic stresses (Mastrodenico and Purcell 2012; Mastrodenico et al. 2013; Van Roekel et al. 2015).
Considering that the proper functioning of shoot organs relies on a fine-tuned balance between the concentration of P and N, soybean plants appear to employ, in response to post-flowering P limitation, a primary strategy of down-regulating BNF to mitigate the risks of nutrient imbalance (Sadras 2006; Vitousek et al. 2010; Li et al. 2023). Herein, this condition was found in P20 and P100 plants, that exhibited a concomitant reduction in the concentration of P and N in the leaves and stems at R5.5. In P-stressed tissues, an impaired synthesis of proteins leads to an increase in the content of free amino acids, which in turn triggers an N-feedback mechanism resulting in the down-regulation of BNF (Schulze 2004). Moreover, given that shoot growth potential drives N requirement of the plants (Voisin et al. 2010), we argue that differences in yield performance posed also an important influence on the control of BNF: while there was no variation in the biomass of vegetative parts, DM of harvestable organs (pods with grains at R5.5 and grains at R8) and HI increased with a sufficient P supply. Indeed, actively filling grains act as strong sinks for N transport from vegetative tissues and plays a critical role on the stimulation of BNF (Rotundo et al. 2014). Such a phenomenon might, in turn, explain the exacerbation of the difference in shoot N content between P-deficient and -sufficient plants from R5.5 to R8. Interestingly, P-induced DM production increases the demand for N, which in turn enables BNF to persist and potentially contribute to higher crop yields and grain N content (Cabeza et al. 2024).
Additional insights into how nutrient deficiencies impact BNF potential of grain legume crops can be obtained with the assessment of nodulation parameters (Kouas et al. 2005; Zhong et al. 2024; Koutroubas et al. 2023). In our study, such an evaluation was performed at a critical stage coinciding with the peak of N demand (Bender et al. 2015; Gaspar et al. 2017b) and stabilization of nodules production in soybean (Gray et al. 2012). It was found that nodule number, nodule DM and average nodulation were severely affected in P20 and P100 plants, most likely because a lower plant’s N demand, as a response to P limitation in the rooting medium, induces a decreasing on nodule production (Divito and Sadras 2014). However, P-deficient plants exhibited shoot N content per nodule that was either higher or similar compared to the P-sufficient ones and there was no variation in the concentration of P and N in the nodules across treatments. These findings suggest that, in order to cope with the damaging effects of late P limitation on the process of N2 fixation, soybean plants tend to optimize the allocation of P to the formed nodules for proper functioning (Sulieman and Tran 2015; Valentine et al. 2017).
Finally, our results revealed also that the condition of post-flowering P deficiency impacted negatively the efficiency by which N is partitioned to the grains, as indicated by the decline in NHI at the physiological harvest. Nevertheless, this phenomenon was not translated into a reduced NUtE because a comparable decreasing occurred in grain N concentration (Manschadi et al. 2014; Rotundo et al. 2014). There is positive relationship between the partitioning of P and N toward reproductive tissues in grain crops (Ma et al. 2016), which explains the fact that the harvest indexes of these nutrients responded in parallel to P supply regimes. However, compared to N, the nutritional status of P of harvestable organs seems to be more sensitive to the effects of post-flowering P deficiency. For instance, in addition to the decline in P concentration of reproductive structures at R5.5, the reduction in grain P concentration was higher than that of N at R8 (32% versus 9%); moreover, P concentration in pod-shells decreased under P deficiency, but an opposite response occurred in the case of N. These contrasting outcomes contribute to elucidate the finding that while NUtE efficiency remained unaltered across nutrition treatments, PUtE was higher under P limitation despite the reduction in PHI. It is worth noting even though the decrease in grain concentration of P and N ameliorated yield penalty of soybean facing post-flowering P deficiency, such a phenomenon could further also affect seed quality and seedling vigor (Wilson et al. 2014; Rose et al. 2020).
5 Conclusions
Our study highlights the critical role of P availability during the reproductive phase on N nutrition relationships of soybean. While P deficiency during such a period of plant development does not affect the conversion of acquired N into yield formation, it severely harms the process of N2 fixation. Accordingly, there is no evidence to support the hypothesis that impaired N utilization efficiency exacerbates the detrimental effects of hindered N2 fixation on crop productivity under post-flowering P deficiency. Moreover, the damages caused by low-P on N acquisition cannot be directly ascribed to poor nodule functioning, as evidenced by the lack of variation in the content of N2 fixed per nodule and concentration of P and N in this organ. Then, the decreasing in nodule production along the reproductive phase of soybean due to diminished N requirement is a primary factor governing N2 fixation potential. In this context, optimizing strategies to maximize N acquisition should involve approaches that ensure a proper supply of P during the reproductive phase of soybean. Also, our findings shed light on the need of addressing the complex interplay between P and N in soybean production systems for advances in crop nutrient management and agricultural sustainability.
Data Availability
Data available on request from the authors.
References
Bender RR, Haegele JW, Below FE (2015) Nutrient uptake, partitioning, and remobilization in modern soybean varieties. Agron J 107:563–573. https://doi.org/10.2134/agronj14.0435
Bulgarelli RG, Marcos FCC, Ribeiro RV, de Andrade SAL (2017) Mycorrhizae enhance nitrogen fixation and photosynthesis in phosphorus-starved soybean (Glycine max L. Merrill). Environ Exp Bot 140:26–33. https://doi.org/10.1016/j.envexpbot.2017.05.015
Cabeza RA, Schulze J, Salinas-Roco S, Morales-González A, Amigo R, Pérez-Díaz R, Carrasco B, Contreras-Soto R, Maldonado C, Pedreschi R, Espinoza S, del Pozo A (2024) The inhibition of N2 fixation by nitrogen is attenuated by the P supply, altering the plant metabolism. Environ Exp Bot 222:105762. https://doi.org/10.1016/j.envexpbot.2024.105762
Ciampitti IA, Salvagiotti F (2018) New insights into soybean biological nitrogen fixation. Agron J 110:1185–1196. https://doi.org/10.2134/agronj2017.06.0348
De Groot CC, Marcelis LF, van den Boogaard R, Kaiser WM, Lambers H (2003) Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil 248:257–268. https://doi.org/10.1023/A:1022323215010
Divito GA, Sadras VO (2014) How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crops Res 156:161–171. https://doi.org/10.1016/j.fcr.2013.11.004
Duarte FCN, Cipriano MAP, de Andrade SAL, Zambrosi FCB (2022) Foliar application of phosphite reduces grain weight of soybean facing postflowering phosphorus deficiency. J Plant Nutr Soil Sci 186:30–37. https://doi.org/10.1002/jpln.202200124
Farmaha BS, Fernández FG, Nafziger ED (2012) Soybean seed composition, aboveground growth, and nutrient accumulation with phosphorus and potassium fertilization in no-till and strip‐till. Agron J 104:1006–1015. https://doi.org/10.2134/agronj2012.0010
Fehr WR, Caviness CE (1977) Stages of soybean development. Cooperative Extension Service, Agriculture and Home Economics Experiment Station, Iowa State University, Ames, IA
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciênc Agrotec 35:1039–1042. https://doi.org/10.1590/S1413-70542011000600001
Gan H, Jiao Y, Jia J, Wang X, Li H, Shi W, Peng C, Polle A, Luo ZB (2016) Phosphorus and nitrogen physiology of two contrasting poplar genotypes when exposed to phosphorus and/or nitrogen starvation. Tree Physiol 36:22–38. https://doi.org/10.1093/treephys/tpv093
Gaspar AP, Laboski CA, Naeve SL, Conley SP (2017a) Phosphorus and potassium uptake, partitioning, and removal across a wide range of soybean seed yield levels. Crop Sci 57:2193–2204. https://doi.org/10.2135/cropsci2016.05.0378
Gaspar AP, Laboski CA, Naeve SL, Conley SP (2017b) Dry matter and nitrogen uptake, partitioning, and removal across a wide range of soybean seed yield levels. Crop Sci 57:2170–2182. https://doi.org/10.2135/cropsci2016.05.0322
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224. https://doi.org/10.4141/P00-093
Gray SB, Strellner RS, Puthuval KK, Ng C, Shulman RE, Siebers MH, Rogers A, Leakey ADB (2012) Minirhizotron imaging reveals that nodulation of field-grown soybean is enhanced by free-air CO2 enrichment only when combined with drought stress. Funct Plant Biol 40:137–147. https://doi.org/10.1071/FP12044
Howard DD, Essington ME, Tyler DD (1999) Vertical phosphorus and potassium stratification in no-till cotton soils. Agron J 91:266–269. https://doi.org/10.2134/agronj1999.00021962009100020014x
Israel DW, Rufty TW Jr (1988) Influence of phosphorus nutrition on phosphorus and nitrogen utilization efficiencies and associated physiological responses in soybean. Crop Sci 28:954–960. https://doi.org/10.2135/cropsci1988.0011183X002800060018x
Kouas S, Labidi N, Debez A, Abdelly C (2005) Effect of P on nodule formation and N fixation in bean. Agron Sustain Dev 25:389–393. https://doi.org/10.1051/agro:2005034
Koutroubas SD, Damalas CA, Fotiadis S, Markopoulos T (2023) Species, cultivar and seasonal effects on nodulation and nitrogen utilization of spring mediterranean grain legumes. J Soil Sci Plant Nutr 23:4463–4473. https://doi.org/10.1007/s42729-023-01364-7
Laira MD, Andrade SA, Silveira NM, Machado EC, Ribeiro RV, Zambrosi FCB (2023) High post-flowering phosphorus status promotes the tolerance of soybean to terminal heat stress. Environ Exp Bot 215:105501. https://doi.org/10.1016/j.envexpbot.2023.105501
Latifinia E, Eisvand HR (2022) Soybean physiological properties and grain quality responses to nutrients, and predicting nutrient deficiency using chlorophyll fluorescence. J Soil Sci Plant Nutr 22:1942–1954. https://doi.org/10.1007/s42729-022-00785-0
Li SX, Wang ZH, Stewart BA (2011) Differences of some leguminous and nonleguminous crops in utilization of soil phosphorus and responses to phosphate fertilizers. Adv Agron 110:125–249. https://doi.org/10.1016/B978-0-12-385531-2.00003-7
Li H, Wang L, Zhang Z, Yang A, Liu D (2022) Effect of phosphorus supply levels on nodule nitrogen fixation and nitrogen accumulation in soybean (Glycine max L). Agronomy 12:2802. https://doi.org/10.3390/agronomy12112802
Li Q, Philp J, Denton MD, Huang Y, Wei J, Sun H, Li Y, Zhao Q (2023) Stoichiometric homeostasis of N: P ratio drives species-specific symbiotic N fixation inhibition under N addition. Front Plant Sci 14:1076894. https://doi.org/10.3389/fpls.2023.1076894
Liang G, Hua Y, Chen H, Luo J, Xiang H, Song H, Zhang Z (2023) Increased nitrogen use efficiency via amino acid remobilization from source to sink organs in Brassica napus. Crop J 11:119–131. https://doi.org/10.1016/j.cj.2022.05.011
Ma BL, Zheng ZM, Morrison MJ, Gregorich EG (2016) Nitrogen and phosphorus nutrition and stoichiometry in the response of maize to various N rates under different rotation systems. Nutr Cycl Agroecosyst 104:93–105. https://doi.org/10.1007/s10705-016-9761-6
Manschadi AM, Kaul HP, Vollmann J, Eitzinger J, Wenzel W (2014) Developing phosphorus-efficient crop varieties-an interdisciplinary research framework. Field Crops Res 162:87–98. https://doi.org/10.1016/j.fcr.2013.12.016
Marmagne A, Jasinski S, Fagard M, Bill L, Guerche P, Masclaux-Daubresse C, Chardon F (2020) Post-flowering biotic and abiotic stresses impact nitrogen use efficiency and seed filling in Arabidopsis thaliana. J Exper Bot 71:4578–4590. https://doi.org/10.1093/jxb/eraa011
Marmagne A, Masclaux-Daubresse C, Chardon F (2022) Modulation of plant nitrogen remobilization and postflowering nitrogen uptake under environmental stresses. J Plant Physiol 277:153781. https://doi.org/10.1016/j.jplph.2022.153781
Mastrodomenico AT, Purcell LC (2012) Soybean nitrogen fixation and nitrogen remobilization during reproductive development. Crop Sci 52:1281–1289. https://doi.org/10.2135/cropsci2011.08.0414
Mastrodomenico AT, Purcell LC, King CA (2013) The response and recovery of nitrogen fixation activity in soybean to water deficit at different reproductive developmental stages. Environ Exp Bot 85:16–21. https://doi.org/10.1016/j.envexpbot.2012.07.006
Matei G, Benin G, Woyann LG, Dalló SC, Milioli AS, Zdziarski AD (2017) Agronomic performance of modern soybean cultivars in multi-environment trials. Pesq Agropec Bras 52:500–511. https://doi.org/10.1590/S0100-204X2017000700004
Medina IR, da Rocha GH, Pereira EG (2022) Limitations in grain yield and carbon partitioning differs in soybean (Glycine max (l.) merr.) Cultivars with contrasting photosynthetic phosphorus-use efficiency. J Soil Sci Plant Nutr 22:3914–3924. https://doi.org/10.1007/s42729-022-00940-7
Mirriam A, Mugwe J, Raza MA, Seleiman MF, Maitra S, Gitari HH (2022) Aggrandizing soybean yield, phosphorus use efficiency and economic returns under phosphatic fertilizer application and inoculation with Bradyrhizobium. J Soil Sci Plant Nutr 22:5086–5098. https://doi.org/10.1007/s42729-022-00985-8
O’hara GW, Boonkerd N, Dilworth MJ (1988) Mineral constraints to nitrogen fixation. Plant Soil 108:93–110. https://doi.org/10.1007/BF02370104
Ortez OA, Tamagno S, Salvagiotti F, Prasad PV, Ciampitti IA (2019) Soybean nitrogen sources and demand during the seed-filling period. Agron J 111:1779–1787. https://doi.org/10.2134/agronj2018.10.0656
Prudent M, Vernoud V, Girodet S, Salon C (2016) How nitrogen fixation is modulated in response to different water availability levels and during recovery: a structural and functional study at the whole plant level. Plant Soil 399:1–12. https://doi.org/10.1007/s11104-015-2674-3
Qiao Y, Tang C, Han X, Miao S (2007) Phosphorus deficiency delays the onset of nodule function in soybean. J Plant Nutr 30:1341–1353. https://doi.org/10.1080/01904160701555325
Qin L, Zhao J, Tian J, Chen L, Sun Z, Guo Y, Lu X, Gu M, Xy G, Liao H (2012) The high-affinity phosphate transporter GmPT5 regulates phosphate transport to nodules and nodulation in soybean. Plant Physiol 159:1634–1643. https://doi.org/10.1104/pp.112.199786
Rose TJ, Raymond CA (2020) Seed phosphorus effects on rice seedling vigour in soils differing in phosphorus status. Agronomy 10:1919. https://doi.org/10.3390/agronomy10121919
Rotundo JL, Borrás L, De Bruin J, Pedersen P (2014) Soybean nitrogen uptake and utilization in Argentina and United States cultivars. Crop Sci 54:1153–1165. https://doi.org/10.2135/cropsci2013.09.0618
Rufty TW Jr, Mackown CT, Israel DW (1990) Phosphorus stress effects on assimilation of nitrate. Plant Physiol 94:328–333. https://doi.org/10.1104/pp.94.1.328
Sa TM, Israel DW (1995) Nitrogen assimilation in nitrogen-fixing soybean plants during phosphorus deficiency. Crop Sci 35:814–820. https://doi.org/10.2135/cropsci1995.0011183X003500030030x
Sadras VO (2006) The N: P stoichiometry of cereal, grain legume and oilseed crops. Field Crops Res 95:13–29. https://doi.org/10.1016/j.fcr.2005.01.020
Santachiara G, Salvagiotti F, Rotundo JL (2019) Nutritional and environmental effects on biological nitrogen fixation in soybean: a meta-analysis. Field Crops Res 240:106–115. https://doi.org/10.1016/j.fcr.2019.05.006
Schulze J (2004) How are nitrogen fixation rates regulated in legumes? J Plant Nutr Soil Sci 167:125–137. https://doi.org/10.1002/jpln.200320358
Singh SK, Reddy VR, Fleisher DH, Timlin DJ (2018) Phosphorus nutrition affects temperature response of soybean growth and canopy photosynthesis. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.01116
Sulieman S, Tran LS (2015) Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci 239:36–43. https://doi.org/10.1016/j.plantsci.2015.06.018
Telles TS, Nogueira MA, Hungria M (2023) Economic value of biological nitrogen fixation in soybean crops in Brazil. Environ Technol Innov 31:103158. https://doi.org/10.1016/j.eti.2023.103158
The SV, Snyder R, Tegeder M (2021) Targeting nitrogen metabolism and transport processes to improve plant nitrogen use efficiency. Front Plant Sci 11:628366. https://doi.org/10.3389/fpls.2020.628366
Valentine AJ, Kleinert A, Benedito VA (2017) Adaptive strategies for nitrogen metabolism in phosphate deficient legume nodules. Plant Sci 256:46–52. https://doi.org/10.1016/j.plantsci.2016.12.010
Van Roekel RJ, Purcell LC, Salmerón M (2015) Physiological and management factors contributing to soybean potential yield. Field Crops Res 182:86–97. https://doi.org/10.1016/j.fcr.2015.05.018
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Vengavasi K, Pandey R (2018) Root exudation potential in contrasting soybean genotypes in response to low soil phosphorus availability is determined by photo-biochemical processes. Plant Physiol Biochem 124:1–9. https://doi.org/10.1016/j.plaphy.2018.01.002
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Voisin AS, Munier-Jolain NG, Salon C (2010) The nodulation process is tightly adjusted to plant growth. An analysis using environmentally and genetically induced variation of nodule number and biomass in pea. Plant Soil 337:399–412. https://doi.org/10.1007/s11104-010-0536-6
Wilson EW, Rowntree SC, Suhre JJ, Weidenbenner NH, Conley SP, Davis VM, Casteel SN (2014) Genetic gain × management interactions in soybean: II. Nitrogen utilization. Crop Sci 54:340–348. https://doi.org/10.2135/cropsci2013.05.0339
Yang J, Xin X, Zhong X, Yang W, Zhang X, Ding S, Rem G, Zhu A (2023) The fate of fertilizer-derived phosphorus under different long-term fertilization regimes: a phosphate oxygen isotope study. Sci Total Environ 856:159263. https://doi.org/10.1016/j.scitotenv.2022.159263
Zhong Y, Tian J, Li X, Liao H (2023) Cooperative interactions between nitrogen fixation and phosphorus nutrition in legumes. New Phytol 237:734–745. https://doi.org/10.1111/nph.18593
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Contributions
FA performed the experiments and analyzed the data. SA and FZ contributed to supervision, conceptualization, analyzing and interpretation of the data. All the authors contributed to writing, reviewing, editing and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almeida, F.M., Andrade, S.A.L. & Zambrosi, F.C.B. Post-Flowering Phosphorus Deficiency Negatively Impacts Biological Fixation but not Nitrogen Utilization Efficiency of Soybean. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01975-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01975-8