Abstract
Half-sandwich titanium complexes, specifically Cp*TiCl3 and Cp*Ti[O(2,6-iPr2-Ph)]Cl2, were investigated as catalytic precursors for the synthesis of ethylene/propylene copolymers and ethylene/propylene/5-ethylidene-2-norbornene terpolymers. For this purpose, a variety of activators were tested: modified polymethylaluminoxane; boron-containing compounds such as B(C6F5)3 and Ph3CB(C6F5)4 in combination with triisobutylaluminum (TIBA); isobutylaluminoxane (IBAO), and isobutylaluminum aryloxide (2,6-tBu2,4-Me-PhO-)AliBu2 (AlBHT). In the copolymerization of ethylene and propylene, the catalysts exhibited high activity when activated by MMAO-12 and TIBA+Ph3CB(C6F5)4 but low activity with TIBA+B(C6F5)3 and AlBHT. With IBAO as an activator, these catalysts were found to be totally ineffective. The catalysts exhibited low activity in terpolymerization. It was further revealed that more than one type of active sites was generated in the catalytic systems: these sites were responsible for simultaneous formation of low-molecular-weight and ultrahigh-molecular-weight polymers. The composition of the copolymers as well as their thermophysical and physicomechanical properties were shown to depend on the type and composition of the catalytic system.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
ABBREVIATIONS
AlBHT—Isobutylaluminum aryloxide, (2,6-tBu2,4-Me-PhO-)AliBu2,
GPC—Gel permeation chromatography,
DSC—Differential scanning calorimetry,
IBAO—Isobutylaluminoxane,
XRD—X-ray diffraction analysis,
EPM—Ethylene–propylene copolymer,
EPDM—Еthylene–propylene–diene terpolymer,
Cp—Cyclopentadienyl,
Cp*—Pentamethyl cyclopentadienyl, C5(CH3)5,
MAO—Methylaluminoxane,
MMAO-12—Modified methylaluminoxane,
P—Propylene,
TIBA—Triisobutylaluminum, AliBu3,
E—Ethylene,
ENB—5-Ethylidene-2-norbornene,
Mw—Weight-average molecular weight,
Mn—Number-average molecular weight.
Ethylene–propylene (EPM) and ethylene–propylene–diene (EPDM) copolymers are elastomeric polymers widely demanded and extensively used in the automotive, rubber, and cable industries, as well as in the manufacture of construction materials, impact-resistant plastics, etc.
The production of EPM and EPDM is based solely on catalytic coordination polymerization processes. The catalyst type plays a key role in the formation of EPM and EPDM with desired compositions and molecular weights, and these parameters are critical to the operating characteristics such as microstructure, crystallinity, and glass transition temperature, as well as the molecular-weight and thermophysical properties of the products. There are various classes of catalytic systems suitable for the production of EPM and EPDM, including those produced on an industrial scale [1–3].
Constrained geometry catalysts (CGCs) contain a η5-Cp ligand and a donor ligand (Don) linked by a bridging group. The bridging group reduces the Cp–M–Don angle (M = Ti, Zr, Hf) by about 20°–30° compared to sandwich metallocene complexes Cp–M–Cp, thus ensuring high availability of a transition metal in the active site and a high degree of comonomer incorporation (up to 20 mol % of 1-octene) [1, 4, 5]. The Don is linked to the transition metal by a σ-bond and ensures the high stability of this catalyst type at elevated temperatures (up to 160–180°C).
Non-bridged mono-Cp titanium complexes are even less sterically hindered. For example, in the copolymerization of ethylene with 1-hexene at 0 to –78°C and atmospheric pressure, with the composition of comonomers being varied, the catalytic complex Cp*TiMe3/B(C6F5)3 has produced high- and ultrahigh-molecular-weight copolymers with ethylene content varying within about 5–85% (although this catalytic system exhibited moderate or even low activity) [6]. Bavarian et al. [7] investigated catalytic systems like Cp*TiMe3/B(C6F5)3 in the terpolymerization of ethylene, propylene, and 5-ethylidene-2-norbornene. With moderate catalytic activity, a high-molecular-weight copolymer with up to 40 mol % propylene and up to 10 mol % diene was produced at –18°C and a total comonomer pressure of 1 atm.
Cp*TiMe3/MAO is one of the most efficient catalytic systems for the synthesis of syndiotactic polystyrene [8]. However, further research is needed with regard to the role of catalytic intermediates both in this type of catalysts (TiII, TiIII, or TiIV) and in systems where some other activators are used. A series of NMR/EPR examinations of Cp*TiMe3/B(C6F5)3, Cp*TiMe3/[Ph3C][B(C6F5)4], and Cp*TiCl2,3/MAO for styrene polymerization have demonstrated that in catalytic systems of this kind, more than one type of active sites can be generated simultaneously [9, 10].
Half-sandwich non-bridged titanium complexes with donor aryloxy ligands are structurally similar to the phosphinimides, ketimides, guanidinates, iminoimidazolinates, and amidinates manufactured by ARLANXEO and extensively used for commercial rubber production [3]. These complexes, first synthesized and investigated by a research group led by Prof. Nomura in 1998 [11, 12], are now well-known as catalysts for the polymerization of ethylene as well as the copolymerization of ethylene with higher α-olefins and cyclic olefins [11–16]. The half-sandwich complexes activated by MAO and perfluorophenylborates are distinguished by high activity in polymerization and high affinity for α-olefins and cyclic olefins. The effects of organoboron activators on the catalytic performance of Cp*TiMe2(O-2,6-iPr2C6H3) in the polymerization of 1-hexene were investigated in [16]. With B(C6F5)3 as a cocatalyst, the catalytic activity in the polymerization of 1-hexene was extremely low, but it was significant in the presence of Ph3CB(C6F5)4. The reaction between Cp*TiMe2(O-2,6-iPr2C6H3) and B(C6F5)3 was shown to lead to catalyst decomposition and/or formation of several compounds even at –70°C, whereas no decomposition was observed in the reaction between the complex and Ph3CB(C6F5)4. To the best of our knowledge, available publications offer no data on the use of catalytic aryloxide titanium complexes in ethylene/propylene copolymerization or ethylene/propylene/5-ethylidene-2-norbornene terpolymerization for the synthesis of EPM or EPDM.
The purpose of the present study was to investigate and compare the catalytic performance of non-bridged titanium complexes such as Cp*TiCl3 and Cp*TiCl2(O-2,6-iPr2C6H3) in the synthesis of bipolymers (ethylene/propylene) and terpolymers (ethylene/propylene/5-ethylidene-2-norbornene) for the production of EPM and EPDM, with organoaluminum and organoboron compounds as activators. The study was further aimed at identifying the effects of the catalyst type on the copolymerization kinetics and the copolymer properties. To activate chlorine-containing titanium complexes, we additionally used some activators specially developed in our previous studies such as IBAO [17] and aryloxide AlBHT, which is one of the series of isobutylaluminum aryloxides that exhibited high activating capacity for dimethylated metallocene precursors in the homopolymerization of ethylene and propylene, as well as in ethylene/propylene copolymerization and ethylene/propylene/5-ethylidene-2-norbornene terpolymerization [18–22].
EXPERIMENTAL
Reagents. We used high purity grade solvents that were dried and stored over 4 Å molecular sieves. Oxygen-sensitive and moisture-sensitive compounds were handled in an atmosphere of 99.998%-pure argon. Ethylene and propylene (manufactured by the Moscow Refinery, Russia) were dried by passing through a 4 Å activated molecular sieve column. Cp*TiCl3 (Dalchem, Russia) was used as received. MMAO-12, TIBA, [Ph3C][B(C6F5)4], and B(C6F5)3 (Sigma-Aldrich Corp., USA) were also used without pretreatment. ENB (Sigma-Aldrich Corp., USA) was dried and stored over 4 Å molecular sieves.
Synthesis of Cp*TiCl2(O-2,6-iPr2C6H3). This complex was synthesized in accordance with the modified procedure described in [11]. Cp*TiCl3 was mixed with phenol (OH-2,6-iPr2C6H3) in toluene at a molar ratio of 1 : 2.5. The reaction was carried out by stirring the resultant solution at 70°C for 5 h. To remove the evolved HCl, the reaction solution was bubbled with argon. The reaction completeness was monitored by 1H NMR spectroscopy. A red powder was prepared by distilling off toluene and was recrystallized in a dichloromethane/heptane (1 : 3) solvent mixture at 5°C to obtain needle-like red crystals of the target complex.
1H NMR Cp*TiCl2(O-2,6-iPr2C6H3) (CDCl3, δ, ppm): 1.22 (d, 12H, (CH3)2CH–), 2.18 (s, 15H (C5(CH3)5)), 3.17 (m, 2H (CH3)2CH–), (d, 1H, Ar-H), (t, 2H, Ar-H).
Synthesis of IBAO. IBAO was synthesized by controlled hydrolysis of TIBA [17]. A toluene solution of TIBA precooled to –78°C was added to a specific amount of water (free of dissolved air) based on an Al/H2O molar ratio of 2 : 1. The resultant mixture was stirred for 15 min, then slowly heated to room temperature for 40 min. The total hydrolysis time was 1 h. The IBAO was prepared 2 h before the polymerization.
Synthesis of AlBHT. Aryloxide (AlBHT) was synthesized in accordance with the procedure we previously described in [18].
Copolymerization of ethylene and propylene. The copolymerization was carried out in a 200 mL stainless steel reactor. The reactor was evacuated at 50°C for 1 h, filled with argon, and cooled to room temperature. A vial with a weighed sample of the appropriate titanium complex (catalyst precursor) was placed in the reactor under an argon flow. The reactor was evacuated and, after introducing a toluene solution of the activator, heated to 30°C. The calculated amounts of first propylene and then ethylene were successively injected to achieve an E/P molar ratio of 0.7 : 1 in the solution and a specific monomer pressure in the gas phase. The catalyst was introduced into the solution by crushing the sample vial to initiate the polymerization. A constant pressure was maintained by adding ethylene. The reaction was terminated by introducing a 5% ethanol solution of HCl into the reactor. The polymer was washed with a water/ethanol mixture, filtered, and dried at 60°C in a vacuum oven to constant weight.
Terpolymerization of ethylene, propylene, and 5-ethylidene-2-norbornene. For the ternary copolymerization of ethylene, propylene, and 5-ethylidene-2-norbornene, we followed the same procedure as for the ethylene/propylene copolymerization except that the required amount of 5-ethylidene-2-norbornene was introduced into the reactor simultaneously with the toluene solution of the activator.
Characterization of copolymers. Specimens of copolymers were analyzed by GPC, DSC, IR spectroscopy, and XRD.
The gel chromatograms of the specimens were recorded on a Waters GPCV 2000 chromatograph using a PLgel 5 µm MIXED-C column in 1,2,4-trichlorobenzene at 135°C. The molecular weights of the copolymers were evaluated based on the universal calibration curve taking into account the constants K and α from the Mark–Houwink equation for polystyrene and polyethylene in 1,2,4-trichlorobenzene.
The melting point and specific heat of fusion of the copolymers were derived from DSC data. The DSC measurements were performed on a DSC 822E calorimeter (Mettler Toledo, Switzerland) using STARe 15 software. The thermograms were recorded through three heating–cooling–heating cycles in an inert atmosphere with the temperature being varied between –100 and +170°C at a rate of 10°C/min. Table 1 presents the characteristic melting points and thermal effects of melting transitions for the second heating.
The copolymer compositions were derived from the IR spectra of polymer films in accordance with the procedures described in [23, 24] using a PerkinElmer Spectrum 100 FTIR spectrometer.
The XRD patterns of copolymer films were recorded on an ARLX'TRA diffractometer (Thermo Fisher Scientific, Switzerland) equipped with a solid-state detector using θ−θ geometry in the 5°–60° with a step of 0.2°/min and a counting time of 1 s/step.
Stress–strain testing of polymer specimens. The stress–strain tests of the copolymer specimens were run using a 2166 P-5 Plastics Testing Machine at room temperature in accordance with ISO 37 (Dietype 1). The strain rate was 10 mm/min. The physicomechanical properties of the specimens were determined by averaging the test data for five copolymer specimens. The elongation at break (EL) of the copolymers was evaluated by the formula: EL = (l1 – l0)/l0×100, where l1 is the length of the broken specimen measured by summing the lengths of the two fragments being merged; and l0 is the initial length of the specimen.
RESULTS AND DISCUSSION
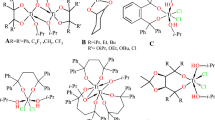
The titanium complexes used in this investigation of ethylene/propylene (binary) and ethylene/propylene/5-ethylidene-2-norbornene (ternary) catalytic copolymerization are schematically illustrated below:

Catalytic properties of Ti–Cl and Ti–1 in binary and ternary copolymerization using various activators. Copolymer properties. The catalytic properties of Ti–Cl and Ti–1 in ethylene/propylene copolymerization and ethylene/propylene/5-ethylidene-2-norbornene terpolymerization using various activators, as well as the properties of the copolymers produced are summarized in Table 1.
When Ti–Cl was activated by MMAO-12 ([(CH3)0.95(n-C8H17)0.05AlO]n), the activity of this catalytic system in ethylene/propylene copolymerization (run 1) was 192 kg of the copolymer per mole of titanium per hour per atmosphere pressure (kgpolymer molTi–1 h–1 atm–1). At a comonomer molar ratio of 0.7 : 1 in the reaction medium, the copolymer contained about 7 mol % of propylene. During the terpolymerization at a comonomer molar ratio of 4.7 : 4.3 : 1 (run 2), with other conditions being equal, an almost five-fold drop in the activity was observed. This could be associated with diene reactivity, which is lower than that of propylene, and/or with possible catalyst deactivation reactions. Despite the lower propylene to ethylene molar ratio in the reaction medium in the terpolymerization case (0.91 versus 1.43 for copolymerization), the propylene content in the copolymer increased to 10.5 mol %. The diene content was about 1 mol %. When Ti–Cl was activated by TIBA+Ph3CB(C6F5)4, the catalytic activity in copolymerization (run 3) was about double that with MMAO-12, but it dropped by a factor of more than ten in terpolymerization (run 4). The propylene content was about 9.3 mol % in the bipolymer and about 7.3 mol % in the terpolymer, roughly consistent with the decrease in its ratio to ethylene in the initial solution. The Ti–Cl catalytic system with bulk activators (borate and MMAO) provided a markedly lower incorporation of diene, probably due to the more strong steric limitations for the entrance of diene into active sites. A similar pattern was observed in terpolymerization over Cp*TiMe3/B(C6F5)3 at low temperatures [7]. Furthermore, similar diene effects in terpolymerization (specifically, an about 10-fold drop in terpolymerization activity after diene introduction) were reported when metallocene catalysts were activated by MAO [25] or by TIBA+borate [26].
The kinetic curves of copolymerization and terpolymerization over Ti–Cl/MMAO-12 and Ti–Cl/TIBA+Ph3CB(C6F5)4 are shown in Fig. 1. In the presence of diene, the most pronounced deactivation was observed for the catalytic system with the borate activator.
Specific ethylene consumption (Qsp) over time using Ti–Cl activated by MMAO-12 and TIBA+Ph3CB(C6F5)4 in binary and ternary copolymerization: (a) MMAO-12/Ph3CB(C6F5)4; and (b) TIBA+Ph3CB(C6F5)4. The curve numbers correspond to the run numbers in Table 1.
In ethylene/propylene copolymerization over Ti–Cl/TIBA+Ph3CB(C6F5)4, a homogeneous copolymer with Mw = 55 000 and a narrow polydispersity index (Mw/Mn = 2.2) was produced (run 3). Activation by MMAO-12 resulted in the formation of a copolymer partially soluble (88%) under GPC conditions with Mw = 17 000 and a low polydispersity index (Mw/Mn = 1.9, run 1). The insoluble ultrahigh-molecular-weight fraction of the copolymer was formed on active sites of a different type, likely resulting from transformation of the titanium cation complex during the copolymerization. In terpolymerization over Ti–Cl activated both by MMAO-12 and TIBA+Ph3CB(C6F5)4, the copolymers had higher heterogeneity (with soluble fractions accounting for 70 and 51%, respectively). These terpolymers exhibited higher molecular weights of soluble fractions and higher polydispersity indices than the binary copolymers (runs 2 and 4). Figure 2 presents the molecular weight distribution (MWD) curves of the soluble fractions of the binary and ternary copolymers produced using Ti–Cl with various activators. Metallocene catalysts activated by MAO and TIBA+borate are known to promote the formation of homogeneous copolymers with low polydispersity indices [24, 25]. This suggests that, in the catalytic systems under study, the titanium complex was transformed with the generation of various active sites responsible for the formation of low-molecular-weight and high-molecular-weight copolymers.
MWD curves of soluble fractions of bipolymers and terpolymers produced using Ti–Cl with various activators. The curve numbers correspond to the run numbers in Table 1.
A comparison of the plots in Figs. 1 and 3, in combination with the data of Table 1, shows that, both with MMAO-12 and TIBA+Ph3CB(C6F5)4 as activators, Ti–1 exhibited very high activity in the copolymerization of ethylene and propylene. The activity of Ti–1 exceeded that of Ti–Cl (other conditions being equal) by more than an order of magnitude (runs 5 and 7 vs. runs 1 and 3 in Table 1). Moreover, the complex with the aryloxide ligand exhibited a noticeably better stability during copolymerization (Fig. 3). When activated by TIBA+B(C6F5)3, both the activity and stability of the system were extremely low, consistent with the behavior of its methylated analogue, Cp*TiMe2(O-2,6-iPr2C6H3), previously demonstrated in the polymerization of 1-hexene with similar activation [16]. The Ti–1 catalyst also exhibited very poor activity in terpolymerization. In binary copolymerization, the complex with the aryloxide ligand clearly provided a markedly higher degree of propylene incorporation than Ti–Cl (cf. run 2 (7.3% P) vs. run 5 (19.7% P) and run 4 (9.3% P) vs. run 7 (11.0% P), see Table 1). The Ti–1 catalytic systems with the activators known to be effective for dimethylated metallocenes, specifically AlBHT [18, 21] and IBAO [17], were tested in ethylene/propylene copolymerization. As a result, the catalyst exhibited low activity in combination with AlBHT and turned out to be totally ineffective with IBAO (runs 12 and 13 in Table 1). The poor performance of the activators can likely be explained by the fact that they are able to activate only alkylated metal complexes.
Specific ethylene consumption (Qsp) over time using Ti–1 activated by MMAO-12, TIBA+Ph3CB(C6F5)4, and TIBA+B(C6F5)3. The curve numbers correspond to the run numbers in Table 1.
A distinctive feature of copolymers produced over Ti–1 activated by MMAO-12 and TIBA+Ph3CB(C6F5)4 is the bimodality of their GPC curves. As noted above, the copolymers obtained using Ti–Cl were likewise marked with fractional heterogeneity, i.e., the presence of an insoluble ultrahigh-molecular-weight fraction (runs 1, 2, and 4 in Table 1). In the case of Ti–1, the copolymer specimens were completely dissolved in 1,2,4-trichlorobenzene (TCB) at 135°C. Figure 4 illustrates the GPC data for some copolymers. The peak resolution and fractional composition are indicated in Table 1. The data of Fig. 4 and Table 1 clearly show that bulk activators such as MMAO-12 and Ph3CB(C6F5)4 promoted the formation of bimodal copolymers with polydispersity indices of about 2.0 for each peak. This serves as evidence of two different types of active sites, one being responsible for the formation of a high-molecular-weight polymer (with Mw of nearly 1×106 g/mol) and the other for the formation of a low-molecular-weight polymer (Mw ranging between 33×104 and 45×104 g/mol). The contribution of each peak is indicated in Table 1.
MWD curves of copolymers produced using Ti–1 with various activators. The curve numbers correspond to the run numbers in Table 1.
Figure 5 illustrates the differential fractional compositions of the ethylene/propylene copolymers produced using Ti–1 with various activators. Interestingly, the contribution of low-molecular-weight fractions, namely Fraction 4 (Mw = 5×104–1×105 g/mol) and Fraction 5 (1×104–5×104 g/mol), was significantly greater for the copolymer in run 10 (see Table 1). This was the case with stronger bounded B(C6F5)3-based counterions that provided the lowest propylene incorporation (6 mol %). Fraction 3 was predominant, with its content being similar (1×105–5×105 g/mol) in all three copolymers. The copolymers synthesized with the bulk activators MMAO-12 and Ph3CB(C6F5)4 had similar content of high-molecular-weight fractions: 1×106–1×107 g/mol for Fraction 1 and 5×105–1×106 g/mol for Fraction 2, far in excess of the content of the same fractions in the copolymer obtained with B(C6F5)3. Bearing in mind the considerable loss of activity (by a factor of about 35) and propylene incorporation capacity, these data indicate that the probability of chain transfer to Ti–1/B(C6F5)3 active sites was markedly higher than in the cases of less strong bounded counterions.
Content of fractions soluble under GPC conditions in E/P copolymers produced using Ti–1 with various activators. Fractions (Mw range, g/mol): (1) 1×106–1×107; (2) 5×105–1×106; (3) 1×105–5×105; (4) 5×104–1×105; and (5) 1×104–5×104. The copolymer numbers correspond to the run numbers in Table 1.
It is worth noting that the heterogeneity of most copolymers was manifested not only in the GPC data but also in the thermophysical properties. The first-heating DSC curves mostly contained several endothermic peaks (Fig. 6). The peak intensity was markedly lower for terpolymers (except for run 11). During recrystallization (the second DSC heating), the number of peaks was reduced due to the disappearance of low-temperature peaks. The melting points (column “Tmelt” in Table 1) were derived from the maxima of endothermic peaks after recrystallization. The specific heat of fusion (column ‘ΔH’ in Table 1) was evaluated based on the total heat effect.
Thermograms of E/P and E/P/ENB copolymers produced using Ti–1/TIBA+Ph3CB(C6F5)4 (runs 7 and 8 in Table 1).
The crystallinity (column “χ” in Table 1), determined as the ratio between the XRD-measured intensities of the crystalline and amorphous phases, was higher (up to 54%) in the Ti–Cl-catalyzed copolymerization cases. Intriguingly, among the copolymers produced in the presence of Ti–Cl, the specimens with low molecular weights and small content of the insoluble (ultrahigh-molecular-weight) fraction had greater crystallinity, likely due to a greater mobility of low-molecular-weight chains involved in recrystallization.
The crystallite sizes (column “D” in Table 1) were derived from the XRD spectra using the Scherrer equation. We clearly see that they are small and independent of the crystallinity. Below it is demonstrated that neither crystallinity nor crystallite size are decisive factors for the physicomechanical properties of these copolymers.
The copolymers obtained using Ti–1, which also exhibited fractional heterogeneity although they lacked an ultrahigh-molecular-weight fraction, possessed a markedly lower crystallinity (about 20–30%). The only exception was that in the cases of adding boranes to Ti–1 (runs 9 to 11, Table 1), the crystallinity substantially increased when diene was introduced into the polymer chain (Fig. 7). This affected the strength properties of the terpolymers.
XRD patterns of E/P and E/P/ENB copolymers produced using Ti–1/TIBA+B(C6F5)3 (runs 9 and 11 in Table 1).
The stress–strain curves and physicomechanical properties of the copolymers are provided in Fig. 8 and Table 1. We see that, in the initial strain region, the copolymers produced using Ti–Cl (and distinguished by 40–60% crystallinity compared to 20–30% in the specimens obtained over Ti–1) generally exhibited a higher elastic modulus. The initial elastic strain regions of the copolymers obtained with Ti–1 depended on various factors such as copolymer type, crystallinity, and catalytic system. The only exception was the ethylene/propylene copolymer obtained over Ti–Cl/MMAO-12 (run 1). The specimen with the lowest content of the GPC-insoluble fraction (12%), the lowest molecular weight (Mw = 17.000), and the highest crystallinity (54%) was broken without reaching the yield stress at a strain of about 5%. The terpolymer produced with the same catalytic system (run 2: soluble fraction 70%; Mw = 25 000; crystallinity 43%) reached a yield stress of 7 MPa at a strain of 36%, but further increasing the strain caused brittle fracturing. The other copolymers remained elastic even when the strain reached above the yield stress; their properties depended on the copolymer type, crystallinity, and catalytic system (see their σb and εb values in Table 1).
Stress–strain curves of copolymers and terpolymers produced over Ti–Cl and Ti–1 with various activators. The curve numbers correspond to the run numbers in Table 1.
Introducing diene into the reaction mixture had a major effect on the stress–strain curves. In all cases with both catalysts, it improved the strength properties of the copolymers (runs 3 and 4). The appearance of or increasing the content of an insoluble fraction in the copolymers obtained using Ti–Cl increased the tensile stress (runs 2 vs. 4) and decreased the elongation at break (runs 3 vs. 4).
Ti–1 promoted the formation of completely soluble polymers. The stress–strain curves of the terpolymers produced over Ti–1 with MMAO-12 and boron-containing activators (runs 6 and 11) are similar in shape to those of elastomers, with the tensile elongation at break (EL) being about 190% and about 300%, respectively. Increasing the B/Ti ratio (runs 9 and 10) also affected the mechanical properties: these binary copolymers exhibited breaking elongations up to 770%.
Finally, it should be noted that some other activators such as IBAO [17] and isobutylaluminum aryloxides [18, 19], known to be effective for dimethylated metallocene complexes, exhibited inadequate performance in the catalytic systems that contained the half-sandwich titanium complexes under study. No copolymers were formed over these catalytic systems with IBAO. The ethylene/propylene copolymer obtained over Ti–1 activated by AlBHT exhibited elastomeric properties to a greater extent. At low crystallinity (below 10%), the copolymer exhibited a low yield stress and fairly high strength properties, with the tensile elongation at break being about 180%. This behavior of the polymer can likely be explained by the large number of chain entanglements in the amorphous portion of the polymer.
The present study demonstrated that the use of various half-sandwich titanium complexes with various organoaluminum and organoboron activators in binary and, especially, ternary copolymerization offers an opportunity to produce copolymers with a wide range of properties. We expect that further research will be focused on the synthesis and characterization of the catalytic properties of new half-sandwich titanium complexes with the aryloxy moieties of ligands being varied. Further research should also investigate the effects of copolymerization conditions (e.g., activator type, comonomers ratio, temperature, etc.) on the production of EPM and EPDM that would satisfy the desired operating characteristics.
CONCLUSIONS
This study investigated ethylene/propylene copolymerization and ethylene/propylene/5-ethylidene-2-norbornene terpolymerization over half-sandwich titanium complexes Cp*TiCl3 (Ti-Cl) and Cp*TiCl2(O-2,6-iPr2C6H3) (Ti-1) with various activators, such as MMAO-12, TIBA+Ph3CB(C6F5)4, TIBA+B(C6F5)3, isobutylaluminoxane, and isobutylaluminum aryloxide. The activity of Ti–Cl activated by TIBA+Ph3CB(C6F5)4 was double that with MMAO-12; in contrast, Ti–1 exhibited comparable activity for both activator types. In binary copolymerization, Ti–1 proved to be more stable than Ti–Cl with both activators. In terpolymerization, all the catalytic systems exhibited an order of magnitude lower activity than in binary copolymerization. In combination with (2,6-tBu2, 4-Me-PhO-)AliBu2 (otherwise referred to as AlBHT and known to be an effective for dimethylated metallocene activator), Ti–1 exhibited low activity in binary copolymerization; this complex was totally ineffective when activated by IBAO. The copolymers obtained over both catalytic complexes activated by MMAO-12 and boron-containing activators were characterized by fractional heterogeneity (i.e., an ultrahigh-molecular-weight copolymer and a low-molecular-weight copolymer being formed simultaneously), bimodal molecular weight distribution curves, and multimodal peaks in DSC thermograms. This serves as evidence of more than one type of active sites generated in the catalytic systems.
These findings show the potential for further research to achieve the following goals: to synthesize and characterize the catalytic properties of new half-sandwich titanium complexes with the aryloxy moieties of ligands being varied; to determine the underlying causes of the multimodal properties of the copolymers; to assess the feasibility of the activation of methylated complexes by IBAO and isobutylaluminum aryloxides; and to optimize copolymerization conditions (activator type, comonomers ratio, temperature, etc.) for the production of EPM and EPDM that would satisfy the desired operating characteristics.
REFERENCES
Baier, M.C., Zuideveld, M.A., and Mecking, S., Angew. Chem. Int. Ed., 2014, vol. 53, pp. 9722–9744. https://doi.org/10.1002/anie.201400799
Klosin, J., Fontaine, P.P., and Figueroa, R., Acc. Chem. Res., 2015, vol. 48, pp. 2004–2016. https://doi.org/10.1021/acs.accounts.5b00065
Bravaya, N.M., Faingol’d, E.E., Sanginov, E.A., and Badamshina, E.R., Catalysts, 2022, vol. 12, pp. 704–718. https://doi.org/10.3390/catal12070704
Stevens, J.C., Timmers, F.J., Wilson, D.R., Schmidt, G.F., Nickias, P.N., Rosen, R.K., Knight, G.W., and Lai, S.-Y., Patent EP 0416815 A2, 1991.
Braunschweig, H. and Breitling, F.M., Coord. Chem. Rev., 2006, vol. 250, pp. 2691–2720 https://doi.org/10.1016/j.ccr.2005.10.022
Murray, M.C. and Baird, M.C., J. Polym. Sci., Part A: Polym. Chem., 2000, vol. 38, pp. 3966–3976.
Bavarian, N., Baird, M.C., and Parent, J.S., Macromol. Chem. Phys., 2001, vol. 202, pp. 3248–3252.
Tomotsu, N., Ishihara, N., Newman, T.H., and Malanga, M.T., J. Mol. Catal. A: Chem., 1998, vol. 128, pp. 167–190. https://doi.org/10.1016/S1381-1169(97)00171-4
Williams, E.F., Michael C. Murray, M.C., and Baird, M.C., Macromolecules, 2000, vol. 33, pp. 261–268. https://doi.org/10.1021/ma991006f
Bryliakov, K.P., Semikolenova, N.V., Zakharov, V.A., and Talsi, E.P., J. Organomet. Chem., 2003, vol. 683, pp. 23–28. https://doi.org/10.1016/S0022-328X(03)00232-8
Nomura, K., Naga, N., Miki, M., Yanagiand, K., and Imai, A., Organometallics, 1998, vol. 17, pp. 2152–2154. https://doi.org/10.1021/om980106r
Nomura, K., Naga, N., Mikiand, M., and Yanagi, K., Macromolecules, 1998, vol. 31, pp. 7588–7597. https://doi.org/10.1021/ma980690f
Nomura, K., Liu, J., Padmanabhan, S., and Kitiyanan, B., J. Mol. Catal. A: Chem., 2007, vol. 267, pp. 1–29. https://doi.org/10.1016/j.molcata.2006.11.006
Zhao, W. and Nomura, K., Catalysts, 2016, vol. 6, pp. 175–182. https://doi.org/10.3390/catal6110175
Zhao, W., Yan, Q., Tsutsumi, K., and Nomura, K., Organometallics, 2016, vol. 35, pp. 1895–1905. https://doi.org/10.1021/acs.organomet.6b00242
Nomura, K. and Fudo, A., Inorg. Chim. Acta, 2003, vol. 345, pp. 37–43. https://doi.org/10.1016/S0020-1693(02)01371-3
Bravaya, N.M., Panin, A.N., Faingol’d, E.E., Saratovskikh, S.L., Babkina, O.N., Zharkov, I.V., and Perepelitsina, E.O., Polym. Bull., 2016, vol. 73, pp. 473–491. https://doi.org/10.1007/s00289-015-1505-2
Faingol’d Е.Е, Bravaya, N.M., Panin, A.N., Babkina, O.N., Saratovskikh, S.L., and Privalov, V.I., J. Appl. Polym. Sci., 2015, vol. 133, pp. 43276. https://doi.org/10.1002/app.43276
Faingol’d, Е.Е., Zharkov, I.V., Bravaya, N.M., Panin, A.N., Saratovskikh, S.L., Babkina, O.N., and Shilov, G.V., J. Organomet. Chem., 2018, vol. 871, pp. 86–95. https://doi.org/10.1016/j.jorganchem.2018.07.001
Faingol’d Е.Е, Saratovskikh, S.L., Panin, A.N., Babkina, O.N., Zharkov, I.V., Garifullin, N.O., Shilov, G.V., and Bravaya, N.M., Polymers, 2021, vol. 220, p. 123559. https://doi.org/10.1016/j.polymer.2021.123559
Faingol’d, E.E., Saratovskikh, S.L., Panin, A.N., Babkina, O.N., Zharkov, I.V., Kapasharov, A.T., Bubnova, M.L., Shilov, G.V., and Bravaya, N.M., Polymers, 2023, vol. 15, p. 487. https://doi.org/10.3390/polym15030487
Faingol’d, E.E., Saratovskikh, S.L., Panin, A.N., Babkina, O.N., Zharkov, I.V., Kapasharov, A.T., Lashmanov, N.N., Shilov, G.V., and Bravaya, N.M., Polyolefins J., 2023, vol. 10, pp. 205–210. https://doi.org/10.22063/poj.2023.3417.1270
ASTM D3900-05a. Standard Test Methods for Rubber-Determination of Ethylene Units in Ethylene-Propylene Copolymers (EPM) and in Ethylene-Propylene-Diene Terpolymers (EPDM) by Infrared Spectrometry, 2010.
Cran, M.J. and Bigger, S.W., Appl. Spectros., 2003, vol. 57, pp. 928–932. https://doi.org/10.1366/000370203322258887
Sobhanmanesh, K. and Hajizadeh, A., Iranian Polymer J., 2004, vol. 13, pp. 257–262. https://www.sid.ir/paper/85918/en
Ali, A., Moradian, J.M., Naveed, A., Aziz, T., Muhammad, N., Maouche, C., Guo, Y., Yaseen, W., Yassen, M., Haq, F., Hassan, M., Fan, Z., and Guo, L., Polymers, 2022, vol. 14, pp. 3239. https://doi.org/10.3390/polym14163239
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within governmental order (project no. 124013000722-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faingol’d, E.E., Saratovskikh, S.L., Zhukov, S.A. et al. Ethylene/Propylene Copolymerization and Their Terpolymerization with 5-Ethylidene-2-norbornene over Catalytic Systems Based on Half-Sandwich Titanium Complexes with Organoaluminum and Organoboron Activators. Pet. Chem. 64, 500–510 (2024). https://doi.org/10.1134/S0965544124010158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544124010158