Abstract
The synthesis and olefin polymerization behavior of a new TADDOL-based Ti(IV) complex, (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetrakis[bis-(3,5-trifluoromethyl)phenyl]-1,3-dioxolane-4,5-dimethanolato-titanium(IV) dichloride, are described. Upon activation with MAO, this complex polymerized ethylene, producing ultra-high molecular weight linear polyethylene (UHMWPE) with activities up to 4500 kg mol (Ti)−1 [C2H4]−1 h−1 atm−1 and molecular weights up to 3.25 × 106. The optimal temperature for UHMWPE synthesis was 50 °C. This complex is also capable of copolymerizing ethylene with 1-hexene and 1-octene, giving high molecular weight copolymers with α-olefin incorporation up to 7.8%. The copolymers, obtained with a different ratio of comonomers, are statistical, according to the analysis of the 13C NMR spectra. The reaction parameters that influenced the copolymerization behavior, such as comonomer concentration, reaction temperature and [Al]/[Ti] molar ratio, are examined in detail. Furthermore, high catalytic activities up to 12,531 kg mol(Ti)−1 [C2H4]−1 h−1 atm−1 were observed in copolymerization of ethylene and 1-hexene or 1-octene with the 2/MAO catalytic system. The obtained copolymers possess high molecular weights (Mw = 1.4 × 106—ethylene/1-hexene and 1.86 × 106—ethylene/1-octene) with broad MWD (Mw/Mn = 3.04–8.23) and high comonomer incorporation degrees (up to 6.2 mol% of 1-hexene and 7.8 mol% of 1-octene). Depending on the synthesis conditions, it is possible to form both a statistical copolymer and a block copolymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
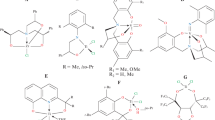
Polyolefins account for more than 50% in weight of the produced polymers and remain at the top of the global production of synthetic polymers [1]. The ever-growing demand for new synthetic polymer materials is driving the development of new catalytic systems. Among a myriad of post-metallocene catalysts created in recent decades [2,3,4,5,6], complexes in which the ligand environment is formed exclusively from alkoxo-oxygen atoms are extremely rare. (Examples of such structures are shown in Fig. 1.)
Probably this is due to the low catalytic activity of such systems upon their activation by the conventional for non-metallocene cocatalysts (alkylaluminoxanes, alkylaluminum or alkylaluminum chlorides). For example, the diolate complex B (Fig. 1), activated with Et3Al2Cl3, catalyzed the formation of low molecular weight PE with productivity not exceeding 30.4 kg PE g(Ti)−1 h−1 [7].
However, when using mixtures of alkylaluminum chlorides and organomagnesium compounds proposed by Kissin et al. [8, 9], quite effective catalytic systems are formed. We have successfully used titanium dichloride and alkoxide complexes with 1,4- and 1,2-diolate ligands (complexes B–E) to produce disentangled UHMWPE [10, 11]. Based on the assumption that higher catalytic activity can be expected from complexes with a less stable 7-membered chelate ring, we used the tetraaryl-1,3-dioxolane-4,5-dimethanols (TADDOL) derivative as ligands. TADDOLs, containing two adjacent diarylhydroxymethyl groups in a trans relationship on a 1,3-dioxolane ring, were introduced by Seebach et al. [12, 13] and found wide application in asymmetric synthesis both as ligands and as organocatalysts [14]. We have previously shown that (TADDOL)TiCl2 complexes effectively catalyze the polymerization of ethylene and propylene with the formation of high molecular weight polymers [15,16,17,18,19]. The maximum catalytic activity in ethylene polymerization with titanium TADDOLates (compounds F, Fig. 1) with a binary cocatalyst was shown by a complex containing perfluorophenyl fragments [19].
The aim of the present work is to verify the hypothesis that an increase in the acidity of the TADDOL’s hydroxy groups will increase the catalytic activity of the corresponding complexes, as is the case for 1,2-diolate complexes [11]. In this work, we use the TADDOL ligand with four strong electron-withdrawing CF3 substituents in phenyl fragments, because introduction of fluorine into the ligands was expected to increase the Lewis acidity of the catalyst complex [20, 21]. For a reliable analysis of the effect of substituents on catalytic activity, we used MAO as the cocatalyst. The obtained catalytic system exhibits relatively low activity that avoids limitations due to mass transport [22]. We previously showed that structurally close complexes (compounds F, Fig. 1), activated by MAO, form only trace amounts of polymer [19].
Experimental
Catalysts and synthetic methods
All manipulations with air-sensitive materials were performed with rigorous exclusion of oxygen and moisture in oven-dried Schlenk glassware on a dual manifold Schlenk line, interfaced to a high-vacuum line. Argon and ethylene of special-purity grade (Linde gas) were dried by purging through a Super Clean™ Gas Filters.
NMR spectra were recorded on Bruker Avance-400 instrument. Deuterated solvents (CDCl3, THF-d8) were degassed by freeze–pump–thaw vacuum cycles and stored over 3 Å molecular sieves. Chemical shifts are reported in ppm and were determined by reference to the residual solvent peaks. All coupling constants are given in hertz. Air-sensitive NMR spectra were recorded in J. Young tubes with Teflon valve plugs. IR spectra were recorded on a Magna-IR 750 spectrophotometer. Elemental analysis was performed by the microanalytical laboratory at A. N. Nesmeyanov Institute of Organoelement Compounds.
Hexane was distilled over Na/benzophenone, and the water content was periodically controlled by Karl Fischer coulometry by using a Metrohm 756 KF apparatus. Methylaluminoxane (Sigma-Aldrich) was used as 7 wt% solution in toluene. Diisopropyl 2,3-O-isopropylidene-l-tartrate was synthesized according to the previously described procedure [23].
(4R,5R)-2,2-Dimethyl-α,α,α′,α′-tetrakis[bis-(3,5-trifluoromethyl)phenyl]-1,3-dioxolane-4,5-dimethanol (1)
The synthesis was carried out under argon. A solution of n-butyllithium (2.68 mL, 6.69 mmol) was added at − 78 °C to a solution of 3,5-bis-trifluoromethyl-phenyl bromide (1.30 g, 4.46 mmol).
The reaction mixture was heated to room temperature and kept at ~ 20 °C for 4 h. Then, the reaction mixture was cooled to − 78 °C and a solution of diisopropyl 2,3-O-isopropylidene-l-tartrate (0.274 g, 1.00 mmol) in diethyl ether (30 mL) was added dropwise. After warming to room temperature, the reaction mixture was refluxed for 24 h and neutralized with a saturated solution of NH4Cl. The organic layer was separated, the solvent was evaporated, and the residue was recrystallized from hexane. Yield 1.19 g (13%), m.p. 192 °C (dec.), [α]D = + 187.3 (c 0.5, CHC13). Found (%): C, 46.30; H, 2.16, F, 45.09. C39H22F24O4 (1010). Calculated (%): C, 46.35; H, 2.19, F, 45.12. 1H NMR (d-THF), δ: 8.04 (s, 6 H, Ar); 7.91 (s, 2 H, Ar); 7.90 (s, 4 H, Ar); 4.58 (s, 2 H, CH); 2.56 (s, 2 OH, CH); 1.04 (s, 6 H). 19F NMR (d-THF), δ: − 65.58. 13C NMR, δ: 23.92, 24.18, 26.03, 65.97, 66.19, 76.73, 81.17, 110.76, 121.98, 122.09, 124.69, 127.69, 128.62, 130.40, 130.73, 130.88, 131.06, 131.21, 131.39, 131.54, 145.04, 147.32. FT-IR (KBr): 652, 570 cm−1 ν(Ti–O).
(4R,5R)-2,2-dimethyl-α,α,α′,α′-tetrakis[bis-(3,5-trifluoromethyl)phenyl]-1,3-dioxolane-4,5-dimethanolato titanium(IV) dichloride (complexes 2)
A 2.5 M solution of butyllithium in hexane (0.17 mL, 0.42 mmol) was added dropwise with stirring under argon to a cooled (− 78 °C) solution of ligand 1 (0.20 mL, 0.20 mmol) in toluene (10 mL). The temperature of the reaction mixture was slowly brought to ambient temperature; the mixture was stirred for 4 h and cooled to − 78 °C. A solution of TiCl4 (0.024 mL, 0.20 mol) was added, and the mixture was again warmed to ambient temperature. After 3 h, the reaction mixture was filtered, the solvent was evaporated, and the product was recrystallized from toluene. Yield 0.16 g (64%), m.p. 249 °C (dec)., [α]D = + 97.9 (c 0.5, CHC13). Found (%): C, 43.26; H, 2. 82, Cl, 5.64, Ti, 3.80. C39H20Cl2F24O4Ti.2C3H8OH (1246.10). Calculated (%): C, 43.33; H, 2. 91, Cl, 5.68, Ti, 3.84. 1H NMR (d-THF), δ: 8.15–8.06 (m, 12 H, Ar); 4.85 (s, 2 H, CH); 3.86 (s, 2 H, CH); 1.10 (s, 12 H), 0.69 (s, 6 H). 19F NMR (d-THF), δ: − 63.67. 13C NMR, δ: 23.92, 24.12, 24.32, 24.52, 26.26, 62.03, 65.58, 66.27, 80.95, 88.94, 112.36, 121.75, 121.98, 122.09, 122.15, 124.30, 124.96, 127.30, 127.33, 127.57, 129.37, 130.01, 130.34, 130.65, 131.00, 131.06, 131.40, 131.72, 132.05, 145.63, 150.63.
Ethylene polymerization and copolymerization were carried out in hexane in a 0.5 L stainless steel reactor equipped with a mechanical stirrer. The reactor was kept under vacuum for 1 h at 90 °C before each experiment and then cooled to 20 °C, filled with dry argon and hexane, the cocatalyst (MAO), and in the case of copolymerization, the desired comonomers were introduced into it. The reactor was heated to a specified temperature, and the reaction mixture was saturated with ethylene. After saturation of the solvent with ethylene, polymerization was started by breaking the sealed glass ampule with pre-catalyst inside the reactor. The PE was kept constant throughout each run; the ethylene loss was compensated for by introducing additional gas from a high-pressure vessel. After a prescribed time, the ethylene gas feed was stopped, and 10% HCl solution in ethanol was added to terminate the polymerization reaction. The polymer was isolated by filtration, washed with ethanol and dried at 60 °C for 12 h in a vacuum oven.
Preparation of block copolymer of ethylene and 1-octene
The preparation of polyoctene-b-poly(ethylene-co-1-octene) block copolymer was attempted by a sequential addition polymerization procedure. To prepare a polyoctene segment, to the stainless steel reactor prepared as described in the previous section, the cocatalyst MAO (1.04 mL of 2.7% solution in toluene, Aldrich) and the 1-octene (80 mL) were introduced. The reactor was heated to 50 °C, and the polymerization of 1-octene was started by breaking the sealed glass ampule with pre-catalyst inside the reactor. After 1 h, a sample of the reaction mass was taken for analysis and the solvent (100 mL of hexane) was introduced of the reaction and the entire solution was saturated with ethylene to produce a poly(ethylene-co-1-octene) segment. The PE was kept constant throughout each run; the ethylene loss was compensated for by introducing additional gas from a high-pressure vessel. After a prescribed time, the ethylene gas feed was stopped, and 10% HCl solution in ethanol was added to terminate the polymerization reaction. The polymer was isolated by filtration, washed with ethanol and dried at 60 °C for 12 h in a vacuum oven.
Polymer evaluation methods
DSC was performed with a differential scanning calorimeter DSC 204 F1 Phoenix («NETZSCH») in helium atmosphere. The analyses were performed at the heating rate of 10 °C min−1 in the temperature range of 50–200 °C. The heating cycle was run twice. In the first scan, samples were heated and then cooled to room temperature. In the second scan, samples were reheated at the same rate. The characteristic melting temperatures and the heat of fusion for UHMWPE are given according to the first and second heating. For copolymers, only the results of the second scan were reported because the first scan was influenced by the mechanical and thermal history of samples.
Viscosity-average molecular weight of synthesized UHMWPE samples was calculated with the Mark–Houwink equation: Mv = 5.37 × 104 [η]1.37 [24], where Mv = viscosity-average molecular weight (g mol−1); [η] = intrinsic viscosity in decalin at 135 °C (dL g−1); [η] = (2ηsp − 2lnηr)1/2/0.056 (ηsp—specific viscosity decalin at 135 °C; ηr—relative viscosity in decalin at 135 °C; ηr = ηsp + 1).
Gel permeation chromatographic (GPC) analysis of polymers was carried out at 135 °C with a Waters GPCV-2000 chromatograph equipped with two columns (PLgel, 5 μ and Mixed-C, 3007.5 mm) and a refractometer. 1,2,4-Trichlorobenzene was used as a solvent; the elution rate was 1 mL min−1. Molecular weights (MWs) of polymer products were determined using the universal calibration dependence relative to polystyrene standards with a narrow MW distribution: for polystyrene К = 2.88 × 10−4, α = 0.64; for PE, К = 6.14 × 10−4, α = 0.67.
13C NMR spectra of ethylene/1-octene and ethylene/1-hexene copolymers (~ 5 wt% solutions in o-dichlorobenzene) were recorded at 100 °C on a Bruker Avance-400 spectrometer at 10.613 MHz. The relaxation delay was 15 s; the number of scans varied from 500 to 2000. The signal assignment in the 13C NMR spectra was based on the literature data [25, 26].
IR spectra of polymers (thin films) were recorded on a PerkinElmer Spectrum 100 spectrophotometer. 1-Hexene content in the ethylene/l-hexene copolymer was estimated by FT-IR method using the A 1379CH3 /A 1369CH2 absorbance ratios [27] in accordance with the methodology [28].
The degree of crystallinity was calculated using DSC data, as the ratio between crystalline peak area and the entire area under the curve in the 2θ region (15°–35°), excluding support and air dissipation.
Results and discussion
Synthesis of (4R,5R)-2,2-dimethyl-α,α,α′,α′-tetrakis(3,5-bis-(trifluoromethyl)-phenyl)-1,3-dioxolan-4,5-dimethanol 1 was performed according to reported methods [21]. Direct interaction of ligand 1 with dichloro(diisopropoxy)titanium (TiCl2(OiPr)2) results in the one step formation of titanium(IV) dichloride complex 2 (Scheme 1). The complex contains two coordinated isopropanol molecules, which are eliminated upon cocatalyst addition to yield vacant coordination sites, as in the previously described cases [19]. The results of elemental analysis, the 1H, 19F, 13C NMR and IR data of complex 2 are consistent with the structure proposed in Scheme 1.
Thus, the signals of OH protons, observed at 2.56 ppm in the spectrum of the ligand 1, disappear in the 1H NMR spectrum of the complex 2, but signals of coordinated isopropanol molecules appear at 3.86 (–CH–), 1.10 ppm (–CH3).
The 19F NMR spectrum of the complex and the ligand contains a signal typical for CF3 fragments (− 65.58 ppm—for 1 and − 63.67 ppm—for 2). In the IR spectrum of the complex 2, valence vibrations of the Ti–O bond are observed at 550 cm−1 and 620 cm−1.
We have evaluated catalytic activity of compound 2 in ethylene polymerization. The complex 2 produces almost linear polyethylene, as indicated by the low branching degree determined by 13C NMR and IR analysis. PE samples were insoluble in hot 1,2,4-trichlorobenzene so molecular weight was determined by viscometric method in decalin; the viscosity-average molecular weights are in the range of 1.94–3.25 × 106, which allows to attribute it to UHMWPE. An increase in the reaction temperature from 30 to 50 °C is accompanied by a 30% increase in the activity, but further increase in the temperature to 70 °C leads to the deactivation of the catalytic system (Fig. 2a). These data indicate moderate thermal stability of the catalytic system.
The melting points of the polyethylene samples were measured by DSC (Table 1). The melting points are in the range of 141–143 °C at first heating run, which significantly exceeds the usual values for polyethylene. Such high values of Tm, as well as the thermal exo-effect observed on the DSC curves, are typical for disentangled UHMWPE [29, 30].
The 2/MAO catalytic system produces UHMWPE with moderate productivity (1500–4500 kg mol(Ti)−1 [C2H4]−1 h−1 atm−1), compared to structurally related TADDOL pre-catalysts F [19], as well as complexes with 1,2- and 1,4-diolate ligands [10, 11] and derivatives of 2-hydroxymethylphenol [31, 32], that were practically inactive in the presence of MAO (Fig. 1. Compounds A–F). Since the catalytic activity of the titanium(IV) TADDOL complexes has a tendency to increase with the increased acidity of the hydroxyl groups [11], we have compared the catalytic performances of complex 2 with other structurally related titanium(IV) TADDOL complexes F (Fig. 1). However, the studied complexes 2 (Scheme 1) and F (Fig. 1) do not obey this trend.
The results obtained in entries 1–3 of Table 1 are promising, so we have studied the catalytic behavior of this system in the copolymerization of ethylene with higher α-olefins. 1-Octene was used as the monomer, since the ethylene–octene copolymer (EOC), a relatively novel polyolefin elastomer, developed by Dow Chemical Company with metallocene catalysis, is attracting a lot of attention from both research and industry. Among ethylene-based elastomers, EOC is characterized by an excellent compatibility with polyolefins, such as polyethylene (PE) and polypropylene (PP), lower level of crystallinity and a higher flexibility. Based on these properties, EOC is currently being used as an impact modifier of PP to replace ethylene propylene rubber (EPR) and ethylene propylene diene rubber (EPDM) [33].
Insofar as the maximum activity (4500 kg mol−1 of Ti [C2H4] h atm.) was recorded during ethylene polymerization at 50 °C; the optimization of the copolymerization conditions was carried out at this temperature. Copolymerization was performed at a variety of feed ratios (runs 4–7, Table 1; Fig. 2b) to investigate how the different concentrations of comonomer affect the catalytic activity and properties of the copolymers.
The increase in 1-octene content in the feed is beneficial both to productivity and the comonomer inclusion, while the molecular weight of the polymer is reduced. In this case, the comonomer synergistic effect is clearly evident: The productivity of this system in copolymerization is about twice higher than those observed in ethylene homopolymerization (entry 7 vs 2).
Unlike the polymerization process, the maximum productivity of the catalytic system in copolymerization is achieved at 30 °C. An increase in temperature to 50–70 °C is accompanied by a significant decrease in activity (entries 6, 11–12, Table 1, Fig. 2a). The polymer with higher MW (1.86 × 106, entry 11) was produced at the lowest polymerization temperature (30 °C).
The DSC analysis of the copolymers shows one sole melting point in the range of 125.4–139.8 °C, which is significantly lower than the melting points of homopolymers (140.4–143.2 °C).
Analysis of the copolymer samples by GPC revealed that catalytic system 2/MAO produces high molecular weight copolymers. (Mw values are in the range 1.69 × 105–1.86 × 106.) All copolymers have a broad molecular weight distribution (Table 1, Fig. 3). The latter is explained by the presence of several types of active sites in the catalytic system.
The effect of [Al]/[Ti] molar ratio on the catalytic activity and properties of the resulting polymer was investigated. The activity grows with the ratio increase up to [Al]/[Ti] = 1000:1 reaching 10,063 kg mol(Ti)−1 [C2H4]−1 h−1 atm−1 (Table 1, entry 9); further growth up to 2000:1 leads to a decrease in activity. Such a phenomenon can be well clarified by the influence of the Al concentration on the termination of polymer chains. Interestingly, the degree of inclusion of the comonomer monotonically increases with growth in [Al]/[Ti] molar ratio, reaching a maximum—7.8% at molar ratio 2000:1.
The preparation of polyoctene-b-poly(ethylene-co-1-octene) block copolymer was attempted by a sequential addition polymerization procedure: To prepare a polyoctene segment, 1-octene was added to the activated complex and after 1 h, a sample of the reaction mass was taken for analysis. According to GPC data, the polyoctene segment had the following characteristics: Mw: 1.59 × 103; Mn: 1.11 × 103; Mw/Mn: 1.42. After that, the ethylene feed was started to produce a poly(ethylene-co-1-octene) segment [34]. As a result, a semicrystalline high molecular weight polymer was obtained with high efficiency 10,875 kg mol(Ti)−1 [C2H4]−1 h−1 atm−1 (Table 1, entry 13). The content of 1-octene was amounted to 2.5%; the polymer is characterized by a rather narrow MWD − 2.87 (the smallest value in this series). The composition and structure of ethylene/1-octene block copolymer was confirmed by 13C NMR using the method of Randall et al. [25, 26] (Fig. 4).
13C NMR (10.613 MHz) spectrum of ethylene/1-octene copolymer produced with 2/MAO (Table 1, entry 13, T = 50 °C; [Al]/[Ti] = 500; [C8H16] = 9.0 mass%)
The values of r1 = 10.48 and r2 = 0.2358 calculated according to [26], indicate that reactivity of these monomers differs in ≈ 44 times. The consequence of this is the block nature of the resulting copolymer: r1 × r2 = 2.47. The presence of long ethylene sequences in polymer chains leads to the formation of a crystalline phase based on PE.
The copolymerization of ethylene with 1-hexene takes place with twice productivity compared to ethylene/1-octene copolymerization (12,531 and 6075 kg mol(Ti)−1 [C2H4]−1 h−1 atm−1; Table 1, entries 5 vs 14), which is obviously explained by the higher reactivity of the 1-hexene comonomer.
Conclusion
We have synthesized and fully characterized a new TADDOLate Ti(IV) complex—(4R,5R)-2,2-dimethyl-α,α,α′,α′-tetrakis-[bis-(3,5-trifluoromethyl)phenyl]-1,3-dioxolane-4,5-dimethanolato-titanium(IV) dichloride. This complex displayed moderate catalytic activity toward ethylene polymerization in the presence of MAO as a cocatalyst and produced UHMWPE with Mw 1.94–3.25 × 106 even at elevated reaction temperature.
The reaction conditions in ethylene copolymerization (the temperature, ethylene/α-olefin and Al/Ti ratios) at a constant concentration of complex 2 have been optimized. Comonomer incorporation and polymer MW can be controlled in a wide range by the variation of the reaction parameters. Both the productivity of the catalytic system and the degree of comonomer content increase with increasing concentration of the latter in the feed mixture.
This effect of the [Al]/[Ti] ratio on the composition of the copolymers can be explained by two reasons: 1—With an increase in the Al/Ti molar ratio above 500, the chain transfer reaction to the cocatalyst (MAO) becomes noticeable; 2—there is a change in the composition and structure of the active center(s).
The obtained results confirm our assumption that the introduction of electron-withdrawing fluorine-containing substituents into the ligand structure is accompanied by an increase in the catalytic activity of the corresponding titanium complexes.
References
Ittel SD, Johnson LK, Brookhart M (2000) Late-metal catalysts for ethylene homo- and copolymerization. Chem Rev 100:1169. https://doi.org/10.1021/cr9804644
Gibson VC, Spitzmesser SK (2003) Chemistry of the lanthanides using pyrazolylborate ligands. Chem Rev 103:283. https://doi.org/10.1021/cr980461r
Makio H, Kashiwa N, Fujita T (2002) Catalysts for the living insertion polymerization of alkenes: access to new polyolefin architectures using Ziegler–Natta chemistry. Adv Synth Catal 344:1. https://doi.org/10.1002/1615-4169(200207)344:5%3c477:AID-ADSC477%3e3.0.CO;2-6
Makio H, Fujita T (2009) Development and application of FI catalysts for olefin polymerization: Unique catalysis and distinctive polymer formation. Acc Chem Res 42:1532. https://doi.org/10.1021/ar900030a
Lamberti M, Mazzeo M, Pappalardo D, Pellecchia C (2009) Mechanism of stereospecific polymerization of α-olefins by late-transition metal and octahedral group 4 metal catalysts. Coord Chem Rev 253:2082. https://doi.org/10.1016/j.ccr.2009.02.014
Brylyakov KP (2007) Post-metallocene catalysts for olefin polymerisation. Russ Chem Rev 76:253. https://doi.org/10.1070/RC2007v076n03ABEH003649
Matsukawa N, Ishii S, Furuyama R, Saito J, Mitani M, Makio H, Tanaka H, Fujjita T (2003) Polyolefin structural control using phenoxy-imine ligated group 4 transition metal complex catalysts. e-Polymers. https://doi.org/10.1515/epoly.2003.3.1.258
Kissin YV, Nowlin TE, Mink RI, Brandolini AJ (2000) A new cocatalyst for metallocene complexes in olefin polymerization. Macromolecules 33:4599. https://doi.org/10.1021/ma992047e
Kissin YV, Mink RI, Brandolini AJ, Nowlin TE, Polym J (2009) AlR2Cl/MgR2 combinations as universal cocatalysts for Ziegler–Natta, metallocene, and post‐metallocene catalysts. Sci Part A Polym Chem 47:3271. https://doi.org/10.1002/pola.23391
Ch Gagieva S, Tuskaev VA, Fedyanin IV, Buzin MI, Vasil’ev VG, Nikiforova GG, Afanas’ev ES, Zubkevich SV, Kurmaev DA, Kolosov NA, Mikhaylik ES, Golubev EK, Sizov AI, Bulychev BM (2017) Novel titanium(IV) diolate complexes: synthesis, structure and catalytic activities in ultra-high molecular weight polyethylene production. J Organomet Chem 828:89. https://doi.org/10.1016/j.jorganchem.2016.11.026
Tuskaev VA, Gagieva SCh, Kurmaev DA, Khrustalev VN, Dorovatovskii PV, Mikhaylik ES, Golubev EK, Buzin MI, Zubkevich SV, Nikiforova GG, Vasil’ev VG, Bulychev BM, Magomedov KF (2018) Novel titanium(IV) complexes with 1,2-diolate ligands: synthesis, structure and catalytic activities in ultra-high molecular weight polyethylene production. J Organomet Chem 877:85. https://doi.org/10.1016/j.jorganchem.2018.09.014
Seebach D, Beck AK, Heckel A (2001) TADDOLs, their derivatives, and TADDOL analogues: versatile chiral auxiliaries. Angew Chem Int Ed 40:92. https://doi.org/10.1002/1521-3773(20010105)40:1%3c92:aid-anie92%3e3.0.co;2-k
Pellissier H (2008) Use of TADDOLs and their derivatives in asymmetric synthesis. Tetrahedron 64:10279–10317. https://doi.org/10.1016/j.tet.2008.08.029D
Seebach D, Plattner DA, Beck AK, Wang YM, Hunziker D (1992) On the mechanisms of enantioselective reactions using α,α,α′,α′-tetraaryl-1,3-dioxolane-4,5-dimethanol (TADDOL)-derived titanates: differences between C2-and C1-symmetrical TADDOLs—facts, implications and generalizations. Helv Chim Acta 75:2171. https://doi.org/10.1002/hlca.19920750704
Belokon Y, Gagieva S, Sukhova T, Dmitriev AB, Lyssenko KA, Bravaya NM (2005) Titanium(IV) chloride complexes with chiral tetraaryl-1,3-dioxolane-4,5-dimethanol ligands as a new type of catalysts of ethylene polymerization. Russ Chem Bull 54:2348. https://doi.org/10.1007/s11172-006-0121-6
Rishina LA, Galashina NM, Gagieva SC, Tuskaev VA, Kissin YV (2009) Single-center vs. multi-center post-metallocene catalysts for propylene polymerization. Eur. Polym. J. 45:2951
Rishina LA, Galashina NM, Gagieva SCh, Tuskaev VA, Kissin YV (2011) Vysokomol Soedin Ser B 53:284 (Polym Sci B (Engl Transl) 53:42 (2011))
Rishina LA, Galashina NM, Gagieva SC, Tuskaev VA, Kissin YV (2013) Cocatalyst effect in propylene polymerization reactions with post-metallocene catalysts. Eur Polym J 49:147
Tuskaev VA, Gagieva SC, Maleev VI, Borissova AO, Solov’ev MV, Starikova ZA, Bulychev BM (2013) Titanium(IV) and zirconium(IV) chloride complexes on the base of chiral tetraaryl-1, 3-dioxolane-4, 5-dimetanol ligands in the polymerization of ethylene: the promoting role of lithium and magnesium chloride. Polymer 54:4455
Hintermann L, Perseghini M, Beilstein AT (2011) Development of the titanium–TADDOLate-catalyzed asymmetric fluorination of β-ketoesters. J Org Chem 7:1421. https://doi.org/10.3762/bjoc.7.166
Seebach D, Beck AK, Dahinden R, Hoffmann M, Kuehnle FNM (1996) Croat Chem Acta 69:459
Lin S, Tagge CD, Waymouth RM, Nele MR, Collins S, Pinto JC (2000) Kinetics of propylene polymerization using bis (2-phenylindenyl) zirconium dichloride/methylaluminoxane. J Am Chem Soc 122:11275
Carmack M, Kelley CJ (1968) Synthesis of optically active Cleland’s reagent [(–)-1,4-dithio-l-threitol]. J Org Chem 33(5):2171. https://doi.org/10.1021/jo01269a123
Kurtz SM (2004) The UHMWPE Handbook, “Ultra high molecular weight polyethylene in total joint replacement”. Elsevier, New York, p 397
Hsieh ET, Randall JC (1982) Monomer sequence distributions in ethylene-1-hexene copolymers. Macromolecules 15:1402. https://doi.org/10.1021/ma00233a036
Randall JC (1989) A review of high resolution liquid 13carbon nuclear magnetic resonance characterizations of ethylene-based polymers. J Macromol Sci Part C Polym Rev 29:201–317. https://doi.org/10.1080/07366578908055172
Nowlin TE, Kissin YV, Wagner KP (1988) High activity Ziegler–Natta catalysts for the preparation of ethylene copolymers. J Polym Sci Polym Chem 26:755. https://doi.org/10.1002/pola.1988.080260307
Kissin YV (1995) Molecular weight distributions of linear polymers: detailed analysis from GPC data. J Polym Sci Polym Chem 33:227. https://doi.org/10.1002/pola.1995.080330205
Kurtz MS (2004) Ultra-high molecular weight polyethylene in total joint replacement. In: Kurtz SM (ed) The UHMWPE Handbook. Elsevier, Amsterdam
Michler GH, Seydewitz V, Buschnakowski M, Myasnikowa LP, Ivan’kova EM, Marikhin VA, Boiko YM, Goerlitz SJ (2010) Correlation among powder morphology, compactability, and mechanical properties of consolidated nascent UHMWPE. Appl Polym Sci 118(2):866–875. https://doi.org/10.1002/app.32346
Solovev MV, Gagieva SCh, Tuskaev VA, Bravaya NM, Gadalova OE, Khrustalev VN, Borissova AO, Bulychev BM (2011) Novel titanium(IV) complexes with 2,4-di-tert-butyl-6-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl) phenol in ethene polymerization. Russ Chem Bull Int Ed 60:2227. https://doi.org/10.1007/s11172-011-0342-1
Tuskaev VA, Gagieva SCh, Solov’ev MV, Kurmaev DA, Kolosov NA, Fedyanin IV, Bulychev BM (2015) Coordination compounds of titanium(IV) and 2-hydroxymethyl-phenol derivatives: their synthesis, structure and catalytic activity in ethylene and 1-hexene polymerization. J Organomet Chem 797:159. https://doi.org/10.1016/j.jorganchem.2015.08.017
Chum PS, Swogger KW (2008) Olefin polymer technologies—history and recent progress at The Dow Chemical Company. Prog Polym Sci 33:797. https://doi.org/10.1016/j.progpolymsci.2008.05.003
Furuyama R, Mitani M, Mohri J, Mori R, Tanaka H, Fujita T (2005) Ethylene/higher α-olefin copolymerization behavior of fluorinated bis (phenoxy–imine) titanium complexes with methylalumoxane: synthesis of new polyethylene-based block copolymers. Macromolecules 38:1546. https://doi.org/10.1021/ma0481104
Acknowledgements
This work was financially supported by the Russian Science Foundation (Project No. 18-13-00375). The synthesis of UHMWPE was financially supported by the Russian Science Foundation (Project No. 16-13-10502). NMR and elemental analysis were performed with the financial support from Ministry of Science and Higher Education of the Russian Federation using the equipment of Center for molecular composition studies of INEOS RAS, Russia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gagieva, S.C., Tuskaev, V.A., Saracheno, D. et al. Ethylene homopolymerization and copolymerization with 1-hexene and 1-octene catalyzed by titanium(IV) dichloride TADDOLate complex activated with MAO. Polym. Bull. 78, 1967–1979 (2021). https://doi.org/10.1007/s00289-020-03195-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03195-3