Abstract
State of the art in the field of using biodegradable lubricating materials is considered, and data on recent achievements in their use are summarized. The major factors influencing the progress of studies in this field are associated with the environmental regulations becoming more and more stringent. Along with the use of poly(alkylene glycol) and ester liquids, raw materials of vegetable origin attract considerable interest of researchers developing biodegradable oils; cellulose can be used as a promising thickener for multipurpose lubricants.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1. INTRODUCTION
1. INTRODUCTION
The environment pollution with lubricating materials is a pressing problem for all the countries, despite the development of new oils and lubricants and organizational measures up to adoption of the corresponding laws [1, 2]. To solve this problem, attempts are made in the following directions:
– development of lubricating materials with prolonged operation life, desirably covering the whole time of functioning of the working unit;
– regeneration of spent oils and lubricants, followed by the use of the regenerated materials as a feedstock for new productions;
– development of oils and lubricants that rapidly degrade in the natural environment.
Japanese car manufacturers gained particular success in the first direction; they use in bearings “eternal” lubricants, mainly based on polyureas.
As for regeneration of spent lubricants and oils, there are several technologies, some of which have been implemented in practice. The progress in this field is slow because of problems with the collection of spent oils and with their sorting, and also because of unclear financial perspectives of the reprocessing. As a result, lubricating materials get into soil and water bodies not only at emergencies, but also because of irresponsibility of owners of cars and other machines. In any case, the ability of spent lubricants to degrade to relatively harmless products under the action of sunlight, water, atmospheric oxygen, microorganisms, and other natural factors is of crucial importance. In the ideal case, the degradation is completed by bacteria that convert the pollutants to carbon dioxide, water, and a certain amount of an inassimilable residue.
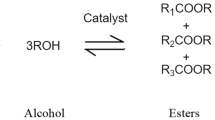
Let us pass to the third direction. Almost any lubricating material consists of at least two components: an oil base constituting the major fraction of the products and additives (in particular, thickeners) whose concentration can reach 30%. Oils recovered from vegetable oil cultures, poly(alkylene glycols), and ester liquids synthesized by reactions of polyhydric alcohols with carboxylic acids or, on the contrary, of di- and polybasic acids with aliphatic alcohols are used as biodegradable oil base. Evaluation of their biodegradability by the CEC Code: CELS-103-12 method gives the following values (percents of the initial amount) [3]:
– petroleum oils, 20–40;
– vegetable oils, 90–98:
– ester liquids, 65–100.
Poly-alpha-olefin oils (PAOs) are also classed with biodegradable products [4]. n-Decene chains constituting the PAO “comb” are actually linear paraffins and are assimilated by bacteria similarly to them. In addition, PAOs contain no aromatic hydrocarbons, which are not noticeably assimilated by microorganisms.
Biodegradation in one of the steps, often final, in elimination of lubricating materials from the environment. This process necessarily involves atmospheric oxygen (required for the activity of aerobic bacteria), water, solar radiation, and other factors responsible for preparation of readily assimilable nutrition for bacteria. Therefore, degradation pathways of different lubricating materials in the nature are different. Hydrocarbons can be ranked in the following order with respect to the rate of assimilation with microorganisms:
n-paraffins > iso-paraffins, naphthenes > aromatic hydrocarbons.
Therefore, as indicated above, petroleum oils from selective refining. containing aromatic hydrocarbons, degrade most difficultly; the next are “hydrocracking” oils of group III with decreased content of aromatic hydrocarbons; and, finally, poly-α-olefin oils degrade most readily. Ester liquids first undergo hydrolysis, and the alcohols and acids released in the process are readily soluble in water. Polyglycol liquids are miscible with water in any ratio and, being heavier than water, migrate deep into the soil, reaching aquifers where they become invulnerable to bacteria, and we can only hope that they will be diluted with a large amount of water. However, poly(alkylene glycols) themselves are not very toxic.
Naturally, pollution of the environment with vegetable oil has no catastrophic consequences. In water and soil, oils undergo hydrolysis to form glycerol and higher aliphatic acids, which form with time calcium, sodium, and other soaps; their hydrocarbon moiety is readily assimilated by bacteria.
Luna et al. [5] compare methods for determining the oxidation resistance of lubricating oils and their biodegradability. The biodegradability of vegetable oil was predicted with the aid of the biokinetic model without using microorganisms. The half-life of biodegradable vegetable oil was about 25 days, which is considerably shorter compared to petroleum oils.
There are also products termed “biosynthetic.” These are compounds obtained synthetically but from purely natural feedstock. This term, which somewhat contradicts the law of composition preservation, appeared to be convenient from the practical viewpoint, emphasizing the product origin, which is important in the environmental sense. For example, such products include plastic lubricant thickeners based on modified lignin or cellulose and products of ethylene glycol esterification with methyl esters of fatty acids obtained from vegetable cultures (sunflower seeds, soybeans, jatropha, palmchrist), which can be used as components of lubricating materials [6].
2. BASE VEGETABLE OILS
Base vegetable oils are an ideal product from the viewpoint of the impact on the nature. They are nontoxic and do not suppress the activity of plants and living bodies. Vegetable oils consist to 94–96% of triglycerides of С10–С20 fatty acids and, like any esters, are readily hydrolyzed to form relatively harmless С10–С20 acids and alcohols. It is also important for the economy of any country that the development of technical vegetable oil industry creates the effect of an economic multiplier, stimulating the development of plant growing and related branches of economy. However, vegetable oils have certain drawbacks preventing their direct use as lubricating materials: unsatisfactory temperature properties (congealing points), oxidizability, and tendency to carbonization in an engine. To minimize these drawbacks, chemical modification of vegetable oils or use of appropriate additives is recommended [7].
The tribological characteristics of vegetable oils were studied by many researchers. Some vegetable oils were tested on a four-ball friction machine (FBM) according to GOST (State Standard) 9490 in comparison with I-20A petroleum oil. Vegetable oils were found to surpass petroleum oils in tribological properties (Table 1).
This result is attributed to the fact that the mean molecular mass of vegetable oils is lower than that of petroleum oils; therefore, the wedging effect that they exert on the friction surface in the course of formation of a defective layer due to the Rebinder effect (grinding of solids in the presence of surfactants) is lower [8]. However, we can hardly agree with such explanation. The point is that the Rebinder effect is observed when physical characteristics of the substance change, whereas the process occurring in FBM is boundary friction with the formation of a juvenile surface with enormous free energy, which is spent for the formation of a protective film on the friction surface. The material of the surface itself and all materials in contact with it (lubricating medium, air and water dissolved in it, additives) participate in the formation of this film. In these processes, polar molecules of triglycerides are more active than nonpolar or weakly polar molecules of petroleum oil hydrocarbons. Therefore, the lubricating effect of triglycerides is stronger.
However, vegetable oils themselves also differ from each other in the tribological properties. For example, coconut oil has unsatisfactory antiwear characteristics even in comparison with petroleum oils. However, its mixing with effective mustard oil gives a product with satisfactory lubricating ability [9].
Other researchers also confirm better antiwear properties of vegetable oils [10]; they note, however, that petroleum oils are characterized by higher resultant pressure of the lubricating liquid layer on the lubricated surfaces (bearing ability of oils) in the elastohydrodynamic friction mode.
The production cost of technical vegetable oils prepared for use as lubricating materials even at large production volumes exceeds the production cost of petroleum oil fractions. Search for cheaper products led to spent culinary oils. However, such oils require processing to remove undesirable impurities that they contain. For example, they are converted by alcoholysis to fatty acid methyl esters, which are admixed in a definite amount to fresh oils [11].
The lubricating properties of vegetable oils, namely, their ability to decrease the wear and friction of the surfaces, can be improved by introducing additives. Additives obtained from natural raw materials are often suggested. For example, 4% trialkyl citrates prepared by reaction of citric acid with С8–С18 alcohols was introduced into sunflower oil. In FBM tests, the wear spot diameter decreased from 650 to 430 μm, and the friction coefficient in the steel/bronze couple (II-5018 friction machine) decreased by a factor of 1.5 [12].
Oxidation resistance of vegetable oils, characterized by the acid number and by the amount of low-molecular-mass acids and precipitate formed by oxidation, is an important characteristic influencing the oil storage conditions and the carbon and slag deposition in the engine and oil system. The oxidation resistance of vegetable oils is lower than that of petroleum and synthetic oils, which is caused by the prevalence of unsaturated compounds, glycerides of unsaturated acids. The larger is the number of multiple bonds in an acid molecule, the easier it is oxidized. The relative rates of oxidation of oleic, linoleic, and linolenic acids with atmospheric oxygen are 1 : 27 : 77 [13]. The fatty acid composition of some vegetable oils attracting the largest developers’ attention is given as example in Table 2.
The concentration of unsaturated bonds can be reduced by hydrogenation [15], but it is better to do this by epoxidation, as it is often done in papers and patents [16, 17]. Oxirane rings are opened by nucleophilic agents such as alcohols and water [18] with the subsequent esterification [19]. As a result, the physicochemical characteristics remain in the admissible ranges, and the oxidation resistance is considerably enhanced. As demonstrated by the example of olive oil, epoxidation improves the lubricating ability of oil owing to participation of active epoxy groups in tribochemical reactions [20]. Polymerization of soybean oil, reducing the concentration of unsaturated bonds, was also suggested [21].
Another traditional method is the use of antioxidant additives. However, new technical solutions were required in this case, because traditional antioxidants show poor performance in vegetable oils. The theoretical basis for the development of essentially novel additives is lacking yet; therefore, studies consist in active screening of diverse compounds, sometimes quite unexpected at first glance, such as N-acylated chitosan, which exhibited stabilizing effect in oxidation of castor oil [22]. Coupling of phenolic acids (gallic, п-hydroxycinnamic with 4-aminodiphenylamine gave products with extremely high stability. The induction period of oxidation of rapeseed, coconut, and epoxidized soybean oils, determined by differential scanning calorimetry, increased on introducing these products by factors of 2.2, 14.0, and 32.0, respectively [23]. Furthermore, FBM tests have shown that the additive tested surpasses widely used zinc dialkyl phosphorodithioate in antiwear performance.
A product with enhanced thermal oxidation resistance was obtained by adding abietic acid pentaerythritol ester as a component to vegetable oils. On adding this ester in 20% amount, the induction period of oxidation of rapeseed and soybean oils increased by 305 and 124%, respectively [24]. It should be noted that this fact is particularly interesting for countries with the developed pulp industry, in particular, for Russia. Abietic acid together with its analogs is the major component of so-called resin acids, waste from production of tall oil fatty acids [25]. These products do not find proper use.
The number of double bonds in the molecule directly affects the oil behavior in storage. The larger is their number, the faster is the oil polymerization manifested as “drying.” With respect to the drying ability, oils are subdivided into drying (flaxseed, hempseed oils), semidrying (sunflower, corn, soybean, cottonseed oils), and nondrying (coconut, palm, rapeseed oils). Apparently, nondrying oils are preferable over drying oils from the viewpoint of storage, but are inferior to them in the low-temperature and tribological properties.
The physical stability is the property that is more important for vegetable oils than for petroleum oils. Unrefined vegetable oils contain as impurities lipids, vitamins, and other compounds. Their amount reaches 4%. However, this amount is sufficient for their separation into a specific phase visible by naked eye. The process is accelerated in the presence of surfactants; the higher is the hydrophilic-lipophilic balance of the surfactant, the faster is the phase separation [26].
The viscosity of vegetable oils corresponds to the viscosity of petroleum oils. Many vegetable oils are close to each other in this parameter. For example, the kinematic viscosity of sunflower, soybean, and cottonseed oils at 40°С is 27.0, 27.5, and 32.1 mm2/s, respectively [13]. Their viscosity–temperature characteristic is quite good. Figure 1 shows the temperature dependence of the kinematic viscosity of unrefined sunflower oil (IV 165) and MS-8 petroleum oil (IV 80). Some characteristics of vegetable oils in comparison with commercial oils of various groups in accordance to API are given in Table 3.
Low-temperature properties. A significant drawback of vegetable oils, noted by all researchers, is high congealing point. Attempts to use depressing and dispersing additives effective for petroleum oils have not given appreciable results. Another approach, dilution with low-temperature liquids, also appeared to be inefficient. Arca et al. [21] described the results of adding some depressing and dispersing additives and oils of groups IV and V to polymerized soybean oil. Introduction of 8 wt % LZ7671A additive led to a 12.5°С depression (Fig. 2).
The required concentration is too high to make the use of additives in vegetable oils economically feasible even at excellent effect. The same is true for diluents (Fig. 3).
Depression of the congealing point of polymerized soybean oil on dilution with (1) PAOM-8 poly-α-olefin oil, (2) ditridecyl adipate, and (3) triisooctyl trimellitate (data of [21]).
The congealing point of vegetable oils can also be decreased by esterification. For example, the congealing point of castor oil was decreased from –15 to –39°С by esterification with 2-ethylhexanol [29]. Another way to improve the low-temperature properties is polycondensation of unsaturated fatty acids with alcohols to form saturated estolides (hydroxy acid anhydrides formed by intermolecular water elimination) of different molecular weights. They have very low congealing points (down to –54°С) and the viscosity index of up to 218 [30].
Cooling properties. The thermal conductivity of vegetable oils at 10–50°С varies in the range 0.16– 0.18 W m–1 K–1 [13]. As expected, this value is somewhat higher than that of petroleum oils (the commonly accepted value is 0.12) but lower than that of polar ester liquids. As for the heat capacities, the heat capacity of unrefined sunflower oil is 1.8–2.0 kJ kg–1 K–1, and that of petroleum oil is 1.7–2.5 kJ kg–1 K–1. Close levels of these properties favor the use of vegetable oils as components of cutting fluids.
The above data on the operation properties of vegetable oils suggest that there are no prospects for using them in lubricating formulations (motor and transmission oils), at least in the foreseeable future. Methods for enhancing the thermal oxidation resistance unacceptably increase the oil cost; furthermore, such oils would require more frequent replacement. However, the use of vegetable oils as hydraulic fluids and oils for two-cycle and rotor–piston engines, as dispersion media for plastic lubricants, and as components of oil-based and water-miscible cutting fluids should be encouraged.
Vegetable oils can also be a feedstock for various additives. For example, alcoholysis of vegetable oil yields ethyl esters of fatty acids, which are then sulfurized with elemental sulfur at 160–165°С for 30–60 min to obtain sulfurization products containing 5–45% sulfur. Additions of such compounds to a casting lubricant improve its antioxidant and antiscuff properties and can be used instead of traditional nonbiodegradable additives.
Food-grade lubricant based on rapeseed oil with 10% bee wax was also studied. The lubricant with rapeseed oil showed better performance compared to I-20A oil, but introduction of wax into rapeseed oil at high loads deteriorated its characteristics. The friction coefficient for the steel/bronze couple was determined with an MTU-2K7 friction machine [31]. Oils based on edible or technical oils (rapeseed, castor), taken individually or in mixtures with poly-α-olefins or poly(ethylene glycols), were also permitted for contact with food [32].
Vegetable oils are considered as promising, e.g., for lubrication of mechanisms of solar power plant panels [22].
An interesting idea was suggested in a patent [33]: use of a mixture of mono-, di-, and triacyl (С16–С22) glycerides, probably produced by incomplete hydrolysis of vegetable oil, as a dispersion medium for lubrication.
Cutting fluids (CFs) based on vegetable oils are actively used as both oil-based [34] and water-miscible concentrates [35]. Many authors note advantages of CFs based on vegetable oils over traditional petroleum oils in cutting of ferrous and nonferrous metals. These advantages are associated with good tribological and cooling properties of vegetable oils, described above [36]. Analysis of original papers shows that canola and jatropha oils attract the most attention as components of CFs for metal cutting [37, 38].
3. LUBRICATING MATERIALS BASED ON POLY(ALKYLENE GLYCOL) (PAG) AND ESTER LIQUIDS
Oils and lubricants based on PAGs and ester liquids are known for a long time and are produced as relatively expensive lubricating materials for special applications. The examples are commercial Russian oils B-3V (ester of pentaerythritol and С5–С9 acids) and DOST (heat-resistant dioctyl sebacate). High cost of such oils prevents their wide use, despite numerous advantages. Therefore, active studies are made to develop cheaper products, mainly at the expense of using cheaper raw materials. For example, polymerization of tetrahydrofuran recovered from biomass (wool) is suggested as a route to poly(alkylene glycols) [39].
In attempts to obtain a lubricant thickened by microcrystalline cellulose, dioctyl sebacate forms an unstable colloidal system. However, a lubricant with good rheological and tribological properties can be obtained on adding a stabilizer, oleophilic montmorillonite [40]. The optimum composition of an ester-based lubricant was determined. It contains 25% clay stabilizing the system and 20% microcrystalline cellulose. These components ensure high antiwear properties: high yield point (15 kPa), heat resistance (drop point >300°C), and low degree of oil separation (10% under a pressure of 100 kPa applied for 30 min) (Table 4).
Lignin was added to poly(ethylene glycol) as a biodegradable additive [41]. However, this polymer has no definite mimic structure, which depends on the kind of wood and recovery methods. It has been found that the lubricating properties of lignin are the better, the broader is distribution of its macromolecules and the higher is the concentration of hydroxy groups.
4. ADDITIVES AND IONIC LIQUIDS
We described above some original additives intended for specific base oils. Below we present a survey of data on general-purpose additives introduced not only into biodegradable products but also into lubricating materials as a whole. These are additives to Low SAPS oils (low content or complete absence of sulfated ash, phosphorus, and sulfur), and also nanosized additives and ionic liquids.
Numerous studies deal with the effect exerted by additives of nanosized metals and their oxides (copper [42], titanium [43], zirconium [44]) and of carbon nanotubes and graphene [45] on the rheological and tribological properties of vegetable oils or lubricants based on vegetable oils [46]. Nanoparticles are introduced into all the lubricating materials, including CFs.
Nanoparticles. The effect of nanoparticles introduced into date oil on the friction coefficient and wear of balls in FBM was studied at loads of 45 and 90 N. The nanoparticles were introduced in concentrations of 0.3 to 1.6 wt %. Figure 4 shows the dependences of the wear rate on the sliding velocity [42].
It was found that introduction of 0.9 wt % copper nanoparticles into date oil decreased the wear. Examination of the balls used in FBM by scanning electron microscopy (SEM) showed that the surface grooves formed on introducing 0.9 wt % nanoparticles were less deep than those formed using the oil without nanoparticles (Fig. 5).
SEM images of the surface of balls tested at a load of 95 N: (a) date oil without additives and (b) date oil with 0.9 wt % copper nanoparticles (adapted from [42]).
Similar results were obtained when introducing graphene into a lubricant based on natural wax. Graphene was introduced by ultrasonic mixing at 80°С for 2 h. The lubricating characteristics were evaluated with an Optimol SRV-IV tribometer (ball over disc) in the temperature interval 50–300°С at loads of 100–300 N [46]. Figure 6а shows the dependence of the friction coefficient on the applied load in tests of lubricant samples with and without graphene additive.
The lubricant without graphene does not operate at a load of 300 N. Figures 6b and 6c show how the friction coefficient and wear rate of steel depend on the testing temperature at a load of 250 N. The friction coefficient with the graphene-containing lubricant is somewhat lower than that with the graphene-free lubricant throughout the examined temperature interval. On the other hand, the steel wear rate with the graphene-containing lubricant is considerably lower than that with the graphene-free lubricant throughout the examined temperature interval. Figure 7 schematically illustrates the mechanism of the action of graphene-free and graphene-containing lubricants at room temperature and elevated temperatures.
Schematic illustration of the mechanisms of the lubricant action at (a) room temperature and (b) elevated (150°C) temperature (adapted from [46]).
At room temperature, the wax-based lubricant ensures the formation of a lubricating film for steel contact pairs, and extensive formation of grooves and of triboreaction products, iron oxides, is observed. Under the same conditions, the graphene-containing lubricant is readily distributed over the surface and retains the fragmented multilayer graphene, which results in the formation of an excellent lubricating film. This film protects the steel from the oxidation, decreases the wear, and increases the limiting load for the samples of the graphene-containing lubricant compared to the graphene-free sample. This is confirmed by analysis of the surface morphology and composition. At a high temperature (150°C, Fig. 7b), the sample of the graphene-free lubricant melts and partially ensures the boundary lubrication; hence, in this case the mechanism of the wear of steel discs involves abrasion and oxidation. The graphene-containing lubricant at high temperature exhibits good heat resistance and ensures high lubricating ability.
Nagabhooshanam et al. [47] described a CF based on canola oil methyl ester with the addition of 0.5 to 1.5% ZrО2 nanoparticles to improve the tribological properties. Introduction of 0.5% ZrО2 nanoparticles ensured a 37.2% decrease in the mean friction momentum compared to oil-based CFs for metal machining.
Introduction of tenth fractions of percent of various nanoparticles into an oil or lubricant increases the oil viscosity and improves the antiwear and antifriction characteristics. The effect of adding copper nanoparticles on the physicochemical characteristics of dioctyl sebacate was studied [48]. The results common for all the dispersion media were obtained: improvement of antiwear properties, viscosity index, etc. Synergism with phenolic antioxidants and antagonism with amine-based antioxidants were also noted. In addition, Oganesova et al. [49] recently published a review on nanoparticles in lubricating materials. Also, a comprehensive review on the synthesis and use of ionic liquids has been published [50].
In accordance with the Low SAPS concept, additives with the minimal content of sulfur, phosphorus, and ash-forming components are being sought for. Without dwelling on palliative solutions such as replacement of zinc by magnesium in dialkyl phosphorodithioates, let us consider more novel approaches. Major attention is paid to compounds containing two “allowed” heteroatoms: nitrogen and, to a greater extent, oxygen. For example, esters prepared by the reaction of 2-pyrone-5-carboxylic acid with aliphatic alcohols, when introduced in an amount of 1% into petroleum oil, showed significant antiwear effect under the testing conditions: The wear decreased by 60%, and the friction coefficient, by 25% [51].
Often researchers consider natural products as a feedstock for new formulations. As judged from the number of publications, this approach has a high potential. Extracts of some plants (e.g., Chamaerops humilis Mediterranean palm), containing large amounts of gallic acid, catechols, and other polyphenols, showed excellent antioxidant properties [52].
Ionic liquids as additives to lubricating materials are of interest primarily because of two features: unique solvency and high catalytic activity. The first feature ensures compatibility of chemically dissimilar components in a lubricating material, and the second feature, rapid occurrence of tribochemical reactions with the formation of strong protective films. The lubricating properties of ionic liquids are being very actively studied, and almost all the studies confirm their high tribological potential, in particular, as additives to biosynthetic dialkyl sebacate [53], to petroleum and synthetic motor oils [54], to esters, and to sunflower oil [55].
Nagendramma et al. [53] studied the effect of introducing 2 wt % ionic liquids based on glutamic and aspartic acids (structures 1 and 2):

The ionic liquids were homogenized by thorough stirring on a magnetic hot plate at 30°C for 20 min. They were readily dissolved in the base oil, ester liquid, with the formation of a transparent homogeneous mixture that did not undergo phase separation before and after the tests. The tribological properties of the mixture were studied with an FBM. Introduction of 2 wt % ionic liquids into the base oil did not lead to sharp changes in the physicochemical properties of the mixtures but led to a decrease in the mean diameter of the wear spot and in the friction coefficient (Table 5).
Anchutkin et al. [54] used as ionic liquids compounds containing one of the following anions: salicylatoborate, mandelatoborate, malonatoborate, succinatoborate, glutaratoborate, or adipatoborate, and one of the following cations: tetraalkylphosphonium, choline, imidazolium, or pyrrolidinium. Wear tests were performed at room temperature (22°C) with a Nanovea tribometer in accordance with ASTM G99 (friction couple: ball–aluminum disc). 0.1 mL of a lubricating material was applied onto the disc. 15W-50 motor oil was used as a reference sample. Experiments were performed at loads of 20 and 40 N, sliding length of 1000 m, wear path length of 20 mm, and velocity of 0.2 m/s. The friction coefficient was recorded throughout the experiment. After completing the tests, the aluminum disc wear depth was measured with a Dektak 150 probe profilometer. With 15W-50 motor oil, the wear depth was 1.369 and 8.686 μm at loads of 20 and 40 N, respectively. The ionic liquids tested considerably decreased the wear of aluminum used in the study, in particular, at a high load (40 N). For example, the wear depth for the aluminum disc lubricated with trihexyltetradecylphosphonium bis(malonato)borate was 0.842 and 1.984 μm at loads of 20 and 40 N, respectively. All the tested ionic liquids also showed lower friction coefficients compared to 15W-50 motor oil. For example, the mean friction coefficients with trihexyltetradecylphosphonium bis(malonato)borate were 0.066 and 0.067 at loads of 20 and 40 N, against 0.093 and 0.102 for the motor oil at 20 and 40 N, respectively.
It was also noted that introduction of methyltrioctylammonium trifluoroacetate ionic liquid into sunflower oil considerably enhanced the thermal oxidation resistance of the oil.
It is also interesting that, on introducing ionic liquids containing phosphonium cations into poly-α-olefin oil, plastic strain was observed in the friction couple under the action of external forces [56]. This fact suggests modification of the composition of the friction couple material. This factor can be both positive and negative depending on the value of the strain [19]. As for potentialities of ionic liquids, there may be problems with their intrinsic toxicity. This issue is insufficiently understood [50, 54] and is a matter of discussions, but in any case the toxicity is a property of a particular substance rather than of ionic liquids as a whole.
The major component of plastic lubricants affecting the environment pollution is the base oil. Therefore, formulations with the above-considered biodegradable liquids are being developed. However, sometimes a problem of combining an oil base with a thickener arises. Traditional plastic lubricants most frequently contain as thickeners lithium, calcium, and other soaps, which can be considered harmless for the nature. However, soaps are not always compatible with the new dispersion media, especially with vegetable oils. For example, cellulose [57], chitinol, and polypropylene were suggested for thickening of castor oil. Il’in et al. [58] used cellulose as a thickener for triethyl citrate when preparing low-temperature plastic lubricants. A characteristic of plastic alloys is their effective viscosity measured at 25°С and shear rate of 10 s–1: The higher is its level, the higher are the temperatures at which this lubricant can operate. The antiwear activity of lubricants is expressed in the friction and wear coefficients measured using a ball–plate friction couple (ball diameter 6.35 mm, 440S steel) at a linear velocity of 1.53 m/s and a friction force of 100 N. The results are shown in Table 6. At a thickener concentration of 7–15%, the lower temperature limit of the lubricant operation is –55°С, and the biodegradability evaluated using the modified Sturm test (OECD 301В, ISO 14852) in all the cases exceeds the threshold level of 60%, which allows these lubricants to be characterized as readily and completely biodegradable.
The problem of choosing a thickener is partially solved by using mixed media, in particular, rapeseed and castor oils [59] or vegetable oils with hydrocracking petroleum oils [60].
Influence of the conditions of the Arctic zone on the choice of lubricating materials. Many factors that favor degradation of oils and lubricants practically do not operate under the conditions of Extreme North. Low temperatures, ice cover, and weak insolation do not promote biodegradation of lubricating materials. However, there are also positive factors: enormous amount of water capable of dispersing undesirable impurities to harmless concentrations; enormous amount of phytoplankton that can incorporate hydrocarbons and other pollutants into trophic chains; finally, there is a hope that new strains of microorganisms capable of assimilating harmful substances will appear (Fig. 8). However, all these issues still remain to be studied in detail [61].
Schematic diagram of Arctic conditions influencing the crude oil biodegradation (adapted from [61]).
Thus, studies in the field of biodegradable lubricating materials largely consist in examining the potentialities of products of natural origin. Developers’ interest is primarily focused on oils isolated from oil-producing plants and on products obtained by processing wood and other kinds of biomass. Extracts from some plants contain chemically active substances such as polyhydric phenols and oxygen-containing heterocycles. The advantages of vegetable oils are their relatively high tribological characteristics, high viscosity index, available renewable resources, and, obviously, nontoxicity. However, they also have serious drawbacks: unsatisfactory oxidation resistance and poor low-temperature properties. Easy oxidizability is eliminated by epoxidation of double bonds of hydrocarbon chains in triglycerides with the subsequent transformations of oxirane rings. However, the problem with high congealing points of vegetable oils is more difficult to solve. The traditional approach, introduction of depressing additives, does not give the desirable effect. Today the problem is solved by mixing vegetable oils with low-temperature liquids based on esters or with poly-α-olefins. However, a simpler organizational solution can be expected: seasonal use of commercial oils and lubricants in regions with warm and moderate climate. As for northern regions, lubricating materials for them will be produced on the basis of readily biodegradable ester and poly(alkylene glycol) liquids.
Thickeners for plastic oils are of less concern. In most cases, these are either readily assimilable soaps or nontoxic nonsoap products: clays, pigments, polyureas, etc. Soaps of toxic metals like barium or lead have virtually come out of use. Nevertheless, alternatives are being considered. In accordance with the trend toward complete use of natural resources, the potentialities of cellulose are being examined; we believe that cellulose has good prospects as a component of multipurpose lubricants.
As for additives to oils and lubricants, the search will inevitably lead to compounds that will differ essentially from modern compounds and will be based on natural substances; however, knowledge in this field is yet insufficient. The use of nanosized particles and ionic liquids based on nontoxic compounds as additives cannot be ruled out.
REFERENCES
TR TS (Technical Regulations of the Customs Union) 030/2012: Requirements to Lubricating Materials, Oils, and Specialty Liquids.
Evdokimov, A.Yu., Lubricating materials in technosphere and biosphere, Tribologiya – mashinostroeniyu: Trudy XI Mezhdunarodnoi nauchno-tekhnicheskoi konferentsii (Tribology for Mechanical Engineering: Proc. XI Int. Scientific and Technical Conf.), Moscow: Inst. for Computer Research, 2016, pp. 67–68.
Parenago, O.P., Abstracts of Papers, Mezhdunarodnaya nauchno-tekhnicheskaya konferentsiya “Polimernye kompozity i tribologiya” (Int. Scientific and Technical Conf. “Polymer Composites and Tribology”), Gomel, June 25–28, 2019, p. 8.
Parenago, O.P., Safieva, R.Z., Antonov, S.V., Stenina, N.D., and Lyadov, A.S., Petrol Chem., 2017, vol. 57, no. 12, pp. 1144–1146. https://doi.org/10.1134/S0965544117060238
Luna, F.M.T., Rocha, B.S., Rola, E.M., Albuquerque, M.C.G., Azevedo, D.C.S., and Cavalcante, C.L., Ind. Crops Prod., 2011, vol. 33, no. 3, pp. 579–583. https://doi.org/10.1016/j.indcrop.2010.12.012
Attia, N.K., El-Mekkawi, S.A., Elardy, O.A., and Abdelkader, E.A., Fuel, 2020, vol. 271, article 117578. https://doi.org/10.1016/j.fuel.2020.117578
Zainal, N.A., Zulkifli, N.W.M., Gulzar, M., and Masjuki, H.H., Renew. Sustain. Energy Rev., 2018, vol. 82, part 1, pp. 80–102. https://doi.org/10.1016/j.rser.2017.09.004
Grigor’ev, A.Ya., Kovaleva, I.N., and Kudritskii, V.G., Tribologiya – mashinostroeniyu: Trudy XI Mezhdunarodnoi nauchno-tekhnicheskoi konferentsii (Tribology for Mechanical Engineering: Proc. XI Int. Scientific and Technical Conf.), Moscow: Inst. for Computer Research, 2016, pp. 55–56.
Sajeeb, A. and Rajendrakumar, P.K., J. Cleaner Prod., 2019, vol. 240, article 118255. https://doi.org/10.1016/j.jclepro.2019.118255
Nazri, Z.H., Rody, M.Z.M., Mohd Fadzli Bin Abdollah, Rafeq, S.A., and Nor Azmmi Bin Masripan, Procedia Eng., 2013, vol. 68, pp. 123–129. https://doi.org/10.1016/j.proeng.2013.12.157
Ameen, N.H.A. and Durak, E., Renew. Energy, 2020, vol. 145, pp. 1730–1747. https://doi.org/10.1016/j.renene.2019.06.117
Dmitriev, V.A., Dorokhova, O.O., Mukhortov, I.V., and Zadorozhnaya, E.A., Sbornik trudov XXX Mezhdunarodnoi innovatsionnoi konferentsii (Proc. XXX Int. Innovation Conf., Moscow, 2019, pp. 240–243.
Nagornov, S.A., Dvoretskii, D.S., Romantsova, S.V., and Tarov, V.P., Tekhnika i tekhnologiya proizvodstva i pererabotki rastitel’nykh masel (Technique and Technology of Production and Processing of Vegetable Oils), Tambov: Tambovskii Univ., 2001.
Shevchenko, E.B., Sukhanberliev, A.I., Abbasov, M.M., and Danilov, A.M., Russ. J. Appl. Chem., 2019, vol. 92, no. 1, p. 166. https://doi.org/10.1134/S10704272190100233
Jian, Z., Patent CN 107723054A, China, 2018.
Borugadda, V.B. and Goud, V.V., Ind. Crops Prod., 2019, vol. 133, pp. 151–159. https://doi.org/10.1016/j.indcrop.2019.01.069
Laijun, T., Patent CN 103805306A, China, 2014.
Rios, Í.C., Cordeiro, J.P., Arruda, B.M.G., Rodrigues, F.E.A., and Ricardo, M.P.S., Ind. Crops Prod., 2020, vol. 145, article 112000. https://doi.org/10.1016/j.indcrop.2019.112000
Dalai, A.K. and Sharma, R., Patent US 9593287, 2016.
Kerni, L., Raina, A., and Haq, M.I.U., Wear, 2019, vols. 426–427, part A30, pp. 819–827. https://doi.org/10.1016/j.wear.2019.01.022
Arca, M., Sharma, B.K., Perez, J.M., and Doll, K.M., Int. J. Sustain. Eng., 2013, vol. 6, no. 4, pp. 326–331, http://dx.doi.org/10.1080/19397038.2012.725430
Gomna, A., N’Tsoukpoe, K.E., Nolwenn Le Pierrès, and Coulibaly, Y., Solar Energy Mater. Solar Cells, 2019, vol. 200, article 109956. https://doi.org/10.1016/j.solmat.2019.109956
Zhao, H., Feng, J., Yu, J.Z.H., and Liu, S., J. Cleaner Prod., 2020, vol. 24, article 118274. https://doi.org/10.1016/j.jclepro.2019.118274
Xu, Z., Lou, W., Zhao, G., Zhang, M., and Wang, X., Tribol. Int., 2019, vol. 135, pp. 213–218. https://doi.org/10.1016/j.triboint.2019.02.038
Fyong, Ch.Kh., Cand. Sci. (Eng.) Dissertation, St. Petersburg, 2003.
Zhang, M., Li, M., and Wu, H., Fuel, 2019, vol. 252, pp. 403–407. https://doi.org/10.1016/j.fuel.2019.04.132
Trofimov, I.L. and Marchuk, S.I., Materialy Х Mezhdunarodnoi nauchno-tekhnicheskoi konferentsii “Prodvizhenie v neftepererabatyvayushchei i neftekhimicheskoi promyshlennosti” (Proc. X Int. Scientific and Technical Conf. “Advances in Oil Refining and Petrochemical Industry”), Lviv, May 18–23, 2020, pp. 64–68.
Danilov, A.M., Vvedenie v khimmotologiyu (Introduction to Chemmotology), Moscow: Tekhnika, 2003.
Luna, F.M.T., Cecilia, J.A., Saboya, R.M.A., Barrera, D., Sapag, K., Rodríguez-Castellón, E., and Cavalcante, C.L., Materials, 2018, vol. 11, article 1764. https://doi.org/10.3390/ma11091764
Soni, S. and Agarwal, M., Green Chem. Lett. Rev., 2014, vol. 7, no. 4, pp. 359–382. https://doi.org/10.1080/17518253.2014.959565
Kovaleva, I.N. and Grigor’ev, A.Ya., Tribologiya – mashinostroeniyu: Trudy XI Mezhdunarodnoi nauchno-tekhnicheskoi konferentsii (Tribology for Mechanical Engineering: Proc. XI Int. Scientific and Technical Conf.), Moscow: Inst. for Computer Research, 2016, pp. 113–114.
Bodachevskii, Yu.S., Pop, G.S., and Zheleznyi, L.N., Tribologiya – mashinostroeniyu: Trudy XI Mezhdunarodnoi nauchno-tekhnicheskoi konferentsii (Tribology for Mechanical Engineering: Proc. XI Int. Scientific and Technical Conf.), Moscow: Inst. for Computer Research, 2016, pp. 23–24.
Beum, K.S., Patent KR 20180031939A, Korea, 2018.
Dorogochinskaya, V.A., Tonkonogov, B.P., Volgin, S.N., Antonov, S.A., Vizhankov, E.M., Zaglyadova, S.V., Mityagin, M.A., Nemets, V.L., Raskin, Yu.E., and Yagoda, M.I., Proizvodstvo i primenenie tekhnicheskikh zhidkostei i spetsial’nykh produktov maslyanykh proizvodstv (Production and Use of Technical Liquids and Specialty Products of Oil Enterprises), Moscow: Ross. Gos. Univ. Nefti i Gaza im. I.M. Gubkina, 2019.
Khisamutdinov, R.M., Pashkov, M.V., Obzherina, L.N., Kiramova, E.A., Galimova, A.A., Danilov, A.M., Bezgina, A.M., Konstantinova, S.Ch., and Ovchinnikov, K.A., Patent RU 2713896, 2020.
Wickramasinghe, K.C., Sasahara, H., Rahim, E.A., and Perera, G.I.P., J. Cleaner Prod., 2020, vol. 257, article 120552. https://doi.org/10.1016/j.jclepro.2020.120552
D’Amato, R., Wang, C., Calvo, R., Valášek, P., and Ruggiero, A., Procedia Manufact., 2019, vol. 41, pp. 145–152. https://doi.org/10.1016/j.promfg.2019.07.040
Sani, A.S.A., Rahim, E.A., Sharif, S., and Sasahara, H., Tribol. Int., 2019, vol. 129, pp. 347–362. https://doi.org/10.1016/j.triboint.2018.08.038
Erwang, X. and Wei, Z., Patent CN 103755948A, China, 2014.
Gorbacheva, S.N., Yarmush, Y.M., and Ilyin, S.O., Tribol. Int., 2020, vol. 148, article 106318. https://doi.org/10.1016/j.triboint.2020.106318
Mu, L., Wu, J., Matsakas, L., Chen, M., and Shi, Y., Int. J. Biol. Macromol., 2019, vol. 129, pp. 564–570. https://doi.org/10.1016/j.ijbiomac.2019.01.175
Singh, Y., Sharma, A., Singh, N.K., and Chen, W.-H., Fuel, 2020, vol. 259, article 116259. https://doi.org/10.1016/j.fuel.2019.116259
Rajaganapathy, C., Vasudevan, D., and Murugapoopathi, S., Materials Today: Proc., 2020. https://doi.org/10.1016/j.matpr.2020.05.032
Shafi, W.K. and Charoo, M.S., Materials Today: Proc., 2020, vol. 26, part 2, pp. 745–749. https://doi.org/10.1016/j.matpr.2020.01.019
Ali, I., Basheer, A.A., Kucherova, A., Memetov, N., and Tkachev, A., J. Mol. Liq., 2019, vol. 279, pp. 251–266. https://doi.org/10.1016/j.molliq.2019.01.113
Xie, M., Cheng, J., Huo, C., and Zhao, G., Tribol. Int., 2020, vol. 150, article 106386. https://doi.org/10.1016/j.triboint.2020.106386
Nagabhooshanam, N., Baskar, S., Prabhu, T.R., and Arumugam, S., Tribol. Int., 2020, vol. 151, article 106510. https://doi.org/10.1016/j.triboint.2020.106510
Guo, Z., Zhang, Y., Wang, J., Gao, C., and Zhang, Z., Tribol. Int., 2020, vol. 141, article 105941. https://doi.org/10.1016/j.triboint.2019.105941
Nanosized additives to lubricating materials, Oganesova, E.Yu., Lyadov, A.S., and Parenago, O.P., Russ. J. Appl. Chem., 2018, vol. 91, no. 10, pp. 1559–1573. https://doi.org/10.1134/S1070427218100014
Singh, S.K. and Savoy, A.W., J. Mol. Liq., 2020, vol. 297, article 112038. https://doi.org/10.1016/j.molliq.2019.112038
White, D., Podolak, K., Kraus, G.A., and Sundararajan, S., Wear, 2020, vols. 442–443, article 203115. https://doi.org/10.1016/j.wear.2019.203115
Zzeyani, S., Mikou, M., Naja, J., Bouyazza, L., and Aiboudi, M., Energy, 2019, vol. 180, pp. 206–215. https://doi.org/10.1016/j.energy.2019.05.007
Nagendramma, P., Khatri, P.K., Thakre, G.D., and Jain, S.L., J. Mol. Liq., 2017, vol. 244, pp. 219–225. https://doi.org/10.1016/j.molliq.2017.08.115
Anchutkin, O.N., Shakh, F.U., and Glavatskikh, S.B., Patent RU 2566364, 2016.
Bodesheim, G., Schmidt-Amelunxen, M., Sohn, D., Grundei, S., and Hoepke, A., Patent RU 2516705, 2014.
González, R., Viesca, J.L., Battez, A.H., Hadfield, M., and Bartolomé, M., J. Mol. Liq., 2019, vol. 293, article 111536. https://doi.org/10.1016/j.molliq.2019.111536
Cortés-Triviño, E., Valencia, C., Delgado, M.A., and Franco, J.M., J. Ind. Eng. Chem., 2019, vol. 80, pp. 626–632. https://doi.org/10.1016/j.jiec.2019.08.052
Il’in, S.O., Yadykova, A.E., Gorbacheva, S.N., and Antonov, S.V., Patent RU 2692090, 2019.
Rongquan, G., Xiaomou, G., and Jiasheng, H., Patent CN 107699328A, China, 2018.
Zhanliang, R., Xianhui, W., and Jingbo, Y., Patent CN 106497651A, China, 2017.
Vergeynst, L., Wegeberg, S., and Mosbech, A., Sci. Total Environ., 2018, vol. 626, pp. 1243–1258. https://doi.org/10.1016/j.scitotenv.2018.01.173
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Translated from Neftekhimiya, 2021, Vol. 61, No. 4, pp. 445–460 https://doi.org/10.31857/S0028242121040018.
Rights and permissions
About this article
Cite this article
Danilov, A.M., Antonov, S.A., Bartko, R.V. et al. Recent Advances in Biodegradable Lubricating Materials (A Review). Pet. Chem. 61, 697–710 (2021). https://doi.org/10.1134/S0965544121050121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544121050121