Abstract
Bio-based lubricants have gained prominence over conventional petroleum-based oils, progressively over the last two decades as biolubricants. This trend is observed in almost every industry that has been dependent on lubricants and oils irrespective of their applications. Factors that initiated and fueled this trend vary from stringent government regulations over petroleum-based oils to the high passed depletion of oil reserves. But the most concerning factor that has fast-tracked the need for biolubricants is the toxic and harmful effect of used petroleum oils has on the environment and ecological factors. It is estimated that nearly 50% of all lubricants produced are introduced to the environment which has spurred the interest in biolubricants. This review discusses various types of eco-friendly bio-lubrications that will become a sustainable and economical alternative to the conventional petroleum-based lubricants by being sourced from renewable resources. Biolubricants are seen to be feasible and versatile lubricants with higher lubricity, lower volatility, higher shear stability, higher viscosity index, higher load-carrying capacity, and superior detergency and dispersancy when compared to petroleum-based lubricants. The review also investigates in detail the poor thermal-oxidative stability, biological deterioration, their poor solidification at low temperatures, and hydrolytic instability as well as mechanical and chemical enhancements that seek to rectify these issues. Furthermore, economical and legislative landscape of biolubricants is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today there are literally thousands of varieties in lubricant formulations of oils, greases, and other functional fluids targeted towards more than 90% of all lubricant applications. The primary function of these lubricants is to decrease friction, transmit energy, protect against corrosion and wear (attrition), remove heat, disperse wear debris, eliminate foreign contaminants, and act as a sealant [1]. The use of natural organic oils and fats derived from plants and animal-based raw materials (e.g., soybean, palm, tallow, and lard) dates back to the earliest days of lubrication for their ability to lower friction and prevent wear. The modern petroleum oil industry began with the first commercial oil well-being drilled in Titusville, PA, USA, in 1859. Over a period of time, this signaled the detrition in research interest and need for natural oils as lubricants. But this was a boon for lubrication technology for industrial application with regard to research on petroleum-based oils to capitalize on its diverse forms for various applications that quickly dominated other oils, such as natural plant and animal-based oils. The introduction of additive along with new chemical synthesis and modification techniques in the mid-1930s further added on to the developmental process of petroleum-based oils that significantly improved on its properties and applicability in terms of load-carrying capacity, lubricity, corrosive protection, and thermal-oxidative stability. With the advancements in technology of petroleum-based oils seen over the past 150 years, it has surpassed natural oils in many aspects mainly due to more time, effort, and money that were dedicated to the research on petroleum-based oils.
At present, petroleum-based oils have established themselves as the universal lubricant for most industrial, commercial, and personal applications. It is estimated that upwards of 50% of all lubricants worldwide enter the environment from spills, improper disposal, accidents, volatility, and total loss applications such as chainsaw oils, two-stroke engines, concrete mold release oils, exhaust fumes in engines, and metal cutting and forming processes [2]. Of these leakage types, the most problematic are the uncontrolled losses via broken hydraulic hoses or accidents whereby large quantities of fluids escape into the environment contaminating soil; surface, ground, and drinking water; as well as the air. It is estimated that 30–40 million tons of lubricant are consumed annually, with 20 million tons of this lubricant entering the environment, totaling to a 55% loss of lubricant [3]. Over 95% of these lubricants entering the environment are petroleum-based and harmful to the environment [4]. As a result of their high toxicity and low biodegradability, petroleum-based lubricants and functional fluids (hydraulic fluids) constitute a considerable threat to the environment. More astonishingly, it is estimated that over 90% of all petroleum-based lubricants could be replaced by biolubricants [5]. Figure 1 shows the global energy consumption by just transportation vehicles which account for a major part of petroleum-based lubricant and oil consumptions. These estimates delineate the substantial potential and need for biolubricants to solve our environmental problems caused by toxic petroleum-based lubricants.
Global energy consumption by transportation vehicles [6]
The three broad categories of lubricants are as follows: (1) mineral oils that are predominantly petroleum-based lubricants and are the most common lubricants; (2) natural oils that are derived from plant-based oils and animal-based fats or tallow; and (3) synthetic oils that include polyalphaolefins (PAOs), synthetic esters, polyalkylene glycols (PAGs), alkylated aromatics, perfluoroalkylpolyethers (PFPEs), among others. In the last three decades, natural and synthetic oils (not mineral oils) derived from bio-based feedstock have seen a resurgence for industrial purposes. The lubrication industry is shifting to become more environmentally responsible with much of the attention centered on ecological conservation and sustainability through the use of bio-based lubricants for industrial purposes to be used as functional fluids. The term “biolubricant” has been ascribed to lubricants that have bio-based raw materials such as plant oils, animal fats, or other environmentally benign hydrocarbons as their main constituents or base. These base constituents render these lubricants as biodegradable and non-toxic to humans and other living organisms, particularly marine environments where the impacts are more severe [7]. For the same reason, one of the major issues being targeted along with the process of development of technologies to incorporate biolubricants as biofuels and/or industrial lubricants is to deter the use of petroleum-based lubricants [8]. In the global lubrication market, the rise in biolubricants is a result of new environmentally friendly initiatives and economic factors such as protecting the environment from toxic substances; the depletion of the world’s crude oil reserves; increasing crude oil prices; and increasingly stringent government regulations regarding use, operation, and disposal of petroleum-based oils [9, 10]. Progressively so the need for biolubricants is also a result of the increase in demand for environmentally friendly lubricants that are less toxic to the environment, renewable, and provide feasible and economical alternatives to traditional petroleum-based lubricants [11, 12].
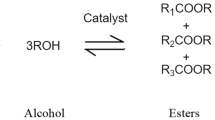
Although mineral oils are classified according to their performance, biolubricants are classified according to their base fluid composition. Traditionally, there are three groups of biolubricants: natural esters (type HETG), synthetic esters (type HEES), and polyglycols (type HEPG). The properties of these groups are listed in Table 1.
-
(1)
Furthermore, a more rudimentary classification of biolubricants will be imposed according to the synthesis process of the lubricant. Biolubricants will be classified into two categories: (1) natural oils and (2) synthetic oils. Natural oils (also known as natural esters) are biolubricants with the base stock consisting of vegetable (plant-based) oils or animal fats. Synthetic oils (or synthetic esters) are biolubricants comprising esters, diesters, genetically modified organisms, perfluoroalkylethers, ionic liquids, polyglycols, or any other lubricant consisting of chemical compounds that are artificially made [14]. Many biolubricants have superior lubricity and wear resistance that exceeds those of petroleum-based lubricants resulting in their increased usage as a base stock for industrial oils and functional fluids, thus facilitating the biolubricant resurgence [15]. The largest drawback to biolubricants particularly natural oils is their poor thermal-oxidative stability and high pour points, which have led to the development of synthetic biolubricants that undergo chemical modifications [15]. Ultimately, biolubricants formulated from bio-based feedstocks should offer the following advantages over petroleum-based oils [16]. Higher lubricity leading to lower friction losses and improved efficiency affording more power output and better fuel economy.
-
(2)
Lower volatility resulting in decreased exhaust emissions.
-
(3)
Higher viscosity indices.
-
(4)
Higher shear stability.
-
(5)
Higher detergency eliminating the need for detergent additives.
-
(6)
High dispersancy.
-
(7)
Rapid biodegradation resulting in decreased environmental and toxicological hazards.
This review provides a summary of biolubricants detailing the types of biolubricants, discussing vulnerabilities, and revealing enhancement techniques. Further investigation examines the biodegradability and economy of biolubricants as well as recent advances in new technologies pertaining to their utilization.
2 Biolubricants
Biolubricants can be derived from a variety of bio-based feedstock, often this is a vegetable oil because they offer natural biodegradability and low toxicity. In other instances, genetically modified natural oils, such as high-oleic sunflower and canola (rapeseed), are being pursued for applications where higher oxidative stability is needed. Synthetic esters derived from natural and artificial resources, i.e., the hydrolysis of solid fats and low-grade waste materials that allows to produce the constituent ester compounds, are also common. Polyglycols (PGs) are another class of synthetic biolubricants similar to synthetic esters, yet PGs are the only type that is water-soluble. This property can be advantageous for biological degradation in water. On the contrary, this poses more of an environmental threat as polyglycol-contaminated water can penetrate more deeply into the soil layers, thus contaminating ground water. For this reason in some countries, PGs are not considered environmentally friendly fluids, thus their discussion will be limited in this review.
Similar to the development of environmentally friendly base fluids, new additives are also being developed. Eco-friendly initiatives necessitate the development of additives for two reasons: (1) existing additives used for mineral oils deteriorate the performance properties in many biolubricants and (2) existing additives contain toxic substances leading to a significant deterioration of the biodegradability of the lubricant as a whole [17–21]. Other biolubricants are composed of environmentally benign solid particles in bio-based oil colloidal suspensions. The formulation of these two-phase lubricants means that biodegradable and low toxicity fluids, i.e., natural oils are combined with biodegradable and low toxicity additives [5].
2.1 Synthetic Esters
Synthetic organic esters are a class of widely used lubricants that were once derived from glycerol molecules found in plant-based oils and animal fats. However, this causes synthetic esters to suffer from similar unsaturated performance issues as natural oils. Now, synthetic esters can be synthesized from long-chain alcohols and acids, where the performance of synthetic esters is superior to natural ester fluids due to a more uniform molecular structure and the use of different alcohols. Using completely saturated esters, the resulting biolubricants tend to exhibit very stable aging characteristics. Additives used specifically for synthetic esters have been developed. Early synthetic esters possessed chemical structures similar to natural oils; however, due to recent advancements, there are now many choices of acids and alcohols available for the production of synthetic esters, affording them a wide variety of technical performance properties. Examples of synthetic esters feedstock include C6–C13 alcohols (i.e., n-hexanol, n-heptanol, isonal, and decanol), C5–C18 mono acids (i.e., valeric, heptanoic, pergalonic, and oleic acid) with neopentyl polyols, such as pentaerythritol (PE), polyol esters, diacids (i.e., adipic acid, azelaic, sebacic, and dodecanedioic) and various dimer acids [22–30].
Esters are a type of biolubricant that provide better low-temperature fluidity, low volatility, high flash points, and improved thermal-oxidative stabilities when compared to natural oils. Similar to natural oils, synthetic esters maintain an affinity for metal surfaces due to their high degree of polarity which affords them the ability to establish monolayers that minimize the surface contact and enhance the tribological properties. Esters are inherently sensitive towards hydrolysis and thermal degradation, and for this reason, their thermal properties have been improved by replacing the glycerol with other polyols such as neopentyl glycol (NPG), pentaerythritol (PE), trimethylolpropane (TMP), trimethylolhexane (TMH), and trimethylolethane (TME) [11, 31–34]. A subgroup of esters known as complex or oligo esters is derived from mixtures of polyols and mono-, di-, and tri-carboxylic acids. Replacing the glycerol molecules with peresters of sugar (i.e., sucrose and sorbitol) yields synthetic esters with enhanced oxidative stability, lubricity, and biodegradability [35]. These improved synthetic esters are strong candidates for replacing mineral oils in the food, pharmaceutical, and cosmetic industries; in fact, these esters could be used in many lubricant applications that require low toxicity due to potential contact with animals or humans [35, 36]. Esters such as the NPG were originally developed for the lubrication of aircraft jet engines, whereas PE esters are derived from C5–C9 carboxylic acids and have found application in gas turbines. Still, other esters such as TMP esters derived from oleic acid have found widespread use as automotive and marine engine oils, compressor oils (as replacement lubricants for hydrofluorocarbon systems), hydraulic fluids, gear oils, and grease formulations. The low toxicity, excellent biodegradability, moderate oxidative stability, and moderate price coupled with the high viscosity and good shear stability make synthetic esters attractive lubricants. Furthermore, the use of appropriate additives to ester molecules improves their performance making them the optimal lubricant of choice for medium-to-heavy lubricant applications [22, 31, 37–39].
Another derivative of the synthetic ester is the diester, a biolubricant derived partly from renewable resources. Synthetic diesters are derived from dicarboxylic acid and monovalent alcohols. The dicarboxylic acid can be prepared from natural sources such as azelaic acid (ozonolysis of oleic acid), sebacic acid, dimeric fatty acid, isostearic acid, or from purely petrochemical sources such as adipic acid or maleic acid. Diesters generally consist of branched alcohols such as 2-ethylhexanol (isooctanol), isodecanol, or guerbet alcohols to offer better low-temperature properties than conventional synthetic ester lubricants. Additionally, branched fatty acids, for example, 12-hydroxystearic acid derived from rhizinoleic acid can be utilized to form diester-based lubricants that also exhibit improved low-temperature properties.
2.2 Natural Oils
The chemical composition of biolubricants derived from oils and fats affords them the ability to be used as fuels and lubricants for various applications. Bio-based oils and fats can be easily sourced from a wide variety of seeds, fruits, nuts, vegetables, animals, and marine life making them readily available, inexpensive, and environmentally benign [8]. Natural oils derived from plant-based oils exhibit characteristics similar to petroleum-based lubricants used in industrial applications for metal-stamping and metal-forming in the sense that they have comparable viscosity and surface tension properties [22, 31]. Many plant-based oils are obtained by expeller methods and solvent extraction processes [11, 37, 40, 41]. There are a wide variety of plant-based oils such as avocado, canola (rapeseed), castor, coconut, corn, cottonseed, olive, palm, palm kernel, peanut, safflower, sesame, soybean, and sunflower, among many others that are native to particular regions of the world. The efficacy of natural oils and fats is determined by their chemical composition, where they predominantly consist of mixtures of fatty acid esters derived from glycerol. Inherently, since these oils and fats are naturally occurring organic substances, their properties differ based on several biological factors such as climate, light, temperature, nutrient availability, humidity, and water as well as being influences based on different extraction methods [11, 40–42]. Despite their biologically inconsistent chemical composition, natural oils have superior wear properties, lower coefficients of friction, excellent biodegradability, renewable feedstocks, sustainable and higher flashpoints, and comparably less ecotoxicity classification than mineral oils [43]. Further, the literature indicates that natural oils derived from plants, with high-oleic acid (> 80%) contents, surpass Group I petroleum-based lubricants at room temperature [44]. When comparing natural oils to mineral and synthetic oils, they have a better lubricity, shear stability, viscosity index, and load-carrying capacity coupled with lower volatility and superior detergency and dispersancy [45].
The molecular structure of the natural oils dictates their characteristic nature which affords superior lubrication properties. This molecular structure of natural oils is defined by the chemical composition consisting approximately 98% triacylglycerol molecules made up of esters derived from glycerol and long chains of polar fatty acids. Minor quantities of mono- and di-glycerols (0.5%), free fatty acids (0.1%), sterols (0.3%), and tocopherols (0.1%) are also present in natural oils [4]. Fatty acids themselves are carboxylic acids with long unbranched aliphatic chains (with 4–28 carbon atoms), composed of hydrogen atoms attached to the carbons, and in many instances contain other organic chemistry-related functional groups, and may be saturated or unsaturated. Due to the large amounts of unsaturated fatty acids in natural oils, they tend to suffer from a poor thermal stability with a limited temperature range.
The boundary lubrication is the condition when fluid films are negligible at the interface and there is considerable asperity contact. The physical and chemical properties of thin surface fluid films are of significant importance as they significantly influence tribological properties. This boundary lubrication and the ability of natural oils to adhere to the metallic surfaces are dictated by the fatty acids present in them. These fatty acids establish a monolayer film using their closely packed polar carboxyl group that can efficiently reduce friction and wear by minimizing the metal-to-metal contact [23]. Research concerning natural oils has centered on understanding the fundamentals of saturated and unsaturated fatty acids. The majority of which is focused on using natural oils as clean-green lubricants along with fatty acids as additives in mineral oils, and bio-based feedstock oils for chemically modified lubricants [22, 31]. Additionally, new additives are being developed to extend the operable temperature range of natural oils to improve their thermal stability. More recently so, lamellar powder particle additives which plainly put are carrier fluids have biolubricants that aid in sliding contact [46].
2.3 Perfluoroalkylethers
High-performance applications that require a combination of high- and low-temperature properties, chemical or oxidative stability, low volatility, material compatibility, inertness, and non-flammability or non-combustibility simultaneously will generally use synthetic lubricants known as perfluoroalkylethers (PFPEs) [47]. PFPEs are a new class of lubricants that are undergoing rapid advances to redesign them to be more environmentally friendly [48–50]. PFPEs are known for their superior thermal and oxidative stability. Once composed entirely of carbon, fluorine, chlorine, and oxygen to produce a colorless, odorless, and completely inert functional fluid, they are now being derived from more environmentally benign feedstock that reduces their environmental impact. One such example is α,ω-dialkoxyfluoropolyethers (DA-FPEs) which are partially fluorinated polyethers that do not contain chlorine atoms and hence they do not contribute to ozone depletion [51]. The improvement in these lubricants is due to the incorporation of two alkoxy-groups that provide reactive sites, which act to minimize the atmospheric lifetime of these functional fluids [51–53]. Typically, PFPEs enter the environment as an aerosol by means of exhaust fumes or volatility. DA-FPEs are more beneficial than PFPEs in the sense that their biological footprint and impact is lower with reduced global warming potential as a result of atmospheric pollution by means of greenhouse gasses in the form of carbon dioxide [51]. Furthermore, perfluorinated ether compounds derived from polymers maintain their properties of typical PFPEs such as high thermal and chemical stability, no acute toxicity, and excellent heat exchange properties [54]. DA-FPEs have alkoxy-groups which allow them to maintain excellent solvent properties with several organic liquids, such as ketones and alcohols [55]. These properties make DA-FPEs a preferable candidate for CFC, perfluorocarbon and halon substitutes in a number of applications, like foaming and fire extinguishing agents, cleaning agents for sophisticated electronic devices, heat transfer fluids, and lubricants in extreme applications [56–58].
2.4 Ionic Liquids
Ionic liquids (ILs) at room temperature have proven to be the forthcoming of a new class of lubricants that are able to overcome the limitations of both petroleum-based lubricants and natural oils [59]. Room-temperature ILs are molten salts, which have organic anion with melting points below 100 °C that are paired with a combination of a bulky, asymmetric organic cation having a liquid range beyond 300 °C. An IL atomic structure is shown in Fig. 2.
This structure is similar to that of a typical lamellar solid particle, held together by weak Van der Waals forces with their anions and the cations forming ionic bonds in the form of layers which in turn build up their liquid lamellar crystal structure. ILs exhibit unique and useful properties such as (1) a broad liquid range (low melting and high boiling point); (2) negligible vapor pressure; (3) non-flammability and non-combustibility; (4) superior thermal stability; (5) high viscosity; (6) miscibility and solubility; (7) environmentally benign (non-toxic); (8) lamellar-like liquid crystal structure; (9) long polar anion–cation molecular chains; and (10) economical costs, which make them well suited as the basis of a new family of biolubricants which have the potential to perform better than their conventional counterparts [47, 60–70].
ILs have been shown to have excellent corrosion and tribocorrosion properties that broaden the range of plausible applications. Newly synthesized ILs based on imidazolium and thiazolium have been shown to have no corrosive effect on steel surfaces. But they were observed to undergo a certain degree of tribocorrosion due to the decomposition of specific cations derived from dicyanamide. However for the same IL bases with [Tf2N], there was the absence of wear, corrosion, and tribocorrosion processes [71, 72].
Some of the well-known ILs with tetrafluoroborate (BF4) or hexafluorophosphate (PF6) anions have good tribological properties, but they have been shown to have corrosive issues. These ILs produce fluorine compounds like hydrogen fluoride due to their hydrophilic properties which make them prone to hydrolysis resulting in a corrosive environment [73]. This led to the research on imidazolium-based ILs that would able to solve these corrosion issues with bis(trifluoromethylsulfonyl)amide (TFSA, [Tf2N]) or tetrafluorotris (pentafluoroethyl) phosphate (FAP) anions [73–75]. These modification aspects of ILs make it a very flexible and customizable form of eco-friendly lubricant and show the possibility of using these new ILs not only as friction reducing and antiwear lubricants but also as corrosion inhibitors, in the line of the most recently described research for IL lubricants [76, 77].
Further, ILs have a chemical composition that is uniform which allows them to be customized to provide a required level of thermal-oxidative stability and lubricity meant for a variety of applications in the aerospace, automotive, manufacturing, and magnetic storage industries. This consistency in chemical composition enables ILs to have physicochemical properties that are readily reproducible. By selecting both the cationic and anionic constituents to be non-toxic, they can be designed to be environmentally friendly [78, 79], not to mention the various methods available to produce ILs from non-petroleum products. Finally, their ability to sustain and perform in various environmental conditions on par with its petroleum counterparts make these new green lubricants more attractive alternative to most oil-based lubricants.
ILs are structurally diverse compounds in the sense of their functionality where they can perform and serve a variety of other purposes than just providing for an efficient lubrication. This is made possible by their inherent tunability of physicochemical characteristics. These compounds are structurally so diverse that it has been estimated to have 1018 different possible combinations of anion and cation moieties [80]. This in itself poses a huge challenge to researchers for IL design. The design process and be significantly be simplified with increasing number of desired properties, but as expected the number of possible ILs decline by a similar magnitude if not more. For example, for a lubricant to be environmentally friendly, highly fluorinated anions cannot be used. But instead, anions based on common food additives (e.g., benzoate and salicylate, are well-known preservatives) or artificial sweeteners (e.g., saccharinate) are utilized. Along the same lines, trihexyltetradecylphosphonium salts (i.e., P666,14) also exhibit anti-microbial properties which can be satisfied by making a similar choice of cations [81, 82]. Also, the idea behind using renewable feedstocks for the preparation of the ILs suggests the use of certain 1,3-dialkylimidazolium cations, such as can be derived from fructose [62, 83, 84]. The structures of these cations and anions are depicted in Table 2 and their atomic structures are shown in Table 3.
2.5 Genetically Modified Lubricants
Genetically modified lubricants are as their name suggests lubricants derived from genetically modified organisms (GMOs). GMOs are organisms whose genetic material has been altered using genetic engineering techniques. In the lubrication industry, genetically modified lubricants are usually natural oils, in particular, plant-based oils such as sunflower, soybean, and canola (rapeseed) oil. Plants are genetically engineered by manipulating the gene sequence within the organisms by causing a gene to be inoperative or “knocked out” or by attaching a gene to an identified region of DNA that initiates transcription of a particular gene thus acting as a “promoter.” Genetically modified vegetable oils often focus on improving the thermal and oxidative stabilities of natural oils by decreasing the linoleic and linolenic acid amounts and increasing the oleic acid amounts, as will be explained further in the proceeding sections [85–87]. Genetically modified vegetable oils also seek to improve upon the cold flow properties of the lubricants where higher proportions of short-chain saturated or long-chain monounsaturated fatty acids lower the pour point [88, 89]. The objective of these manipulations is to create oils with higher degrees of saturation whereby the oil is less susceptible to oxidative deterioration by means of exposed double bonds in the fatty acid molecules [90].
3 Enhancement of Biolubricant Performance
Biolubricant research has focused on thwarting the deficiencies of natural and synthetic oils while seeking to understand the relationship between chemical composition, molecular structure, and enhancements through chemical modifications such as epoxidation, metathesis, acylation, estolide formation, transesterification, and selective hydrogenation of biolubricants with polyols, and additivation [91, 92]. The potential use of biolubricants is far reaching, yet their major disadvantages militating against their widespread use in industrial applications are their sensitivity to hydrolysis, thermal degradation by elimination, and oxidation caused by unsaturated molecular compounds. Investigations into the oxidative limitations of biolubricants have been researched and new techniques have been proposed to enhance the oxidative stability of biolubricants [4]. In some instances, through various chemical manipulations and enhancements biolubricants have been shown to offer higher oxidative stability than traditional petroleum-based lubricants [8, 16, 27, 88, 93–102]. Still, other researchers have investigated the stability of biolubricants when antioxidants are added [103–105]. Nonetheless, chemical modifications to biolubricants by addition reactions to the double bonds constitute one of the most promising processes for obtaining commercially viable products from renewable raw materials. Thus, to facilitate the use of lubricants derived from bio-based materials, additivation, chemical modifications, de novo synthesis, breeding of GMOs, and biotechnology will all play critical roles to ensure adequate functionability and stability as biolubricants and functional fluids [106–108].
3.1 Oxidation
Many natural oils and bio-based feedstock are basically derived from triacylglyceride molecules containing glycerol that contribute to the of fatty acids contents. Figure 3 at location (a) illustrates functional hydroxyl group where tertiary β-hydrogen (secondary hydrogen) get attached to the β-carbon (secondary carbon) due to the presence of glycerol in bio-based materials [4].
An oxidative instability is generated due to the presence of β-hydrogen in the unsaturated fatty acids. This increases the pace at which natural oils oxidize giving rise to a process called autoxidation. It is at this location (at β-hydrogen) that the chemical modification of most bio-based feedstock occurs [11]. Further, the bis-allylic hydrogen, which attaches to the double-bonded carbon atoms in polyunsaturated fatty acids, is particularly susceptible to free radical attacks, peroxide formation, and production of polar oxidation products similar to that of hydrocarbon mineral oils, except at an expedited rate in natural oils [109–112]. The presence of these double bonds in triacylglycerol molecule also leads to oxidative instability as shown in Fig. 3 at location (b) [11, 40, 41, 113–118]. Figure 4 shows how the oxidation stability of vegetable oils is influenced by the number of double bonds by the Rancimat method [119]. Here, the strong dependence of the stability on the amount of double bonds is apparent.
Influence of oxidation stability on the amount of double bonds with the Rancimat method [119]
Additionally, the degree of unsaturation of ester molecules also has a profound effect on the oxidation stability of synthetic lubricants. The effect of oxidation on three different saturated polyol esters is depicted in Fig. 5. The oxidation stability of these fluids was determined by the viscosity increase after an aging process according to the Baader-test (DIN 51587) [119].
Influence of saturation on oxidation stability [119]
As a precaution saturated alcohols and acids are used for the production of lubricants. But the chances of insoluble deposits to be produced are high in the absence of bio-based lubricants that can lead to poor corrosion protection, high viscosity, and oil acidity. Moreover, the existence of unsaturated ester compounds in biolubricants causes hydrolytic degradation, which also increases oxidation [4, 49, 80, 93, 120, 121]. Oxidation stability can also be enhanced by sterically protecting the double bonds by means of branching. The effects of branching on oxidation are similar to the effects of increased hydrolytic stability through branching.
These issues become insignificant in oxygen-free environments due to the critical β-hydrogen and the bis-allylic hydrogen that are immune to oxidative degradation via elimination (e.g., esterification or hydrolysis) and hence can withstand higher temperatures with increased thermal stability of the biolubricants. The tribological properties of natural oils are further influenced by the working environment of biolubricants. This is observable with respect to thermal degradation temperatures, where for an oxygen-free atmosphere the degradation is higher than in an open air oxygenated environment due to the oxidation of oil which lowers the thermal stability [122–124]. For example, a thermogravimetric analysis (TGA) in a nitrogen environment has revealed thermal-oxidative stabilities 20–100 °C higher when compared to a TGA performed in an oxygen environment as performed on fatty acid-based oils by Salih et al. [27], vegetable-based oils by Erhan et al. [28, 95, 102], and synthetic ester base stocks by Salimon et al. [125–129]. To further quantify the susceptibility of the biolubricants to thermal and oxidative vulnerabilities, an unsaturation analysis of biolubricants is often performed. In general, decreasing the unsaturation levels increases the thermal-oxidative stability [130].
3.2 Selective Hydrogenation
As described previously, the multiple double bonds present in polyunsaturated fatty acids is disadvantageous for the utility of biolubricants because of their susceptibility towards oxidation attacks. Monounsaturated fatty acids with only one isolated cis double bond such as oleic acid (C18:1) are considerably more stable towards thermal-oxidation stability by an order of magnitude or more. Despite this, it seems that the ideal situation would be to remove all unsaturation by catalytic hydrogenation. However, this would “harden” the lubricate leaving it with saturated fatty acids such as stearic acid that would turn the (liquid) oil to a (solid) fat rendering it useless as a lubricant or hydraulic fluid. For this reason, it is necessary to leave monounsaturated fatty acids in the oil to ensure optimal lubricity, viscosity, and pour point.
Selective hydrogenation is a process of converting polyunsaturated fatty acids to saturated and monounsaturated fatty acids by removing all or all but one of the double bonds. For example, linolenic acid (C18:3) and linoleic acid (C18:2) would each lose two and one of the cis double bonds, respectively, thus being converted to oleic acid (C18:1) and its positional isomers, each of which carrying only one isolated cis double bond. Hydrogenation can occur through a variety of catalysts [7]. Although these processes are still in their infancy as reactions of linolenic acid (C18:3) and linoleic acid (C18:2) were reduced but not strictly to oleic acid. Some of the reactions isomerized to elaidic acid (trans C18:1) which has properties similar to stearic acid (C18:0). This kind of isomerization is undesirable, and thus rendering the process unsubstantiated. Through continued efforts, this process does have the potential to allow the improvement of readily available natural oils; however, more research is needed.
3.3 Esterification
There are two locations in bio-based lubricants where chemical modifications can be effective (Fig. 3): (a) the first position is at the ester moieties in the triacylglycerides where the β-hydrogen exists and (b) the second position is at the C–C double bonds along the fatty acid chains [27]. Esterification or transesterification is the modification of the ester moieties present in the triacylglycerides, which have glycerol as the alcohol component and have the critical β-hydrogen, which is susceptible to thermal degradation by elimination. In this process, the glycerol is replaced with polyols such TMP, NPG, or PE, effectively creating a synthetic ester. Results indicate that hydrolytic and oxidative stability are increased considerably for biolubricants if the fatty acid portion consists entirely of oleic acid. High-oleic acid vegetable oils have shown high stabilities similar to standard oleic acids (68–72%) with TMP, indicating the feasibility of neat vegetable oils without the need for any modifications. These results are promising and continue to be investigated [28].
3.4 Estolides of Fatty Acids
Estolides are a class of esters derived from natural and synthetic compounds synthesized from fats and oils at the location of the carbon-to-carbon double bond (unsaturation point) where they form a carbocation. The estolide structure consists of a secondary ester linage between one fatty acid acyl molecules to another fatty acid alkyl molecule [126–129]. The carbocation on the estolide can undergo nucleophilic attacks by other fatty acids, with or without carbocation migration along the length of the chain, to form an ester linkage, thus inherently promoting the formation of estolides [50, 131–142]. Estolides can be found in free acids, esters, or triacylglycerides. Estolides were primarily developed to overcome the thermal-oxidative instabilities and poor low-temperature properties of natural oils [143]. In some instances, estolides are enhanced with additives, however at the expense of biodegradability, cost, and toxicity. Recent research in estolides has revealed their synthesis from saturated mono-estolide methyl esters and enriched saturated mono-estolide 2-ethyl hexyl esters from oleic acid, lauric acid, and free estolides [144]. Results indicate that chain length and estolide number affect low-temperature properties and tribological performance.
3.5 Enhancement Through Introduction of Additives
Commercial mineral oils can consist of 10–25% additives depending on the application [145]. Additives are necessary to impart properties that are application specific and beyond what oil base stock can perform. Additives of biolubricants include antioxidants, metal deactivators, detergents, dispersants, corrosion inhibitors, demulsifiers, rust inhibitors, antiwear additives, extreme pressure additives, viscosity improvers, pour point depressants, hydrolysis protection, among others [97, 146–148]. Table 4 shows some of the common additives used with their water pollution classification. Additives are common practice in lubricants, but the toxicity of currently used additives requires research on the development and use of alternative bio-based environmentally benign additives. Thus far, naturally occurring antioxidants such as tocopherol (vitamin E), l-ascorbic acid (vitamin C), esters of gallic acid (lauric alcohol and dodecanol), citric acid derivatives, or lipid-modified ethylenediaminetetraacetic acid (EDTA) derivatives serve as synthetic metal scavengers and provide viable alternatives to the currently used toxic antioxidants [7].
Other biolubricants additives function more as antiwear and extreme pressure additives and consist of environmentally benign solid particulate additives. Green solid lubricants are categorized as “powder lubricants” that have lamellar crystal structures with low interlayer friction [37]. The most prominent examples of these powder lubricants include boric acid (H3BO3) and hexagonal boron nitride (hBN) that have comparable properties to graphite (C), molybdenum disulfide (MoS2), and tungsten disulfide (WS2) [149]. The crystal structure of these lamellar powder lubricants has atoms that are tightly packed with covalent bonding along the same layer and due to weak Van der Waals forces the adjacent layers are far apart rendering observable superior properties to these lubricants [1]. Under sliding contact condition, the presence of these lamellar powder lubricants will minimize asperity contact and wear. This is due to a protective boundary layer generated by the adherence of these powders to the surfaces in contact which acts as a lubricant to accommodate relative surface velocities. During the sliding contact, the lamellar powder layers align themselves parallel to the direction of motion that minimizing friction during sliding motion. These powders are also versatile enough to lubricate in extreme temperature and pressure boundary conditions [31, 33, 39, 41, 150–152].
Although there are many solid powder lubricants that can be used as additives, there are far less that are environmentally benign. Hexagonal boron nitride and boric acid powder represent some of the greener more environmentally friendly additives that are inert to most chemicals [4]. They have been studied extensively because they are highly refractory materials with physical and chemical properties similar to that of graphite [37, 41, 153, 154]. These powder are extremely lubricious with attractive performance-enhancing attributes similar to other lamellar solids, making them attractive alternatives to other inorganic solid lubricants. Boric acid is found naturally; however, boron nitride is not naturally occurring, and it is synthesized from boric oxide or boric acid compounds. Generally, boron-based compounds are extremely stable and do not break down to form other hazardous materials under normal operation; thus, they are safe to handle and feasible to use in industrial applications with no hazardous effects or limitations on their use. There are no reports issued by the National Toxicology Program, International Agency for Research on Cancer, Occupational Safety and Health Administration (OSHA), or American Conference of Government and Industrial Hygienists that indicate boron nitride or other boron compounds are carcinogens or pose any toxic hazard [33]. Boron compounds are not considered hazardous chemicals by the EPA or under the Superfund Amendments and Reauthorization Act (SARA) guidelines, and no regulations exist regarding their use, storage, transport, or disposal. For these many reasons, boron nitride and boric acid can be considered an environmentally benign substance with no limitations on its operational use [155].
Research has indicated that powder-based biolubricants can be problematic because they can be forced out of the contact zone during dry sliding contact [155]. In an attempt to remedy this problem, biolubricant colloidal mixtures composed of powder additives such as boric acid or boron nitride with natural oils such as canola (rapeseed) or soybean oil create a more environmentally friendly lubricant [39]. Here, the natural oil is a bio-based carrier fluid used to circulate the green powder additives allowing them to remain in the contacting pin–disk interface without degrading over time [156, 157]. These powder-based biolubricants demonstrate improved friction and wear reduction and promote ecological sustainability [31, 33, 37, 43].
3.6 Branched Fatty Acids
Biolubricants synthesized from branched fatty acid such as isostearic acid exhibit superior low-temperature behavior such as low pour point, low viscosity, high chemical stability, and high flashpoint. Isostearic acid is procured from the thermal isomerization of polyunsaturated C-18 fatty acids followed by hydrogenation. The branching points are limited to the interior portion of the molecule [59, 158]. In a similar fashion, 12-hydroxystearic acid derived from rhizonoleic acid by hydrogenation can also be used. As previously discussed, removing the double bonds and the glycerol molecules from the triacylglycerides enhances biolubricant performance. Compromises will always be made between tribological performance, oxidative stability, low-temperature performance, and biodegradability. For example, biolubricants synthesized from branched fatty acids maintain low-temperature capabilities that must be present in the oils for many industrial applications; however, branched fatty acids decrease biodegradability of the lubricant.
The foregoing discussion on the enhancements of biolubricants aims at providing an overview of the molecular structure and physical constraints that must be considered when evaluating their performance. In an effort to provide the reader with a broad scope, Fig. 6 summarizes the influence of structure on the chemical and physical properties [13].
3.7 Epoxidation
Epoxidation is the second chemical modification and one of the most important functionalization reactions of the C–C double bonds to improve the oxidative stability, lubricity, and low-temperature behavior of biolubricants [121]. The chemical modification of epoxidized fatty acids is often used as a precursor to ring opening reactions as shown in Fig. 7 [4, 49, 93, 120]. The chemistry of epoxidation of unsaturated fatty acids is a well-known technique for improving the oxidative stability dating back almost 70 years [4]. A number of epoxidation methods have transpired utilizing classical chemical methods via peroxy acids, dioxiranes, peracids, and through the use of alkyl hydroperoxides [126]. Other epoxidation techniques utilize a chemoenzymatic self-epoxidation process as well as an in situ performic acid procedure [143]. In recent attempts to chemically modify the fatty acid chain of natural oils was the development of diester compounds synthesized from oleic acid and common fatty acids [159]. A multi-step process of oleochemical diesters begins with epoxidation, followed by ring opening of epoxidized oleic acid with fatty acids using a p-toluenesulfonic acid (PTSA) as a catalyst to yield mono-ester compounds. The process ends with the esterification reaction of these compounds producing the desired diester compounds [160–165]. These diester compounds have demonstrated enhanced low-temperature behavior due to the increased ability of the long-chain esters to disrupt macrocrystalline formation at low temperatures.
Diagram of various chemical modification techniques for vegetable and animal oils [169]
In addition to epoxidation, a number of other chemical modifications can be employed to manipulate the C–C double bond such as alkylation, radical addition, acylation, enereaction, amino alkylation, hydro aminomethylation, acyloxylation (with the addition of carboxylic acids), co-oligomerization, and hydroformylation [166–168]. Many of these reactions have been investigated using oleic acid esters and other derivatives for their wide applicability and performance enhancements to biolubricants.
4 Evaluating Biodegradability and Ecotoxicity
4.1 Biolubricant Environmental Definitions
Despite the criteria for evaluating biodegradability and ecotoxicity, many lubricants are still deemed biolubricants that are environmentally acceptable even if they are not properly formulated. For example, if a lubricant consists of a bio-based fluid in combination with a toxic additive, the “environmentally friendliness” of the lubricant is thus compromised and should be referred to as not environmentally acceptable, even while the bulk of the fluid is indeed biodegradable, the overall lubricant is not. For this reason, it is useful to classify lubricants according to environmental risk as shown in Table 5 [170].
Moreover, it is equally important to organize the lubricant classifications by environmental risk (Table 6) [7]. According to this categorization, no lubricant can be regarded as truly environmentally friendly, because this would imply an improvement in the environmental conditions. Therefore, one must be content with the fact that a lubricant is environmentally acceptable and that it affects the environment to a less pronounced degree [171]. To this point, several European countries have begun eco-labeling lubricants as environmentally acceptable such as the Blue Angel label in Germany, the Nordic Swan in Nordic countries, the new Euro Margerite eco-label seen in various European countries, and most recently the BioPreferred program in the United States [92, 172–175].
4.2 Evaluation Methods
Since the testing of biolubricants varies substantially from those of conventional petroleum-based lubricants, extensive testing is required. To qualify biolubricants for their intended applications, special laboratory tests are required to ensure that they will withstand the pressures and temperatures encountered while maintaining adequate tribological performance. Many of the conventional chemical and physical test procedures were developed for mineral oils and are not applicable for biolubricants, so many new test procedures have been developed. In particular, new test methods to access the thermal-oxidative stability, biodegradability, and ecotoxicity of biolubricants have been developed. Various test methods exist for evaluating many of the properties of biolubricants. As described previously, biodegradability can be measured by CECL-33-A-93 and OECD 301 tests and ecotoxicity can be measured according to OECD 201 through 213 tests. Other performance tests involve the quantification of a lubricant’s oxidative stability, viscosity, viscosity index, and hydrolytic stability, among many other properties that are pertinent to biolubricants. There are a number of standardized oxidative stability tests for lubricants such as the Baader Oxidation Test (DIN 51553 part 3), Two One-Sided Tests (DIN 51587), Rancimat method, and various Standard Test Methods for Oxidation Stability (ASTM D2112-93, ASTM D2272, and ASTM D943). Kinematic Viscosity and the viscosity index can be studied according to ASTM standards D445 and D2270, respectively. Standard test methods for examining hydrolytic stability are ASTM D2619, known as the ‘beverage bottle test,’ and ASTM D943 where it describes a TOST test measuring hydrolytic stability [7]. More specialized tests exist for particular applications and the needs of a lubricant; however, a detailed summary of all these methods is beyond the scope of this review [176].
4.3 Biodegradability
As the lubrication industry shifts towards the use of “greener” lubricants, two major prerequisites are remaining that biolubricants must meet: (1) a high biodegradability and (2) a low ecotoxicity. One of the primary attributes of bio-based lubricants is their inherent biodegradability. Biodegradation is the chemical dissolution by which organic substances are broken down by the enzymes produced by living organisms. This means that biolubricants based on renewable raw materials derived from CO2 and H2O via photosynthesis, following their use, are ultimately returned to the earth as CO2 and H2O through biodegradation [177]. Organic material can be degraded aerobically, with oxygen or anaerobically, without oxygen. By definition, biodegradation is the chemical transformation of a substance by organisms or their enzymes [178–181]. Biodegradation, once a term reserved for ecology, waste management, and environmental remediation (bioremediation), has now become ubiquitous within the lubrication industry.
Biodegradability of a lubricant can be separated into two types primary and ultimate biodegradation. Primary degradation refers to the disappearance of the original organic compound and may or may not indicate that a substance will biodegrade completely. This method is measured by evaluating the infrared (IR) bands of the C–H bonds through a method listed as CECL-33-A-93 (CEC, Coordinating European Council) [182, 183]. This method of testing biodegradability has yet to become widely accepted due to the obscurity in the compound disappearance [67]. Ultimate degradation also known as total degradation describes the conversion of the original organic compound to carbon dioxide (CO2) and water (H2O) by biodegradation within 28 days. This method is measured by the OECD 301 B (OECD, Organization of Economic Cooperation and Development) test method, which has gained worldwide acceptance [184]. Currently, there are numerous standardized test methods available that have been reviewed for assessing biodegradability, with many biolubricants themselves being tested under a variety of operating conditions [185]. Interestingly, results indicate that biodegradability is not affected by usage and that antioxidant additive has a positive effect.
4.4 Ecotoxicity
Ecotoxicity is an important property in the discussion of biolubricant usage. With upwards of 50% of all lubricants (most being petroleum-based) entering the environment via waste streams, spills, normal usage, and improper disposal, their presence in the environment is toxic with compounding effects that are detrimental to plants, animals, and humans. These effects are strikingly more severe in aquatic ecosystems due to their high sensitivity. For these reasons, it is important to test the aquatic toxicity of lubricants by measuring the extent to which they poison particular environmental species such as green algae, Pseudokirchneriella subcapitata or Desmodesmus subspicatus (OECD 201); freshwater fleas, Daphnia magna (OECD 202-12); rainbow trout minnows, Oncorhynchus mykiss or zebrafish minnows, Brachydanio rerio (OECD 203-13); bacteria, Pseudomonas putida (OECD 209); and laboratory rats, Sprague–Dawley (OECD 401).
Qualification of a lubricant to be classified as biodegradable means the lubricant biodegrades by at least 80% within 28 days (CECL-33-A-93) or by 60% after 28 days (OECD 301B). Toxicity measurements are based on the LD50 value, which is a measurement used to determine the potential impact of the toxic substance on different types of organisms. It provides an objective measure to compare and rank the toxicity of substances based on the median lethal dose (LD) of a substance, or the amount required to kill 50% of a given test population. In ecotoxicity studies, a lubricant is deemed eco-friendly (non-toxic) if its LD50 value is greater than 1000 ppm (LD50 > 1000 ppm).
5 Limitations of Biolubricants
Bio-based lubricants have varying properties such as thermal-oxidative stability, viscosity, viscosity index, and low-temperature behavior that are dependent on the structure of the molecules and the triacylglycerol composition. Despite the many favorable attributes of biolubricants, the largest drawback to them are their poor thermal-oxidative stability, solidification at low temperatures (high pour points), biological (bacterial) deterioration, and hydrolytic instability (aqueous decomposition), and inconsistent chemical composition [60]. Additionally, many biolubricants particularly ester-based oils are susceptible to rapid oxidative degradation due to the presence of free fatty acids and the presence of double bonds in the carbon chains of the ester molecules.
5.1 Lubrication Mechanisms
Many biolubricants are amphiphilic in nature as they are composed of molecules with polar heads that are hydrophilic and non-polar hydrophobic carbon chains [186]. These oils are primarily water insoluble due to the presence of the long hydrocarbon chains in the molecule. Depending on the type of bio-based feedstock, functional groups such as epoxies and hydroxides and various polar and non-polar groups might be present in the hydrocarbon portion of the molecule which can impact the tribological properties as well as other important properties such as oxidation, low-temperature stability, and rheology [187]. The amphiphilic properties of biolubricants affect the boundary lubrication or additive properties, while rheological or fluid properties affect the hydrodynamic properties. For these reasons, the chemical composition of biolubricants is important because the combination of amphiphilic and rheological properties affects the performance of these lubricants in the boundary, hydrodynamic, and mixed-film lubrication regimes [188]. The boundary lubrication condition has negligible fluid film with considerable asperity contact; hydrodynamic or full film lubrication condition has surfaces separated by a relatively thick film of lubricant with negligible asperity contact; and mixed-film lubrication regime deals with the condition of partial fluid film between the surfaces and occasionally the asperity comes in contact.
When lubricants operate in the boundary lubrication regime, their performance is often impacted by the ability of adsorption and tribo-chemical reactions to occur on the metal surface [13, 189–193]. Adsorption refers to the ability of lubricant molecules specifically polar groups of molecules to attach to the friction surface, minimize asperity contact, and reduce the friction and wear as the interface. The ability of a lubricant to adsorb onto a surface is quantified using free energy of adsorption terms.
When lubricants operate in the hydrodynamic or mixed-film lubrication regime, their performance is often impacted by the ability to form tribo-chemical reactions in the tribo-interface. These reactions form tribo-films from the chemical reactions of the lubricants themselves and/or with other materials (e.g., oxygen, moisture, and metal) in the interface. Tribo-chemical reactions often occur as a result of high temperatures, pressures, and shearing of the lubrication process. This process in the interface is highly volatile and so complex that it is not fully understood. As a result, tribo-chemical reactions are often mistakenly blamed for mechanical failures resulting from oil degradation by oxidation and the generation of friction polymers [185].
5.2 Fatty Acid
The structures of the fatty acids affect the properties of the biolubricants in terms of thermal-oxidative stability, viscosity, viscosity index, and low-temperature behavior. By increasing the length of the carbon chain, the fatty acid becomes more oily or fatty and increasingly less water-soluble. The short non-branched fatty acid chains having approximately 6 carbon atoms are more water-soluble due to the presence of the polar –COOH groups [61, 194]. If in the carbon chain every carbon atom is attached to two hydrogen atoms, except those at the ends of the chain, which are attached to three hydrogen atoms, then the fatty acid is considered to be a fully saturated, geometrically configured in a linear shape, and referred to as a saturated fatty acid. When hydrogen atoms are missing from adjacent carbon atoms, the carbons share a double bond instead of a single bond and have a non-linear structure. This type of fatty acid is called unsaturated fatty acids. These acids have lower thermal properties than saturated fatty acids as shown in Table 7 with the boil point values. The fatty acid is polyunsaturated if multiple double bonds occur. Therefore, the classification of fatty acids is saturated, monounsaturated, and polyunsaturated with subcategories of diunsaturated and triunsaturated depending on the number of double bonds present. [62]. Investigations have revealed that the most important unsaturated fatty acids contained in natural oils are oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3). The most important saturated fatty acids are palmitic acid (C16:0) and stearic acid (C18:0) [4]. These fatty acids percentages are shown in Fig. 8 for several common vegetable oils. In this figure, safflower oil is a GMO. As can be seen from Fig. 8, natural oils are composed predominantly of oleic acid and linoleic acid with small trace amounts of other acids [195].
Table 7 details the characteristics of the most important fatty acids found in the natural oils [4]. From Table 7, it can be seen that as the number of double bonds increases as denoted by the lipid number, the degree of unsaturation increases and the boiling point decreases. All of these properties influence the tribological performance of a lubricant when used as a base stock or additive enhancement.
5.3 Thermal-Oxidative Stability
Previous research shows that with a minimal content of polyunsaturated fatty acids (i.e., linoleic acid) in natural oils it is possible to maintain a good thermal-oxidative stability in them [13, 196–200]. The correlation is such that for a low content of polyunsaturated fatty acid a high degree of oxidative stability is observed. Additionally, one double bond of monounsaturated fatty acids like oleic acid can not only improve oxidative stability but also can provide good low-temperature and superior tribological properties. A trade-off between thermal-oxidative stability and low-temperature properties can be achieved through either naturally high-oleic acid oils or by genetically modifying the base stock of low oleic acid oils to yield high-oleic acid oils with concentrations above 80%, such as high-oleic acid safflower oil (HOSO), canola oil, sunflower oil, or soybean oil all of which are commercially available and derived from GMOs [7]. An alternative method would be to perform chemical modifications of the plant-based oil to achieve the desired stability. Fully saturated esters exhibit excellent oxidative stabilities, while partially unsaturated esters require modifications to be useful as engine and transmission fluids or hydraulic and compressor lubricants [201]. Moreover, fully saturated diester oils are highly stable towards oxidation, show good low-temperature performance, and demonstrate a high viscosity-temperature index. The viscosity can be controlled by moderating the chain length of the dicarboxylic acid which can intern control the thermal stability as well as the hydrodynamic capabilities which influence the tribological performance.
5.4 Hydrolytic Stability
The degree of relative resistance to attack or cleavage of a molecule by water or water vapor is known as hydrolytic stability. This property is strongly dependent on the fatty acid ester and synthetic ester structure of bio-based fluids because the chemical reaction for these molecules is an equilibrium reaction. Bio-based fluids cleave into their alcohol and acid components upon hydrolysis, which directly influences the ester bond. This process is also known as “hydrolytic splitting,” which continues until the chemical equilibrium is restored [13]. Thus, hydrolytic stability of an ester is influenced by its chemical structure. Biolubricants derived from saturated esters with straight-chain components have a higher degree of hydrolytic stability than unsaturated or branched esters. The most stable derivatives are saturated dicarboxylic esters, arguably due to steric effects.
Steric hindrance is a natural phenomenon that occurs when a large quantity of methyl groups within a molecule prevent chemical reactions with other molecules with smaller quantities of methyl groups. This consequence of the ester bond improves hydrolytic stability. This protection is due to the presence of methyl groups instead of hydrogen atoms relative to the ester group. The methyl groups sterically protect the ester bond against an unwanted hydrolytic attack. Depending on the number of methyl groups positioned around the ester group, the reaction rate may be reduced many times. Table 8 illustrates the reaction rate as a function of the amount of α-carbon branching [13, 202].
Biolubricants derived from saturated esters with straight chain components have unsaturated or branched esters. The most stable derivatives are saturated dicarboxylic esters, arguably due to steric effects. Biolubricants demonstrate better hydrolytic stability through the use of linear short-chain mono-alcohols. Even the short alkyl chains in these alcohols yield improvements on the hydrolytic stability. When using branched alcohols larger than eight carbon atoms, the resistance increases to the level of saturated linear mono-alcohols [13]. When using glycerol molecules in synthetic esters, the saturated esters behave with a higher degree of hydrolytic stability than stable unsaturated or branched esters. The level of hydrolytic stability is comparable to the stable mono-esters.
The most stable derivatives are saturated dicarboxylic esters, arguably due to steric effects. Their stability is almost entirely independent of the chain length, branching, and the alcohol components used. Figure 9 depicts hydrolytic stabilities of different ester structures, where a low hydrolytic stability corresponds to a high acid number [13, 203, 204]. It can be seen in the figure that many saturated esters, i.e., saturated linear mono-esters, saturated long-chain mono-esters, saturated tri-glycerin-esters, dicarbon-acid-esters, and saturated polyol esters exhibit superior hydrolytic stability.
Hydrolytic stability of different ester structures [13]
Although biolubricants are often susceptible to hydrolytic stability, it is also this inherent property that affords these lubricants their high biodegradability. This is of special importance since hydrolysis is the starting reaction for biological degradation and reducing the effects of hydrolysis inherently reduces the rate of biodegradability. For this enigma, it follows that protecting an ester bond might be a disadvantage with regard to the ecotoxicological properties, and thus a balance must be maintained when improving hydrolytic stability.
5.5 Viscosity and Viscosity Index
The viscosity properties of biolubricants are important properties that influence the applicability of biolubricants as feasible alternatives to petroleum-based lubricants. Generally, bio-based oils derived from plants or animals are known for their high viscosity indices and can be considered multi-range oils. The influence of viscosity is particularly important when esters are used as lubricants because the acids being esterified do influence the viscosity of the lubricant. The viscosity and viscosity index of a biolubricant tend to increase with increasing chain length of the carboxyl acid or with an increase in molecular weight of the alcohol. In the case of polyols, the viscosity depends on the number of hydroxy functional groups present. Examining the effect of different polyols with identical fatty acid base fluids shows the following series of viscosities where PE (40 mm2/s) > TMH (31 mm2/s) > TMP (27 mm2/s) > TME (24 mm2/s) > Glycerol (20 mm2/s) > NPG (12 mm2/s) as illustrated in Fig. 10 [205–208].
Viscosity of different polyol esters with the same base acid [208]
The viscosity index (VI) describes the dependence of viscosity on the temperature. The higher the VI, the smaller the changes in viscosity over a broader temperature range. The VI is also affected by branching where it has similar effects as double bonds. By increasing the branching in either the carboxyl acid or the alcohol while maintaining a constant carbon number, the viscosity and VI decrease. In contrast, an increase in chain length with the same structure, i.e., no branching, results in an increase in viscosity. As more complex esters and acids are used to synthesize biolubricants, there are diminishing returns, where the correlation between the viscosity and the carbon number declines. This is a result of the viscosity increase being compensated by the branching of the ester molecule and the increasing carbon number [13].
5.6 Unsaturation Number
There is a balance that must be maintained in the biolubricants between the fatty acids to ensure functionality, for example, at room temperature stearic acid is solid in the form of a wax, while oleic acid is a liquid. Hence, an optimal ratio of saturation levels between saturated and unsaturated esters must be maintained to have a functional fluid that can ensure the plant-based oil is liquid at room temperature. The unsaturation number (UN) describes the magnitude of saturation levels. For natural oils, this number is described as the average number of double bonds within a triacylglycerol molecule and hence provides a scale to quantify the concentration fatty acid in natural oils. UN is directly proportional to the degree of unsaturation in the natural oil. From the fatty acid distribution, the UN can be calculated using Eq. (1a) and is summarized in Eq. (1b).
In Eq. (1b), the Cx:y is the lipid number which represents the percentage of fatty acids within the plant-based oil with x representing the chain length and y representing the number of double bonds. Similarly, Eq. (1a, 1b) could be used to calculate the UN for unsaturated esters or any other unsaturated hydrocarbon. Table 9 shows the UN for a variety of plant-base oils. Research has shown that natural oils with lower unsaturation numbers maintain a higher thermal-oxidative stability as well superior tribological properties.
5.7 Low Temperature
Low-temperature performance often refers to the pour point of a fluid. The pour point is the lowest temperature at which the fluid still flows before losing its flow characteristics. Hence, it is necessary to achieve the desired low-temperature properties for most lubricants. Pour points for bio-based lubricants exhibit the same dependencies as observed with viscosity. With esters, short-chain branching of the alcohol with tertiary carbon or hydrogen atoms lowers the pour point; however, this molecular structure also leads to a decrease in oxidative stability of the alcohol. For this reason, neopentyl polyols are advantageous for the production of synthetic ester lubricants because their molecular structure is composed primarily of branched hydroxyl groups. Natural oils should have a low amount of saturated fatty acids and a shorter chain length or a branching chain length for optimal low pour points [126, 209, 210] as shown in Fig. 11. On the contrary, unsaturated fatty acids exhibit excellent low-temperature properties, where monounsaturated fatty acids are optimal when compared to polyunsaturated fatty acids that are susceptible to high oxidation attacks due to the increase in double bonds. Although double bonds positively influence the fatty acids by lowering the pour point as shown in Fig. 11, their vulnerability to oxidation negates much of the potential benefit. Short-chained saturated fatty acids are optimal for their cold flow properties, and as the chain length increases to about 16–18 carbon atoms, these fatty acids become solid at temperatures of 65–75 °C [70]. Unfortunately, many natural oils are composed of palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic (C18:3) as shown in Fig. 8, and thus to achieve the desired low pour point temperatures, maintain oil fluidity, and sustain a high oxidative stability, a compromise must be made. Research has shown that oils with high amounts of oleic acid are the best compromise between sufficient cold flow properties and oxidative stability. High-oleic acid oils such as HOSO has demonstrated low-temperature properties with pour points of −35 °C and TMP polyol esters have demonstrated pour points as low as −50 °C [27, 28].
Dependence of pour point on fatty acid structure [211]
Further investigations into the pour point have shown that the position of the double bonds within the fatty acids has no significant influence on the cold flow properties. However, slight differences can be observed depending on the degree of distortion imparted by the double bonds on the molecules. Depending on the position of the double bond, the distance between molecules increases or decreases and this can influence the pour point. The influence of the double-bond location is shown in Fig. 12 for both the cis-configuration and the trans-configuration [211]. The cis-configuration has the hydrogen atoms on the same side of the double bond, whereas the trans-configuration has the hydrogen atoms on opposite sides of the double bond. As shown in Fig. 12, the stereo configuration of the fatty acids influences the cold flow properties, where the cis-configuration has a consistently lower pour point than the trans-configuration.
Influence of double bonds on the pour point [211]
6 Laws, Regulations, and the State of Biolubricants
Most industrialized countries have laws, which are designed to protect water resources, the ground, workplaces, and the air from pollution. In most countries, except Portugal and Austria, there are no compulsory legislative measures regarding the use of biolubricants. Portugal was the first country to institute a mandate requiring outboard two-stroke engines to run off a two-thirds biodegradable lubricant, the minimum requirements of biodegradability according to CECL-33-T-82. In Austria, the use of a plant-derived lubricant for chainsaw oils is a federal regulation. Recommendations for the use of bio-based lubricants and functional fluids exist in the United Kingdom and Canada [212–214]. In the United States, the Department of Agriculture (USDA) has established guidelines for designating and promoting items made from bio-based products (including plant-based lubricants) through two initiatives: (1) Product Labeling, where the government seeks to qualify products to increase consumer recognition, and (2) the Federal Procurement Preference, where the USDA designates categories of bio-based products. Here, the USDA has a BioPreferred program whose primary focus is to “promote the increased purchase and use of biobased products” [215]. This program aims at creating economic development by generating new jobs and building new markets for farm commodities. The USDA believes that an increase in the development, purchase, and use of bio-based products will reduce the nation’s reliance on petroleum. The underlying goal is to increase the use of renewable agricultural resources in hopes of reducing adverse environmental and health impacts from the nearly 20 million tons of petroleum-based lubricant entering the environment annually. Currently, many state and local community regulations are voluntarily trying to convince contractors and subcontractors to use environmentally acceptable lubricants and functional fluids. However, the decision to use biolubricants remains with the contractor as well as the financial burden.
The price of biolubricants is an issue that affects their widespread use. On average, biolubricants cost approximately three times more than traditional petroleum-based lubricants. Vegetable oils and synthetic esters can range anywhere from 1.5 to 5 times more expensive than mineral oils [5]. For this reason, there must be an economic and environmental balance in order to minimize the cost, where the difference between economic cost and true cost are on the same order of magnitude. This can be accomplished by government funded subsidies for original equipment manufacturers (OEMs) to invest in biolubricants without assuming all of the financial liability.
To bring biolubricants to the forefront of the lubrication industry, there needs to be economic incentives where government legislature puts a value on protecting the environment. Increasing legislative pressure would promote the use of biolubricants. In order for this to occur, lawmakers would have to quantifiably decide, with a similar logic as presented in Table 6, if the release of lubricants into the environment is tolerable or hazardous in the sense of pollution. Without a decision on these terms and the lack of appropriate incentives, the consumer, industrial companies, contractors, and other stakeholders involved in the lubrication industry would have little reason to use the most expensive biolubricants over petroleum-based lubricants. Currently, many lubrication engineers first base their decision to use a lubricant on price, then on performance, and lastly by environmental consideration.
Furthermore, legislative pressure and government subsidiaries are necessary to encourage the use of biolubricants because this will incentivize OEMs to design and test their machinery and hydraulic equipment for use with biolubricants. This is important because it ensures a customer that their equipment is covered under warranty if it uses a biolubricant and it helps drive down prices of bio-based lubricants and functional fluids. For example, currently the market share of biolubricants is 2–5% and many OEMs do not see the immediate value of using a biolubricant because it is more expensive; however, a government subsidy could help encourage more OEMs to create products with biolubricants. In turn, this would reduce the price of biolubricants through increased market saturation.
7 Conclusions
Biolubricants can provide economical and feasible alternatives to petroleum-based lubricants while promoting energy conservation and sustainability. Their ability to be non-toxic and renewable as well as potentially satisfy the combination of environmental, health, economic, and performance challenges of conventional lubricants illustrates the vast potential of bio-based lubricants. When developing bio-based lubricants, a compromise must be maintained between synthesizing a lubricant with optimal environmental and tribological properties as well as ensuring a competitive price. Although many biolubricants often suffer from poor thermal-oxidative stability, solidification at low temperatures, biological deterioration, and hydrolytic instability, there remain many techniques to rectify these drawbacks through a multi-disciplined understanding of the relationships between chemical composition, molecular structure, and various chemical modification techniques. In this review, biolubricants were examined from the macro scale with their deficiencies and advantages being highlighted. Biolubricants have progressed tremendously over the last 30 years and, hopefully, in the next 30 years they can become as prevalent and ubiquitous as mineral oil, thus supporting many of the global environmental initiatives.
References
Menezes PL, Ingole SP, Nosonovsky M, Kailas SV, Lovell MR (2013) Tribology for scientists and engineers. Springer, New York
Naegely PC (1993) Environmentally acceptable lubricants, in seed oils for the future. AOCS Press, Champaign
Mang T, Dresel W (2006) Lubricants and lubrication. Wiley, Weinheim
Schneider MP (2006) Plant-oil-based lubricants and hydraulic fluids. J Sci Food Agric 86(12):1769–1780
IENICA (2004) Biolubricants: Market Data Sheet. http://www.ienica.net/marketdatasheets/biolubricantsmds.pdf
Holmberg K, Andersson P, Nylund N-O, Mäkelä K, Erdemir A (2014) Global energy consumption due to friction in trucks and buses. Tribol Int 78:94–114. doi:10.1016/j.triboint.2014.05.004
Salimon J, Salih N, Yousif E (2010) Biolubricants: raw materials, chemical modifications and environmental benefits. Eur J Lipid Sci Technol 112(5):519–530
Aluyor EO, Obahiagbon KO, Ori-jesu M (2009) Biodegradation of vegetable oils: a review. Sci Res Essays 4(6):543–548
Deffeyes KS (2009) Hubbert’s peak. Princeton University Press, Princeton
Goodstein DL (2004) Out of gas: the end of the age of oil. W.W. Norton, New York
Lovell M, Higgs CF, Deshmukh P, Mobley A (2006) Increasing formability in sheet metal stamping operations using environmentally friendly lubricants. J Mater Process Technol 177(1):87
Li W, Kong XH, Ruan M, Ma FM, Jiang YF, Liu MZ, Chen Y, Zuo XH (2010) Green waxes, adhesives and lubricants. Philos Trans R Soc A Math Phys Eng Sci 368(1929):4869–4890
Totten GE, Westbrook SR, Shah RJ (2003) Fuels and lubricants handbook technology, properties, performance, and testing. ASTM International, West Conshohocken
Backé W (1993) The present and future of fluid power. Proc Inst Mech Eng Part I 207(4):193–212
Kumar A, Sharma S (2008) An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Ind Crops Prod 28(1):1–10
Meier MAR, Metzger JO, Schubert US (2007) Plant oil renewable resources as green alternatives in polymer science. Chem Soc Rev 36(11):1788–1802
Feldmann DG, Remmelmann A (1996) Biologisch schnell abbaubare Hydraulikfluessigkeiten—Ergebnisse von Pruefstandstests und Folgerungen fuer die Anwendung. Aachener Fluidtechnisches Kolloquium 12(1):59–80
Feldmann DG, Kessler M (2002) Fluid qualification tests—evaluation of the lubricating properties of biodegradable fluids. Ind Lubr Tribol 54:117–129
Feldmann DG, Hinrichs J (1997) Evaluation of the lubrication properties of biodegradable fluids and their potential to replace mineral oil in heavily loaded hydrostatic transmissions. ASTM Spec Tech Publ 1310:220
Fessenbecker A, Korff J (1995) Additives for environmentally more friendly lubricant. J Jpn Soc Tribol 40(4):306
Korff J, Fessenbecker A (1993) Additives for biodegradable lubricants. NLGI Spokesm 57(3):19
Fox NJ, Tyrer B, Stachowiak GW (2004) Boundary lubrication performance of free fatty acids in sunflower oil. Tribol Lett 16(4):275–281
Grushcow J, Smith MA (2005) Next generation feedstocks from new frontiers in oilseed engineering. In: ASME conference proceedings 42010, pp 487–488
Grushcow J (2005) High oleic plant oils with hydroxy fatty acids for emission reduction. In: 2005 World Tribology Congress III. American Society of Mechanical Engineers, Washington, DC, pp 485–486
Lundgren SM, Ruths M, Danerlov K, Persson K (2008) Effects of unsaturation on film structure and friction of fatty acids in a model base oil. J Colloid Interface Sci 326(2):530–536
Hu Z-S, Hsu SM, Wang PS (1992) Tribochemical reaction of stearic acid on copper surface studied by surface enhanced Raman spectroscopy. Tribol Trans 35(3):417–422
Salih N, Salimon J, Yousif E (2011) The physicochemical and tribological properties of oleic acid based triester biolubricants. Ind Crops Prod 34(1):1089–1096
Erhan SZ, Sharma BK, Perez JM (2006) Oxidation and low temperature stability of vegetable oil-based lubricants. Ind Crops Prod 24(3):292–299
Koshima H, Kamano H, Hisaeda Y, Liu H, Ye S (2010) Analyses of the adsorption structures of friction modifiers by means of quantitative structure-property relationship method and sum frequency generation spectroscopy. Tribol Online 5(3):165–172
Bowden FP, Leben L, Tabor D (1939) The influence of temperature on the stability of a mineral oil. Trans Faraday Soc 35:900–934
Lovell MR, Menezes PL, Kabir MA, Higgs CF III (2010) Influence of boric acid additive size on green lubricant performance. Philos Trans R Soc A Math Phys Eng Sci 368(1929):4851–4868
Bauccio, M., and American Society for, M. (1993) ASM metals reference book. ASM International, Materials Park
Deshmukh P, Lovell M, Sawyer WG, Mobley A (2006) On the friction and wear performance of boric acid lubricant combinations in extended duration operations. Wear 260(11–12):1295–1304
Menezes PL, Lovell MR, Kabir MA, Higgs CF III, Rohatgi PK (2012) Green lubricants: role of additive size. In: Nosonovsky M, Bhushan B (eds) Green tribology. Springer, Berlin, pp 265–286
Havet L, Blouet J, Robbe Valloire F, Brasseur E, Slomka D (2001) Tribological characteristics of some environmentally friendly lubricants. Wear 248(1–2):140–146
Santos ODH, Morais JM, Andrade FF, Aguiar TA, Rocha Filho PA (2011) Development of vegetable oil emulsions with lamellar liquid-crystalline structures. J Dispers Sci Technol 32(3):433–438
Reeves CJ, Menezes PL, Jen T-C, Lovell MR (2012) Evaluating the tribological performance of green liquid lubricants and powder additive based green liquid lubricants. In: STLE annual meeting & exhibition. STLE, St. Louis
Lundgren SM, Persson K, Mueller G, Kronberg B, Clarke J, Chtaib M, Claesson PM (2007) Unsaturated fatty acids in alkane solution: adsorption to steel surfaces. Langmuir 23(21):10598–10602
Reeves CJ, Menezes PL, Lovell MR, Jen T-C (2013) The size effect of boron nitride particles on the tribological performance of biolubricants for energy conservation and sustainability. Tribol Lett 51(3):437–452
Duzcukoglu H, Sahin O (2011) Investigation of wear performance of canola oil containing boric acid under boundary friction condition. Tribol Trans 54(1):57–61
Erdemir A (1990) Tribological properties of boric acid and boric acid forming surfaces: part 1. Crystal chemistry and self-lubricating mechanism of boric acid. In: Society of tribologists lubrication engineers annual conferrence. Argonne National Labs, Denver, CO, USA
Werman MJ, Neeman I (1987) Avocado oil production and chemical characteristics. J Am Oil Chem Soc 64(2):229–232
Nosonovsky M, Bhushan B (2012) Green tribology biomimetics, energy conservation and sustainability. Springer, Berlin
Bennion M, Scheule B (2010) Introductory foods. Prentice Hall, Upper Saddle River
Biermann U, Metzger JO (2008) Synthesis of alkyl-branched fatty acids. Eur J Lipid Sci Technol 110(9):805–811
Randles SJ (1994) Formulation of environmentally acceptable lubricants. In: 49th STLE annual meeting, Pittsburgh
Shubkin RL (1993) Synthetic lubricants and high-performance functional fluids. Dekker, New York
Uosukainen E, Linko YY, Lamsa M, Tervakangas T, Linko P (1998) Transesterification of trimethylolpropane and rapeseed oil methyl ester to environmentally acceptable lubricants. J Am Oil Chem Soc 75(11):1557–1563
Kodali DR (2003) Biobased lubricants—chemical modification of vegetable oils. INFORM 14:121–143
Wagner H, Luther R, Mang T (2002) Lubricant base fluids based on renewable raw materials—their catalytic manufacture and modification. Appl Catal A 221(1):429
Andersen MPS, Hurley MD, Wallington TJ, Blandini F, Jensen NR, Librando V, Hjorth J, Marchionni G, Avataneo M, Visca M (2004) Atmospheric chemistry of CH3O(CF2CF2O)nCH3 (n = 1-3): kinetics and mechanism of oxidation Initiated by Cl atoms and OH radicals, IR spectra, and global warming potentials. J Phys Chem A 108:1964–1972
Scientific Assessment of Ozone Depletion (2003) 2002, National Oceanic and Atmospheric Administration; National Aeronautics and Space Administration; United Nations Environment Programme; World Meteorological Organization; & European Commission, Washington, DC; Nairobi, Kenya; Geneva, Switzerland; Brussels, Belgium
Wallington TJ, Schneider WF, Sehested J, Bilde M, Platz J, Nielsen OJ, Christensen LK, Molina MJ, Molina LT, Wooldridge PW (1997) Atmospheric chemistry of HFE-7100 (C4F9OCH3): reaction with OH radicals, UV spectra and kinetic data for C4F9OCH2· and C4F9OCH2O2· radicals, and the atmospheric fate of C4F9OCH2O· radicals. J Phys Chem A 101(44):8264–8274
Marchionni G, Avataneo M, De Patto U, Maccone P, Pezzin G (2005) Physical properties of four α,ω-dimethoxyfluoropolyethers. J Fluor Chem 126(4):463–471
Marco A, Patto UD, Giuseppe M (2003) Liquid-liquid extraction of polar organic substances from their aqueous solutions with fluorinated extracting liquids. European Patent EP 1,346,757
Giuseppe, M., and Mario, V., 2003. Perfluoropolyethers (PFPEs) having at least an alkylether end group and respective preparation process. European Patent Application EP 1275678
Marchionni G, Petricci S, Guarda PA, Spataro G, Pezzin G (2004) The comparison of thermal stability of some hydrofluoroethers and hydrofluoropolyethers. J Fluor Chem 125(7):1081–1086
Marchionni G, Maccone P, Pezzin G (2002) Thermodynamic and other physical properties of several hydrofluoro-compounds. J Fluor Chem 118(1–2):149–155
Asadauskas S, Erhan S (1999) Depression of pour points of vegetable oils by blending with diluents used for biodegradable lubricants. J Am Oil Chem Soc 76(3):313–316
Reeves CJ, Garvey SL, Menezes PL, Dietz ML, Jen TC, Lovell MR (2012) Tribological performance of environmentally friendly ionic liquid lubricants. ASME/STLE 2012 international joint tribology conference. STLE, Denver, CO, USA
Bermúdez MD, Jiménez AE, Sanes J, Carrión FJ (2009) Ionic liquids as advanced lubricant fluids. Molecules 14(8):2888–2908
Canter N (2005) Evaluating ionic liquids as potential lubricants. Tribol Lubr Technol 61(9):15–17
Liu W, Ye C, Gong Q, Wang H, Wang P (2002) Tribological performance of room-temperature ionic liquids as lubricant. Tribol Lett 13(2):81–85
Zhou F, Liang Y, Liu W (2009) Ionic liquid lubricants: designed chemistry for engineering applications. Chem Soc Rev 38(9):2590–2599
Battez, HA, Alonso DB, Rodriguez RG, Viesca Rodriguez JL, Fernandez-Gonzalez A, Garrido AH (2011) Lubrication of DLC and tin coatings with two ionic Liquids used as neat lubricant and oil additives. In: Proceedings of the STLE/ASME international joint tribology conference—2011. American Society of Mechanical Engineers, Los Angeles, CA, USA
Freemantle M (2010) An introduction to ionic liquids. RSC Publication, Cambridge
Sheldon RA, Arends I, Hanefeld U (2007) Green chemistry and catalysis. Wiley, Weinheim
Yao M, Liang Y, Xia Y, Zhou F (2009) Bisimidazolium ionic liquids as the high-performance antiwear additives in poly(ethylene glycol) for steel-steel contacts. ACS Appl Mater Interfaces 1(2):467–471
Reeves CJ, Menezes PL, Garvey SL, Jen TC, Dietz ML, Lovell MR (2013) The effect of anion-cation moiety manipulation to characterize the tribological performance of environmentally benign room temperature ionic liquid lubricants. In: 2013 STLE annual meeting and exhibition (STLE2013). Society of Tribologists & Lubrication Engineers, Detroit, MI, USA
Reeves CJ, Menezes PL, Lovell MR, Jen TC, Garvey SL, Dietz ML (2013) The tribological performance of bio-based room temperature ionic liquid lubricants: A possible next step in biolubricant technology. In: 5th World tribology congress—Society of Tribologists & Lubrication Engineers, Torino, Italy
Espinosa T, Sanes J, Bermúdez M-D (2016) New alkylether-thiazolium room-temperature ionic liquid lubricants: surface interactions and tribological performance. ACS Appl Mater Interfaces 8(28):18631–18639
Itoga M, Aoki S, Suzuki A, Yoshida Y, Fujinami Y, Masuko M (2016) Toward resolving anxiety about the accelerated corrosive wear of steel lubricated with the fluorine-containing ionic liquids at elevated temperature. Tribol Int 93(Part B):640–650
Jiménez AE, Bermúdez MD, Iglesias P, Carrión FJ, Martínez-Nicolás G (2006) 1-N-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel–aluminium contacts. Wear 260(7–8):766–782
Somers AE, Howlett PC, Sun J, MacFarlane DR, Forsyth M (2010) Transition in wear performance for ionic liquid lubricants under increasing load. Tribol Lett 40(2):279–284
Minami I, Kita M, Kubo T, Nanao H, Mori S (2008) The tribological properties of ionic liquids composed of trifluorotris(pentafluoroethyl) phosphate as a hydrophobic anion. Tribol Lett 30(3):215–223
Espinosa T, Sanes J, Jiménez A-E, Bermúdez M-D (2013) Surface interactions, corrosion processes and lubricating performance of protic and aprotic ionic liquids with OFHC copper. Appl Surf Sci 273:578–597
Saurín N, Minami I, Sanes J, Bermúdez MD (2016) Study of the effect of tribo-materials and surface finish on the lubricant performance of new halogen-free room temperature ionic liquids. Appl Surf Sci 366:464–474
Quijano G, Couvert A, Amrane A, Darracq G, Couriol C, Le Cloirec P, Paquin L, Carrie D (2011) Toxicity and biodegradability of ionic liquids: new perspectives towards whole-cell biotechnological applications. Chem Eng J 174(1):27–32
Gathergood N, Scammells PJ, Garcia MT (2006) Biodegradable ionic liquids, part III. The first readily biodegradable ionic liquids. Green Chem 8(2):156–160
Wu M, Navarrini W, Spataro G, Venturini F, Sansotera M (2012) An environmentally friendly class of fluoropolyether: alpha-omega-dialkoxyfluoropolyethers. Appl Sci 2(2):351–367
Atefi F, Garcia MT, Singer RD, Scammells PJ (2009) Phosphonium ionic liquids: design, synthesis and evaluation of biodegradability. Green Chem 11(10):1595–1604
Omotowa BA, Phillips BS, Zabinski JS, Shreeve JM (2004) Phosphazene-based ionic liquids: synthesis, temperature-dependent viscosity, and effect as additives in water lubrication of silicon nitride ceramics. Inorg Chem 43(17):5466–5471
Freire MG, Carvalho PJ, Gardas RL, Marrucho IM, Santos LM, Coutinho JA (2008) Mutual solubilities of water and the [C(n)mim][Tf(2)N] hydrophobic ionic liquids. J Phys Chem B 112(6):1604–1610
Smith SA, King RE, Min DB (2007) Oxidative and thermal stabilities of genetically modified high oleic sunflower oil. Food Chem 102(4):1208–1213
Marmesat S, Morales A, Velasco J, Carmen Dobarganes M (2012) Influence of fatty acid composition on chemical changes in blends of sunflower oils during thermoxidation and frying. Food Chem 135(4):2333–2339
Fox NJ, Stachowiak GW (2007) Vegetable oil-based lubricants: a review of oxidation. Tribol Int 40(7):1035–1046
Jayadas NH, Nair KP (2006) Coconut oil as base oil for industrial lubricants—evaluation and modification of thermal, oxidative and low temperature properties. Tribol Int 39(9):873–878
Zeman A, Sprengel A, Niedermeier D, Späth M (1995) Biodegradable lubricants studies on thermo-oxidation of metal-working and hydraulic fluids by differential scanning calorimetry (DSC). Thermochim Acta 268:9–15
Mendoza G, Igartua A, Fernandez-Diaz B, Urquiola F, Vivanco S, Arguizoniz R (2011) Vegetable oils as hydraulic fluids for agricultural applications. Grasas Aceites 62(1):29–38
Rudnick LR, Shubkin RL (1999) Synthetic lubricants and high-performance functional fluids. Marcel Dekker, New York
Eisentraeger A, Schmidt M, Murrenhoff H, Dott W, Hahn S (2002) Biodegradability testing of synthetic ester lubricants–effects of additives and usage. Chemosphere 48(1):89–96
Birova A, Pavlovicov A, Cvenros J (2002) Lubricating oils based on chemically modified vegetable oils. J Synth Lubr 18(4):291–299
King JW, Holliday RL, List GR, Snyder JM (2001) Hydrogenation of vegetable oils using mixtures of supercritical carbon dioxide and hydrogen. J Am Oil Chem Soc 78(2):107–113
Erhan SZ, Adhvaryu A, Sharma BK (2006) Chemically functionalized vegetable oils. Chem Ind 111:361–388
Doll KM, Sharma BK, Erhan SZ (2007) Synthesis of branched methyl hydroxy stearates including an ester from bio-based levulinic acid. Ind Eng Chem Res 46(11):3513–3519
Yunus R, Fakhrul-Razi A, Ooi TL, Iyuke SE, Perez JM (2004) Lubrication properties of trimethylolpropane esters based on palm oil and palm kernel oils. Eur J Lipid Sci Technol 106:52–60
Hwang H-S, Adhvaryu A, Erhan SZ (2003) Preparation and properties of lubricant base stocks from epoxidized soybean oil and 2-ethylhexanol. J Am Oil Chem Soc 80(8):811–815
Verkuijlen E, Kapteijn F, Mol JC, Boelhouwer C (1977) Heterogeneous metathesis of unsaturated fatty acid esters. J Chem Soc 1(7):198–199
Schmidt MA, Dietrich CR, Cahoon EB (2006) Biotechnological enhancement of soybean oil for lubricant applications. Chem Ind 111:389–398
Holser R, Doll K, Erhan S (2006) Metathesis of methyl soyate with ruthenium catalysts. Fuel 85(3):393–395
Erhan SZ, Bagby MO, Nelsen TC (1997) Drying properties of metathesized soybean oil. J Am Oil Chem Soc 74(6):703–706
Saad B, Wai WT, Lim BP (2008) Comparative study on oxidative decomposition behavior of vegetable oils and its correlation with iodine value using thermogravimetric analysis. J Oleo Sci 57(4):257–261
Yanishlieva NV, Marinova EM (2001) Stabilisation of edible oils with natural antioxidants. Eur J Lipid Sci Technol 103:752–767
Kapilan N, Reddy RP, Ashok Babu TP (2009) Technical aspects of biodiesel and its oxidation stability. Int J ChemTech Res 1(2):278–282
Domingos AK, Saad EB, Vechiatto WWD, Wilhelm HM, Ramos LP (2007) The influence of BHA, BHT and TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). Braz Chem Soc 18(2):416–423
International Organization for, S. (2006) Animal and vegetable fats and oils: determination of oxidative stability (accelerated oxidation test). International Organization for Standardization, Geneva
Gertz C, Klostermann S, Kochhar SP (2000) Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur J Lipid Sci Technol 102:543–551
Hu X (2005) On the size effect of molybdenum disulfide particles on tribological performance. Ind Lubr Tribol 57(6):255–259
Huang HD, Tu JP, Gan LP, Li CZ (2006) An investigation on tribological properties of graphite nanosheets as oil additive. Wear 261(2):140–144
Sunqing Q, Junxiu D, Guoxu C (1999) A review of ultrafine particles as antiwear additives and friction modifiers in lubricating oils. Lubr Sci 11(3):217–226
Xiaodong Z, Xun F, Huaqiang S, Zhengshui H (2007) Lubricating properties of Cyanex 302-modified MoS2 microspheres in base oil 500SN. Lubr Sci 19(1):71–79
Düzcükoğlu H, Acaroğlu M (2010) Lubrication properties of vegetable oils combined with boric acid and determination of their effects on wear. Energy Sources Part A 32(3):275–285
Erdemir A, Bindal C, Fenske GR (1996) Formation of ultralow friction surface films on boron carbide. Appl Phys Lett 68(12):1637–1639
Erdemir A (1997) Preparation of ultralow-friction surface films on vanadium diboride. Wear 205(1–2):236–239
Erdemir A, Eryilmaz OL, Fenske GR (1999) Self-replenishing solid lubricant films on boron carbide. Surf Eng 15(4):291–295
Erdemir A, Fenske GR, Nichols FA, Erck RA, Busch DE (1990) Self-lubricating boric acid films for tribological applications. In: Japan international tribology conference, Nagoya, Japan. Argonne National Lab, Lemont
Erdemir A, University of, Chicago (1995) Lubrication from mixture of boric acid with oils and greases. US Patent 5,431,830
Streitwieser A, Heathcock CH, Kosower EM (1992) Introduction to organic chemistry. Prentice Hall, Upper Saddle River
Canter N (2001) It isn’t easy being green-the promise, perils, and progress of environmentally friendly lubricants. Lubr World 9(3):16–21
Salimon J, Salih N, Yousif E (2012) Improvement of pour point and oxidative stability of synthetic ester base stocks for biolubricant applications. Arab J Chem 5(2):193–200
Sharma BK, Stipanovic AJ (2003) Development of a new oxidation stability test method for lubricating oils using high-pressure differential scanning calorimetry. Thermochim Acta 402(1–2):1–18
Gapinski, R. E., Joseph, I. E., Layzell, B. D., and Society of Automotive, E. (1994) A vegetable oil based tractor lubricant. Society of Automotive Engineers, Warrendale
Becker R, Knorr A (1996) An evaluation of antioxidants for vegetable oils at elevated temperatures. Lubr Sci 8(2):95–117
Ohkawa S, Konishi A, Hatano H, Ishihama K (1996) Oxidation and corrosion characteristics of vegetable-base biodegradable hydraulic oils. SAE Trans 104(4):737
Salimon J, Salih N (2009) Oleic acid diesters: synthesis, characterization and low temperature properties. Eur J Sci Res 32(2):216–222
Salimon J, Salih N (2009) Preparation and characteristic of 9, 10-epoxyoleic acid α-hydroxy ester derivatives as biolubricant base oil. Eur J Sci Res 31(2):265–272
Salimon J, Salih N (2009) Improved low temperature properties of 2-ethylhexyl 9(10)-hydroxy-10(9)-acyloxystearate derivatives. Eur J Sci Res 31(4):583–591
Salimon J, Salih N (2009) Substituted esters of octadecanoic acid as potential biolubricants. Eur J Sci Res 31(2):273–279
Biermann U, Friedt W, Lang S, Lühs W, Machmüller G, Metzger UO, Klaas MR, Schäfer HJ, Schneider MP (2008) New syntheses with oils and fats as renewable raw materials for the chemical industry. In: Kamm B, Gruber PR, Kamm M (eds) Biorefineries—industrial processes and products. Wiley, Weinheim, pp 253–289
Salimon J, Salih N, Yousif E (2011) Chemically modified biolubricant base stocks from epoxidized oleic acid: improved low temperature properties and oxidative stability. J Saudi Chem Soc 15(3):195–201
Biermann U, Metzger JO (1999) Friedel-Crafts alkylation of alkenes: ethylaluminum sesquichloride induced alkylations with alkyl chloroformates. Angew Chem Int Ed Engl 38:3675–3677
Metzger JO, Riedner U (1989) Free radical additions to unsaturated fatty acids. Fat Sci Technol 91:18–23
Metzger JO, Linker U (1991) New results of free radical additions to unsaturated fatty compounds. Lipid 93(7):244–249
Metzger JO, Biermann U (1993) Alkylaluminium dichloride induced Friedel-Crafts acylation of unsaturated carboxylic acids and alcohols. Liebigs Ann Chem 1993(6):645–650
Biermann U, Metzger JO (1991) Lewis acid catalyzed additions to unsaturated fatty compounds, II: alkylaluminium halide catalyzed ene reactions of unsaturated fatty compounds and formaldehyde. Lipid 93(8):282–284
Pryde EH (1984) Hydroformylation of unsaturated fatty acids. J Am Oil Chem Soc 61(2):419–425
Frankel E, Pryde E (1977) Catalytic hydroformylation and hydrocarboxylation of unsaturated fatty compounds. J Am Oil Chem Soc 54(11):A873–A881
Xia Z, Kloeckner U, Fell B (1996) Hydroformylation of mono and multiple unsaturated: fatty substances with heterogenized cobalt carbonyl and rhodium carbonyl catalysts. Lipid 98:313–321
Behr A, Laufenberg A (1991) Synthesis of new branched fatty acids by rhodium catalyzed homogeneous oligomerization. Fat Sci Technol 93:20–24
Henkel K (1991) Al, Dusseldorf. Germany Patent
Keller U, Fischer J, Hoelderich WF (2000) New lubricants from renewable resources: ecotoxicities and oxidative characteristics. Oelhydraulik und Pneumatik 4:240–245
Crivello JV, Fan MX (1992) Catalysis of ring-opening and vinyl polymerizations by dicobaltoctacarbonyl. J Polym Sci Polym Chem 30(1):31–39
Isbell TA (2011) Chemistry and physical properties of estolides. Grasas Aceites Grasas y Aceites 62(1):8–20
Brimberg UI, Kamal-Eldin A (2003) On the kinetics of the autoxidation of fats: influence of pro-oxidants, antioxidants and synergists. Eur J Lipid Sci Technol 105(2):83–91
Gordon MH, Kourimska L (1995) The effects of antioxidants on changes in oils during heating and deep frying. J Sci Food Agric 68(3):347–353
Schober S, Mittellbach M (2004) The impact of antioxidants on biodiesel oxidation stability. Eur J Lipid Sci Technol 106(6):382–389
Ruger CW, Klinker EJ, Hammond EG (2002) Abilities of some antioxidants to stabilize soybean oil in industrial use conditions. J Am Oil Chem Soc 79(7):733–736
Farrington AM, Slater JM (1997) Monitoring of engine oil degradation by voltammetric methods utilizing disposable solid wire microelectrodes. Analyst 122(6):593
Clauss FJ (1972) Solid lubricants and self-lubricating solids. Academic Press, New York
Kanakia MD, Peterson MB, Southwest Research Institute, San Antonio, TX, Belvoir Fuels, Lubricants Research F (1987) Literature review of solid lubrication mechanisms. Defense Technical Information Center, Ft. Belvoir
Jamison WE (1972) Structure and bonding effects on the lubricating properties of crystalline solids. ASLE Trans 15(4):296–305
Winer W (1967) Molybdenum disulfide as a lubricant: a review of the fundamental knowledge. Wear 10(6):422–452
Wornyoh EYA, Jasti VK, Higgs CF (2007) A review of dry particulate lubrication: powder and granular materials. J Tribol 129(2):438–449
Peng Q, Ji W, De S (2012) Mechanical properties of the hexagonal boron nitride monolayer: ab initio study. Comput Mater Sci 56:11–17
Lelonis DA, Tereshko JW, Andersen CM (2007) Boron nitride powder: a high-performance alternative for solid lubrication. Momentive Performance Materials, Columbus
Clayton GD, Clayton FE, Allan RE, Patty FA (1991) Patty’s industrial hygiene and toxicology. Wiley, New York
Cermak SC, Isbell TA (2003) Improved oxidative stability of estolide esters. Ind Crops Prod 18(3):223–230
Findley TW, Swern D, Scanlan JT (1945) J Am Chem Soc 67(3):412–414
Rangarajan B, Havey A, Grulke EA, Dean Culnan P (1995) Kinetic parameters of a two-phase model for in situ epoxidation of soybean oil. J Am Oil Chem Soc 72(10):1161
Sonnet PE, Foglia TA (1996) Epoxidation of natural triglycerides with ethylmethyldioxirane. J Am Oil Chem Soc 73(4):461–464
Debal A, Rafaralahitsimba G, Ucciani E (1993) Epoxidation of fatty acid methyl esters with organic hydroperoxides and molybdenum oxide. Fat Sci Technol 95(6):236
Ucciani E, Bonfand A, Rafaralahitsimba G, Cecchi G (1992) Epoxidation of monoenic fatty esters with cumilhydroperoxide and hexacarbonyl-molybdenum. Revue Francaise Des Corps Gras 39(9/10):279
Debal A, Rafaralahitsimba G, Bonfand A, Ucciani E (1995) Catalytic epoxidation of methyl linoleate—cyclisation products of the epoxyacid esters. Fat Sci Technol 97(7/8):269
Semel J, Steiner R (1983) Renewable raw materials in the chemical industry. Nachr Chem Tech Lab 31(8):632–635
Klaas MRG, Warwel S (1996) Chemoenzymatic epoxidation of unsaturated fatty acid esters and plant oils. J Am Oil Chem Soc 73(11):1453
Kende AS, Mckusick BC (1987) Performic acid epoxidation. Chem Eng News 65(35):3–3
Klaas MRG, Warwel S (1999) Complete and partial epoxidation of plant oils by lipase-catalyzed perhydrolysis. Ind Crops Prod 9(2):125–132
Chou TC, Lee SV (1997) Epoxidation of oleic acid in the presence of benzaldehyde using cobalt(II) tetraphenylporphyrin as catalyst. Ind Eng Chem Res 36(5):1485–1490
Tocci L (2004) What does biodegradable really mean? Lubes ‘n’ Greases 10:40–43
Organisation for Economic, Cooperation and Development (2002) Guidance document for the development of OECD guidelines for testing of chemicals. Paris: OECD
Pagga U (1997) Testing biodegradability with standardized methods. Chemosphere 35(12):2953–2972
Willing A (1999) Oleochemical esters—environmentally compatible raw materials for oils and lubricants from renewable resources. Lipid 101(6):192–198
Remmele E, Widmann B (1998) Hydraulic fluids based on rapeseed oil in agricultural machinery-sustainability and environmental impact during use. In: Bartz WJ (ed) 11th international colloquium of industrial and automotive lubrication. TA Esslingen, Esslingen
Battersby N (1992) A correlation between the biodegradability of oil products in the CEC L-33-T-82 and modified Sturm tests. Chemosphere 24(12):1989–2000
Benchaita MT, Lockwood FE (1993) Reliable model of lubricant-related friction in internal combustion engines. Lubr Sci 5(4):259–281
Cermak SC, Isbell TA (2009) Synthesis and physical properties of mono-estolides with varying chain lengths. Ind Crops Prod 29(1):205–213
Johansson LE (1979) Copper catalysts in the selective hydrogenation of soybean and rapeseed oils. I. The activity of the copper chromite catalyst. J Am Oil Chem Soc 56:974–980
Behr A, Doring N, Durowitz-Heil S, Lohr C, Schmidtke H (1993) Selective hydrogenation of multi-unsaturated fatty-acids in the liquid-phase. Fat Sci Technol 95:2–11
Behr A (1990) Homogeneous transition-metal catalysis in oleochemistry. Fat Sci Technol 92:375–388
Fell B, Schafer W (1990) Selective hydrogenation of fats and derivatives using Ziegler-type organometallic catalysts. 1. Selective hydrogenation of methyllinoleat and other dienic compounds with isolated double-bonds. Fat Sci Technol 92:264–272
Haase KD, Heynen AJ, Laane NLM (1989) Composition and application of isostearic acid. Fat Sci Technol 91:350–353
Link W, Spitellar G (1990) Products of the dimerization of unsaturated fatty acids. I: the fraction of monomers obtained by dimerization of pure oleic acid. Fat Sci Technol 92:19–25
Perin G, Alvaro G, Westphal E, Jacob RG, Lenardao EJ, Viana LH, D’Oca MGM (2008) Transesterification of castor oil assisted by microwave irradiation. Fuel 87(12):2838–2841
Battersby NS, Morgan P (1997) A note on the use of the CEC L-33-A-93 test to predict the potential biodegradation of mineral oil based lubricants in soil. Chemosphere 35(8):1773–1779
Adhvaryu A, Biresaw G, Sharma BK, Erhan SZ (2006) Friction behavior of some seed oils: biobased lubricant applications. Ind Eng Chem Res 45(10):3735–3740
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM (2000) Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta 364(1–2):87–97
Adhvaryu A, Erhan SZ, Perez JM (2004) Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 257(3–4):359–367
Jahanmir S, Beltzer M (1986) An adsorption model for friction in boundary lubrication. ASLE Trans 29(3):423–430
Jahanmir S, Beltzer M (1986) Effect of additive molecular structure on friction coefficient and adsorption. J Tribol 108(1):109
Jahanmir S (1985) Chain length effects in boundary lubrication. Wear 102(4):331–349
Beltzer M, Jahanmir S (1987) Role of dispersion interactions between hydrocarbon chains in boundary lubrication. ASLE Trans 30(1):47–54
Beltzer M, Jahanmir S (1988) Effect of additive molecular structure on friction. Lubr Sci 1(1):3–26
Schey JA (1983) Tribology in metalworking: friction, lubrication, and wear. American Society for Metals, Metals Park
Liu X, Zhou F, Liang Y, Liu W (2006) Tribological performance of phosphonium based ionic liquids for an aluminum-on-steel system and opinions on lubrication mechanism. Wear 261(10):1174–1179
Reeves Carlton J, Menezes Pradeep L, Jen Tien-Chien, Lovell Michael R (2015) The influence of fatty acids on tribological and thermal properties of natural oils as sustainable biolubricants. Tribol Int 90:123–134
Sharma BK, Liu Z, Adhvaryu A, Erhan SZ (2008) One-pot synthesis of chemically modified vegetable oils. J Agric Food Chem 56(9):3049–3056
Hwang HS, Erhan SZ (2001) Modification of epoxidized soybean oil for lubricant formulations with improved oxidative stability and low pour point. J Am Oil Chem Soc 78:1179–1184
Adamczewska JZ, Wilson D (1997) Development of ecologically responsive lubricants. J Synth Lubr 14(2):129–142
Coscione AR, Artz WE (2005) Vegetable oil stability at elevated temperatures in the presence of ferric stearate and ferrous octanoate. J Agric Food Chem 53(6):2088–2094
Dunn RO (2005) Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process Technol 86(10):1071–1085
Adhvaryu A, Sharma BK, Hwang HS, Erhan SZ, Perez JM (2006) Development of biobased synthetic fluids: application of molecular modeling to structure-physical property relationship. Ind Eng Chem Res 45(3):928–933
Fessenbecker A, Korff J (1995) Additive fuer oekologisch unbedenklichere Schmierstoffe. Tribol Schmierungstech 42(1):26
Roehrs I, Fessenbecker A (1997) A new additive for the hydrolytic and oxidative stabilization of ester based lubricants and greases. NLGI Spokesm 61(3):10–17
Boyde S (2000) Hydrolytic stability of synthetic ester lubricants. J Synth Lubr 16(4):297–312
Asadauskas S, Perez JM, Duda JL (1996) Oxidative stability and antiwear properties of high oleic vegetable oils. Lubr Eng Ill 52(12):877–882
Honary LAT (1996) An investigation of the use of soybean oil in hydraulic systems. Bioresour Technol 56(1):41–47
Lal K, Carrick V (1994) Performance testing of lubricants based on high oleic vegetable oils. J Synth Lubr 11(3):189
Ullmann F, Gerhartz W (1988) Ullmann’s encyclopedia of industrial chemistry. VCH, Winheim
Jayadas NH, Nair KP, Ajithkumar G (2005) Vegetable oils as base oil for industrial lubricants—evaluation oxidative and low temperature properties using TGA, DTA and DSC. In: World tribology congress. American Society of Mechanical Engineers, Washington, DC, pp 539–540
Quinchia LA, Delgado MA, Franco JM, Spikes HA, Gallegos C (2012) Low-temperature flow behaviour of vegetable oil-based lubricants. Ind Crops Prod 37(1):383–388
Hart H, Schuetz RD (1978) Organic chemistry: a short course. Houghton Mifflin, Boston
Miller S, Scharf C, Miller M (2002) Utilizing new crops to grow the biobased market. ASHS Press, Alexandria
Willing A (2001) Lubricants based on renewable resources-an environmentally compatible alternative to mineral oil products. Chemosphere 43(1):89–98
Torbacke TN, Kopp M (2002) Environmentally adapted lubricants in the Nordic marketplace—recent developments. Ind Lubr Tribol 54(3):109–116
USDA (2013) BioPreferred Program. http://www.biopreferred.gov/
Author information
Authors and Affiliations
Corresponding author
Terminologies and Nomenclature
Terminologies and Nomenclature
No. | Terminologies | Description |
|---|---|---|
1 | 1,3-Dialkylimidazolium cations (DAI) | A class of room temperature ionic liquids described as hydrogen-bonded polymeric supramolecules of the type {[(DAI) x (X) x−n ]n+, [(DAI) x−n (X) x )]n−} n , where X is the anion |
2 | 12-Hydroxystearic acid | A chemical compound classified as a lithium soap. In chemistry, “soap” refers to salts of fatty acids which are key components of lubricating greases |
3 | Acylation | The process of adding an acyl group to a compound |
4 | Acyloxylation | The process of substitution of a hydrogen atom by an acyloxy group |
5 | Additivation | Process treating oils and lubricants by adding additives to them |
6 | Adipic acid or maleic acid | A mixture of cyclohexanol and cyclohexanone called “KA oil,” the abbreviation of ketone-alcohol oil |
7 | Alkyl hydroperoxides | Organic compounds containing the peroxide functional group (ROOR′), with R being an alkyl group and R′ being the hydrogen |
8 | Alkylated aromatics | An alkylated hydrocarbon with sigma bonds and delocalized pi electrons between carbon atoms forming a circle |
9 | Alkylation | Alkylation is the transfer of an alkyl group from one molecule to another |
10 | Amino alkylation | Alkylated amines to form C–N bonds |
11 | Amphiphilic | A chemical compound possessing both hydrophilic (water-loving, polar) and lipophilic (fat-loving) properties |
12 | ASTM D2112-93, ASTM D2272, and ASTM D943 | Standard test methods for oxidation stability as described by ASTM |
13 | ASTM D2619 (beverage bottle test) and ASTM D943 (TOST test) | Standard test methods for examining hydrolytic stability as described by ASTM |
14 | ASTM standards D445 and D2270 | Standard Test Methods for Kinematic Viscosity and the viscosity index as described by ASTM |
15 | Azelaic acid | An organic compound with the formula consisting of saturated dicarboxylic acid existing as a white powder and found in wheat, rye, and barley |
16 | Baader Oxidation Test (DIN 51553 part 3) | A versatile test method for oxidation stability as described by VDMA (Verband Deutscher Maschinen- und Anlagenbau, Mechanical Engineering Industry Association) |
17 | Baader-test (DIN 51587) | A test method for oxidation stability as described by VDMA (Verband Deutscher Maschinen- und Anlagenbau, Mechanical Engineering Industry Association) |
18 | Bis-allylic hydrogen | They are two hydrogen atoms that are bonded to an allylic carbon in an organic molecule |
19 | Branched alcohols | Alcohol compounds which occur in isomeric structures, from a straight-chain primary alcohol to a branched-chain tertiary alcohol |
20 | Branched fatty acids | Saturated fatty acids with one or more methyl branches on the carbon chain |
21 | C5–C18 mono acids | Fatty acids that occur as their esters, commonly triglycerides, which are the greasy materials in many natural oils |
22 | C5–C9 carboxylic acids | Organic compounds that contain one or more carboxyl group. That include amino acids (which make up proteins) and acetic acid (which is part of vinegar and occurs in metabolism) |
23 | C6–C13 alcohols | These are aliphatic alcohol components, with a range (6–13) of carbon chain lengths |
24 | CECL-33-A-93 tests | Standard Test Methods of evaluating the infrared (IR) bands of the C–H bonds as described by CEC, Coordinating European Council |
25 | CECL-33-T-82 | Standard Test Methods of evaluating the minimum requirements of biodegradability as described by CEC, Coordinating European Council |
26 | Chemoenzymatic self-epoxidation process | A synthesis, which combines the flexibility of chemical synthesis and the high selectivity of enzymatic synthesis to obtain complex carbohydrates |
27 | Co-oligomerization | Oligomers derived from more than one species of monomer. An oligomer is one which consists of molecules of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular mass |
28 | DA-FPEs | α,ω-Dialkoxyfluoropolyethers |
29 | Diacids | Adipic acid, azelaic, sebacic, and dodecanedioic |
30 | EDTA | Ethyl-enediamine-tetra-acetic acid |
31 | Elaidic acid (trans C18:1) | An organic compound with 18 carbon fatty acid chains and one double bond of the fatty acid chain. It is an unsaturated fatty acid that is the most widely distributed and abundant fatty acid in nature. It is used commercially in the preparation of oleates and lotions and as a pharmaceutical solvent |
32 | Enereaction | Also known as the Alder-ene reaction is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing multiple bonds (the enophile), in order to form a new σ-bond with the migration of the ene double bond and 1,5 hydrogen shift |
33 | Esterification or transesterification | The modification of the ester moieties present in the triacylglycerides, which have glycerol as the alcohol component and have the critical β-hydrogen, which is susceptible to thermal degradation by elimination |
34 | GMOs | Genetically modified organisms |
35 | HOSO | High-oleic acid safflower oil |
36 | Hydroformylation | Also known as oxo synthesis or oxo process is an industrial process for the production of aldehydes from alkenes |
37 | Hydrolytic degradation | Usually, hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both substance and water molecule to split into two parts. In such reactions, one fragment of the target molecule (or parent molecule) gains a hydrogen ion |
38 | Linoleic acid (C18:2) | A carboxylic acid with an 18-carbon chain and two cis double bonds, with the first double bond located at the sixth carbon from the methyl end |
39 | Linolenic acid (C18:3) | An organic compound with an 18-carbon chain and three cis double bonds classified as a keto acid. It is derived from degradation of cellulose and is a potential precursor to biofuels |
40 | Lipid modifiers | Organic compounds which are considered as lipid additives to modify specific properties of lipids |
41 | Lipid number | The numbers in the lipid name are used to describe the fatty acid chains on the lipid. The numbers are generally presented in the format (number of carbons in fatty acid chain):(number of double bonds in fatty acid chain) |
42 | Metathesis | A bimolecular process involving the exchange of bonds between the two reacting chemical species |
43 | Mono/di/tri/poly unsaturated fatty acids | Fatty acids that have one/two/three/poly double bond in the fatty acid chain with all of the remainder carbon atoms being single-bonded |
44 | Natural esters (type HETG) | Vegetable fluid-based hydraulic fluids |
45 | NPG | Neopentylglycol |
46 | Nucleophilic attacks | Nucleophilic substitution/attack is a fundamental class of reactions in which an electron-rich nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms to replace a leaving group; the positive or partially positive atom is referred to as an electrophile |
47 | OECD 201 through 213 tests and 401 | Standard method of testing the aquatic toxicity of lubricants by measuring the extent to which they poison particular environmental species as described by OECD, Organization of Economic Cooperation and Development |
48 | OECD 301 tests | Standard test methods for measuring total degradation that describes the conversion of the original organic compound to carbon dioxide (CO2) and water (H2O) by biodegradation as described by OECD, Organization of Economic Cooperation and Development |
49 | PAGs | Polyalkylene glycols |
50 | PAOs | Polyalphaolefins |
51 | PE | Pentaerythritol |
52 | PFPEs | Perfluoroalkylethers |
53 | PGs | Polyglycols |
54 | PTSA | p-Toluenesulfonic acid |
55 | Polyglycols (type HEPG) | Polyglycol-based synthetic hydraulic fluids |
56 | Rancimat method | Method to determine the oxidation stability of natural fats and oils, in their pure form as well as in fat-containing foods and cosmetics |
57 | Selective hydrogenation | The process of converting polyunsaturated fatty acids to saturated and monounsaturated fatty acids by removing all or all but one of the double bonds |
58 | Synthetic esters (type HEES) | Ester-based synthetic hydraulic fluids |
59 | TGA | Thermogravimetric analysis |
60 | TME | Trimethylolethane |
61 | TMH | Trimethylolhexane |
62 | TMP | Trimethylolpropane |
63 | Transesterification | The process of exchanging the organic group of an ester with the organic group of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst |
64 | Two One-Sided Tests (DIN 51587)/TOST test | Requirements of the thermal-oxidation stability test (TOST) in accordance with DIN 51587 (German Institute of Standardization) |
65 | USDA | The United States Department of Agriculture |
Rights and permissions
About this article
Cite this article
Reeves, C.J., Siddaiah, A. & Menezes, P.L. A Review on the Science and Technology of Natural and Synthetic Biolubricants. J Bio Tribo Corros 3, 11 (2017). https://doi.org/10.1007/s40735-016-0069-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-016-0069-5