Abstract
A review of the current state of research in the field of oil rheology is presented as the basis for quantitative characterization of oil flow in the pipeline system. Based on recent publications, a picture is presented of the dependence of the rheological properties of oils of various types on their composition. The features of the flow of waxy oils, including the problem of correct assessment and depression of the pour point, have been analyzed. The flow of crystallizing oil has been considered proceeding from the concept of oil as a viscoplastic thixotropic medium. It has been shown how the results of rheological studies can be used to solve technological problems, including the restart problem. The rheology of heavy oil has been considered on the basis of the strategic objective of reducing viscosity to a level that meets the requirements of its transport in existing pipeline systems. Various existing and promising ways to solve this problem are discussed. Particular attention is paid to the role of asphaltenes and the formation of emulsions and their importance in oil rheology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The flow of oil is a natural and inevitable factor in the oil production and refining technology, at least because of the geographic distance between oil fields and consuming enterprises. This raises the complex problem of the transportation of oil and oil refining products, requiring a correct description of their properties for its solution, which is the central task of the rheology of oil as any fluid with a complex composition and structure.
To characterize the rheological properties of oil, general rheology methods are quite applicable [1]. However, a very simplified approach is quite frequently used in many studies: oil is characterized either simply by viscosity measured under certain special conditions; or by the dependence of viscosity on shear rate (considering viscosity as a non-Newtonian fluid); or in terms of some simple model of viscoplastic medium, e.g., the Bingham model taking into account the presence of yield stress and nothing more. Recently, much more advanced studies have been carried out, which will be discussed in the subsequent sections.
The simplified approaches to oil rheology were associated with the absolute dominance in technology of light oil, in which low-molecular-weight paraffins played a determining role. Any problem faced was due only to their crystallization at lower temperatures. However, the recent years have seen a gradual but obvious depletion of available sources of light oil, and, as a consequence of this, an accelerating shift towards the production of heavy oils with their inherent high viscosity and the associated problems of their transport. Various ways to improve the transport characteristics of oil of various types were surveyed in [2]. Both that review and numerous subsequent publications clearly indicate the role played by rheological properties, methods for their measurement, and application of the results of rheological studies in technological practice. Hence the task and purpose of this paper arose to discuss of the current state in the field of rheology in the oil industry, mainly based on publications of the last five years.
The structure of the review offered to the reader is as follows. A brief section that gives an idea of the effect of the composition of oil on its viscosity is followed by two main parts, which are respectively devoted to the rheological properties of light and heavy oil and methods for their controlling. This division is rather arbitrary and follows, rather, the historical tendency with respect to oil production, especially since there are various aspects common to oil of any type; for example, this relates to the role of asphaltenes and the formation of emulsions. Nonetheless, there are problems related to a particular type of oil, and the classification of oils into light (paraffinic) and heavy is adhered to both in the world literature and in technological practice.
VISCOSITY–OIL COMPOSITION CORRELATIONS
The data given below on the dependence of oil viscosity upon the content of the main components (which were borrowed from [3] and summarized in [4]) should be regarded rather as a qualitative illustration. The correlations shown do not take into account the superposition of the influence of various structural factors affecting the viscosity, and the viscosity itself was measured in standardized, but somewhat conditional flow regimes.
The international classification of oils is based on the methodology of the American Petroleum Institute (API) [5], which is based on density ρ, and the density of oil is characterized by API units (degrees) calculated as
The density values in this formula are taken at 60 F (15.6°C).
According to this classification, products with API > 22.3 are called light oil, heavy oil is characterized as one with 10 < API < 22.3, and heavy oil and bitumens with a density higher than water density belong to the API < 10 range.
For the purposes of this review, the most important circumstance is that there is a correlation between the density of oil (which is ultimately determined by its composition) and viscosity. Figure 1 illustrates this correlation.
The dependence of viscosity on the density of the considered group of oils is approximated by the power-law equation (Fig. 2)
in which \(n = 4.20 \pm 0.02\).
A similar qualitative correspondence between the density (in API units) and viscosity for a large array of heavy oils of various origins is given in [6], although the data scatter, especially in the region of high density values approaching to those of bitumens, is very significant and there is not always an unambiguous correlation between viscosity and API density [7].
As typical examples, the viscosity at 35°C of heavy oil produced from 24 wells of the North and 40 wells of the South Athabasca oil fields (Canada) ranges within 35 000–802 000 cP (350–802 Pa s) and 9000–55 000 (90–550 Pa s), respectively [8]. The viscosity of heavy Mexican oil is 15–50 Pa s [7]. The viscosity at 20°C of Russian oil from wells of the Ashal’chinskoe field (Tatarstan) is about 8 Pa s and increases sharply with decreasing temperature to thousands Pa s [9]. The viscosity of oil of the Yarega field (Komi) is 2.84 Pa s at 20°C. Naturally, there are even more viscous samples of heavy oil, passing into the bitumen region.
Accordingly, heavy oil in the range of API gravity values of about 23–18 can still be extracted “in the cold”, whereas production of oil with lower API values requires heating or the use of special technology.
The level of viscosity that would enable the pipeline transport of oil in existing networks is even more important. As a general rule, the permissible maximum level of oil viscosity for pipeline transport is set by agreement between the producer and the consumer. However, in many countries of Europe and Americas (primarily Canada and Venezuela), there are certain certified requirements. Most recommendations specify two acceptable levels of maximum viscosity. In the regulations of the 1980s–1990s, it was 200 MPa s, and this level has been increased to 400 MPa s in the 21st century [6, 10].
The observed significant variation in the viscosity of batches of heavy oil of the same density indirectly indicates that the viscosity depends on various features of the oil composition. This is natural, since any oil is an extremely complex multicomponent system with a different nature of interaction between its components and, hence, a different structural arrangement. Nonetheless, as a general rule, the majority of researchers who studied both model systems and natural oil note that the increase in oil viscosity is determined by the concentration of various high-molecular-weight compounds in it, with asphaltenes playing a decisive role [4, 11–13]. Figure 3 illustrates the comparative role of asphaltenes and resins in the viscosity of domestic oils of various origins. As can be seen, even at an asphaltene concentration of about 4%, oil is grouped with highly viscous heavy oils. However, in this case, the aromatics content in the batches compared also increases. Therefore, in real petroleum products, the effects of various components always overlap, so it cannot be stated that only the concentration of asphaltenes determines the viscosity of the oil. Nevertheless, the role of asphaltenes is undeniable.
In this regard, numerous attempts have been repeatedly made to find correlations between the asphaltene content and the viscosity of oil. In these attempts, huge amounts of experimental data for oils of various origins were usually considered (e.g., 753 samples of California oil [14], oil samples of various origins [15], 26 samples of North Sea oil [16]). A typical example of this kind of correlation is the following empirical formula proposed for 700 samples of Egyptian oil produced from various wells [17]:
where µ is the viscosity determined according to ASTM D-2196 with a Brookfeld viscometer, X1 is the density in API degrees, X2 is the molecular weight determined according to ASTM D-2224, and X3 is the inverse temperature (1/T).
The viscosity of oil (as well as its density) also depends on pressure, a property that may turn out to be significant under real technological conditions [18].
However, it should be borne in mind that the very concept of asphaltenes is ambiguous and depends on the method for determining their concentration. So, it was shown that the removal of asphaltenes from heavy oil with various solvents leads to huge differences in the properties of the separated constituent, which, undoubtedly, is due to the fact that different heavy fractions affecting the rheological properties of crude oil are removed together with asphaltenes isolated from the oil using different methods [19].
An increase in the concentration of saturated hydrocarbons (HCs) naturally contributes to a sharp decrease in viscosity (Fig. 4).
It is these two components, asphaltenes and hydrocarbons, out of thousands of constituents making up actual oil (although they themselves include hundreds of individual substances as well) that have a decisive influence on the properties of oil and will be often referred to in the subsequent discussion.
RHEOLOGICAL PROPERTIES OF LIGHT OIL
Determination of Wax Appearance Temperature (WAT)
Light oil, produced and used for over 150 years, is a Newtonian fluid at sufficiently high temperatures. The situation changes radically with decreasing temperature, and this is due to paraffin waxes contained in very many low-viscosity oils. By waxes we usually mean linear hydrocarbons from C18H38 (octadecane) to C35H72 (pentatriacontane) with a melting point from 45 to 65°C; the density of waxes is 0.880–0.915 g/cm3. Naturally, with decreasing temperature, paraffin waxes crystallize, forming a solid phase, and the oil becomes a multiphase system containing solid inclusions, which become a scourge for oil transportation.
To characterize the temperature at which wax crystallization becomes a factor determining the rheological properties of oil, the concept of pour point or wax appearance temperature (WAT), which is evaluated by various methods, is used. The most obvious methods are the optical observation of cloudiness of the liquid [20]; the use of polarized light, which makes it possible to clearly identify the onset of crystal formation; or even more sophisticated optical methods [21]. Crystallization as a phase transition is uniquely determined using differential scanning calorimetry (DSC) by the position of the crystallization peak. Rheological methods, such as measuring flow curves [22–24] and the temperature dependence of the dynamic modulus [25], are also widely used.
In general, it is difficult to talk about “reliable” WAT values, since the measurement results strongly depend on the accepted measurement procedure and the details of the method used [26–28]. Moreover, the first signs of crystallization do not reflect the real and technologically important point of the change in rheological properties. Therefore, in practice, preference is given to high-quality standardized methods for assessing WAT by loss of flowability. Such methods include monitoring the mobility of an oil sample placed in a test tube [29]. The accuracy of this method is low. In a more advanced version of determining the pour (or congelation point), the temperature dependence of the path length of an oil drop rolling along a groove made in a plate inclined at an angle of 30° to the horizon is measured [30]. The transition from a low-viscosity liquid to a gel state due to the formation of the crystal structure is easily detected by the temperature dependence of the elastic modulus [31]. The temperature at which the viscosity level reaches a certain high limit, for example 700 mPa s, which is not typical of low-viscosity oil, can be taken as the condition for achieving WAT [32]. Sometimes more exotic methods for estimating WAT are used, for example, the temperature dependence of the decay time of the NMR signal associated with viscosity [33].
All these methods are quite representative for comparative evaluations. But they, in principle, do not take into account the kinetic factor, which is extremely important for the rate of crystal formation and, therefore, for changes in the rheological properties of waxy oil. This circumstance has been repeatedly emphasized in the analysis of WAT measurement methods [34]. At the same time, the flow of crystallizing oils in the temperature range below WAT is of great technological interest, and a decrease in the viscosity of waxy oil in the region of relatively low temperatures is an important problem solved by various methods [35].
The WAT value of actual oils depends to a very high degree not only on the concentration of paraffins, but also on their composition, and can vary over a very wide range. In addition to wax, the composition of the precipitated solid phase can also include other components, such as naphthenates, aromatic compounds, and compounds with polar groups [36]. Such components can inhibit crystallization or affect the phase transition temperature [37].
A special role is played by the concentration and composition of asphaltenes, which are often present (although in small quantities) in light oils. For example, when studying the properties of six light oil samples (with API = 38) from Colorado oil fields having the asphaltene content as low as 770 to 2270 ppm (less than 1%), it was found that the change in WAT after removal of asphaltenes is from 40 to −20°С. Moreover, the largest changes in WAT took place in the samples with the lowest asphaltene content, and WAT, like the oil viscosity (at 20°C), changed nonmonotonically depending on the total asphaltene content, thereby indicating the role of not only the total concentration of asphaltenes, but also their composition. Thus, the ratio of the amount of aromatic groups in the peripheral part of asphaltenes to the total aromatics content especially strongly affects the increase in WAT [38, 39]. The wax appearance temperature also depends on the polarity of asphaltenes, which is determined by the concentration of aromatic compounds in them, which contribute to the aggregation of solid fractions. A decrease in polarity contributes to a decrease in the degree of aggregation and a decrease in WAT [40]. The difference in the composition of the asphaltenes of six samples obtained from various oils form Russian oilfields (the oil type was not specified) was also demonstrated using a set of spectral methods [41].
The formation of a crystalline structure is not the only problem during the flow of light oil, since under real conditions of production and transport, oil is subjected to intense mechanical stress (mixing, etc.), which leads to its saturation with gas, facilitates the formation of water-in-oil emulsions, and increases viscosity. This undesirable phenomenon has been relatively poorly studied, although there are published data on the rheological properties of such systems [42–47]. The absence of a sufficiently substantiated rheological model of such complex multicomponent systems does not yet make it possible to construct calculated hydrodynamic relations to describe their flow. However, there is the objective complexity of laboratory modeling of the flow of such systems and measuring their rheological properties.
As the temperature drops below WAT, light oil becomes a viscoplastic medium, which leads not only to clogging of the channel with the precipitated solid phase, but also significant rheological problems for the flow (see below). Of course, these problems can be precluded by raising the temperature above WAT. But, as a general rule, this is not an economically viable method. Therefore, a more reasonable solution is to reduce WAT by the methods described below.
Pour Point Depression
High pour point (or WAT) values of oils adversely affect the technical and economic characteristics of the process of oil transportation because of the need to increase temperature or pressure. However, two phenomena should be distinguished here, which, accordingly, determine methods for improving the flow of waxy oil at low temperatures. First, this is the determination of the thermodynamic temperature of crystallization, and second, the role of the structure of crystalline entities that arise in this process, as their structure very strongly affects the rheology of a two-phase medium.
Cocrystallization. Cocrystallization of paraffins with an added component—pour point depressant (PPD)—is an effective means to reduce WAT. This method is based on the use of a phase diagram of a two-component system, according to which the melting temperature of the mixture shifts to the side of lower values with the addition of certain compounds. Here, the compatibility of components plays a decisive role in the choice of depressants involved in the co-crystallization of paraffins [48].
A huge variety of different compounds are used as depressants. Thus, polymeric compounds of various structures turned out to be effective, for example, maleic anhydride copolymers [49–52], acrylate copolymers [53], vinyl acetate copolymers [54, 55], and mixtures of vinyl acetate copolymers with stabilized asphaltene particles which give a noticeable synergistic effect [56]. To obtain joint crystals with paraffins, nanoparticles were also proposed [57]. However, they most likely play the role of heterogeneous crystallization nuclei.
Control of structure formation. Among the methods for controlling the structuring of formed crystallites, two main methods can be distinguished. One is the prevention of crystallite aggregation and of the complete plugging of the channel, and the other is destruction of the formed aggregates.
Preventing the formation of large polycrystalline aggregates, which in fact can simply block the flow, is an effective way to improve the fluidity of oil at low temperatures. If it is possible to prevent the growth of large crystals, then waxy oil in this case will be a dispersion of solid particles of not too high concentration in a low-viscosity medium; i.e., such oil retains quite satisfactory fluidity. Moreover, it was shown on model samples that the introduction of small amounts of microcrystalline wax can even lead to a decrease in the yield stress of the dispersion [58].
One of the ways to solve this problem is to increase the number of crystallization nuclei, which leads to the growth of small crystallites with simultaneous inhibition of their growth. In particular, polymeric additives (for example, vinyl acetate copolymers [59]) were used as crystallization nuclei. The most effective approach to increasing the fluidity of waxy oils is to combine the enhancement of nucleation with the possibility of cocrystallization [49, 60–64]. A similar approach (modification of nanoparticles with vinyl acetate–olefin copolymers) was proposed to reduce the pour point of diesel fuel [65].
The use of various surfactants has become widespread for controlling the structure of polycrystalline entities. The choice of surfactant depends on the characteristics of the oil composition, since not only paraffins, but also other ingredients are involved in the formation of crystalline structures. Accordingly, anionic [48], cationic [66], and nonionic (including polymeric) surfactants [67] can be the most effective, as well as surfactants of biological origin [68, 69]. The use of surfactants to enhance oil recovery is determined not only by the viscosity of the emulsion, but also by its viscoelastic properties, which change the hydrodynamics of the behavior of oil in the reservoir. Thus, the addition of surfactants forming worm-shaped micelles significantly increases the recovery of oil from tight zones [70]. To increase oil recovery, it was also proposed to use nanoparticles forming Pickering emulsions [71]. However, the most commonly used amphiphilic compounds may not be suitable for all types of oils [72].
Depressants for reducing WAT of waxy oils are currently a commercial product manufactured by a number of companies. According to the brochures, the use, e.g., of the VISCOPLEX@ depressant (Evonik Industries) reduces WAT to −35°C.
To conclude this section, we note that methods for actively affecting the already formed polycrystalline structures are also proposed. For example, electromagnetic microwave treatment can be effective. It can lead to a decrease in WAT by mechanical destruction and, thereby, a decrease in the crystallization temperature of components with the highest molecular weight(asphaltenes) involved in the formation of crystals [73]. It was also reported that ultrasonic treatment possibly contributes to a decrease in viscosity [74].
Waxy Oil Rheology Below Crystal Formation Temperature
To consider the rheological properties and behavior during the flow of crystallizing paraffinic oil, the kinetic factor is of fundamental importance. This means that crystallization and the formation of a crystalline structure with a corresponding change in rheological properties occur in time and the rate of this process depends on temperature. This is illustrated in Fig. 5. Moreover, as will be discussed below, the kinetics of crystallization also depends on the shear rate, i.e. flow conditions.
Time course of gel formation during crystallization of waxy oil—isothermal measurements of the elastic modulus and loss modulus [23].
A direct consequence of this is the dependence of the viscosity of oil on its cooling rate [75, 76]. A reflection of the structuring upon crystallization is an increase in the yield stress σY with cooling, with the growth rate of the yield stress also depending on the shear rate at which crystallization occurs, as shown in Fig. 6 [77].
Patterns of change in yield stress by cooling waxy oil at different cooling rates with applied flow [77].
It is interesting to note that viscosity builds up more slowly at a low cooling rate. This effect is due to the temperature dependence of the crystallization rate, which varies in different manners depending on the cooling rate [78].
Of course, the wax content in oil plays a decisive role in the structuring and viscosity growth processes (Fig. 7) [79].
Viscosity of oil with 0.5 or 10% wax content below the equilibrium crystallization temperature Tcr [79].
However, the fact that the strain rate affects the rate of change in rheological properties (Fig. 6) suggests that not only the degree of crystallinity is essential, but also the resulting structure of aggregates, which can be destroyed by internal stresses arising from the shear.
The effect of structuring, depending on the rate of temperature change and on the change in rheological properties during deformation and at rest (static state when the external load is removed), is shown in Fig. 8, which clearly shows structural hysteresis—mismatch of rheological properties with an increase in shear rate and cessation of deformation [79].
Change in the rate of increase in viscosity (shape of the flow curves) depending on the duration of deformation (indicated by the curves) and the stress at rest of the sample [79] (the shear rate on the abscissa axis). The upper curve with an arrow above it shows an increase in shear rate [79].
Figure 9 presents a generalized picture showing a superposition of the influence of temperature, rate of its change, and the applied strain rate on structuring during crystallization of waxy oil below WAT, where the level of structuring was characterized by the yield stress σY, which is important for technological applications.
Below the yield stress, a gel-like structure appears as evidenced by the absence or very weak dependence of the elastic modulus on frequency [81].
Thus, from the point of view of characterization of the rheological properties, oil below WAT is a “living”, thixotropic, and rheokinetic viscoplastic medium, the properties of which are determined by time-dependent viscosity and yield stress. As already mentioned, the structure of paraffinic oil is determined not only by the degree of crystallinity, but also by such a difficult to quantify parameter as “structure.” Therefore, a conditional parameter, such as the degree of structuring β, is used to describe the state of the medium. It is for this parameter that the following kinetic equation is formulated
This equation takes account of the forward and reverse “reactions” of wax structuring and the role of shear rate in these processes (quantities n, m, α, and k are individual empirical parameters specific to a particular object).
A complete system of rheological equations describing the behavior of waxy oil below WAT was formulated in [24, 82–86], in which rather complicated laboratory procedures for determining the constants included in the rheological equations of state were also considered, as well as the dependences of viscosity and yield stress on the degree of structuring of the medium.
Equations of this kind are used to model two important technological processes that determine the operation of pipeline networks. The first of them is the start of the line or restart after stopping oil pumping for some reason. During shutdown, the structure of the medium is restored and the yield stress increases. The calculation of the starting pressure and pressure distribution along the pipeline length is performed on the basis of the rheological equations of state of the material, wherein it is especially vital to evaluate the starting pressure [86, 87]. It is important to borne in mind that the minimum pressure necessary for restarting is always less than the one calculated from the “static” limiting high value of the yield stress and that taking into account the real history of the pumped waxy oil allows the starting pressure to be correctly evaluated without overestimating it [80].
In technological practice, there are various methods of lowering the starting pressure, for example, injection of nitrogen, which apparently facilitates loosening the structure and reducing its strength.
The second area of application of the complete rheological equations of waxy oil is the calculation of the flow in the pipeline during the deposition of solids on the walls. Whether solids will remain as dispersed particles inside the oil during its flow or settle on the walls depends on their structure, which determines the prevalence of the adhesive or cohesive strength of the aggregates formed. Figure 10 shows that this ratio depends on the duration of wax structuring with decreasing temperature [88].
Relationship between the cohesive and adhesive strengths of the structure of solid components released from waxy oil [88].
The formation of deposits in pipelines is a kinetic process. Models of this phenomenon were considered in [89, 90]. Models of this kind are necessary in practice for calculating pipelines on the basis of data on the rheological properties of oil, since they allow calculating velocity profiles and the dependence of productivity upon a preset pressure for various flow regimes of two-component oil systems below WAT in pipelines.
The formation of solid particles during crystallization also entails a decrease in the occupied volume, and this shrinkage can lead to the formation of voids, which was the case indeed observed in a model experiment [91].
Thus, studying the rheological properties of waxy oils makes it possible to find quantitative answers to a number of technological problems associated with the design and operation of pipelines. Whether the proposed methods are used in actual practice depends on the equipment of the laboratory with sufficient rheological measurement equipment, since further application of the obtained data does not face serious difficulties in view of the modern development of computer technology.
On the Rheology of Gas Hydrates
Due to the need to extend the range of oil production sources, transport systems are developing in low-temperature regions, in particular, for oil transport from offshore and underwater sources. Hence the task of evaluating the rheological properties of oil containing gas hydrates, dispersed systems in which frozen water droplets incorporating the gas phase are formed. They, like polycrystals of waxy oil, are prone to the formation of macroscopic aggregates, which impede the flow up to the formation of plugs. In this regard, there is much in common in the rheological approaches to studying waxy oil and gas hydrates.
Also, as in the case of paraffin crystallization, there can be two different approaches to combating the negative consequences of the formation of gas hydrates, the thermodynamic and structural-kinetic ones. The first of them is based on the addition of a certain liquid that displaces the freezing point to the low-temperature region. It should be noted that a fairly rapid crystallization of water droplets occurs in the temperature range below −38°C [92, 93].
The structural-kinetic method is based on stabilization of droplets with the formation of emulsions, preventing their growth [94]. The stabilization of gas hydrate droplets is due to the creation of a protective layer on their surface, similar to interfacial layers in emulsions. Recently, it has been found that droplet growth is stopped by using nanoparticle-modified stabilizers as surfactants [95, 96]. Droplet growth stabilization with a mixture of conventional surfactants with nanoparticles leads to the formation of stable emulsions. However, in this case, their viscosity increases up to two times at high shear rates, and a structuring effect with a sharp increase in viscosity resembling the transition to the yield stress was revealed at low stresses (on the order of 0.1–1.0 Pa) and a sufficiently high concentration of nanoparticles [97].
Drag Reduction Using Polymer Additives
According to the general laws of hydrodynamics, the increase in the productivity of the pipeline system, which is so desirable for oil pumping technology, by increasing the pipe diameter and/or increasing the pressure, leads to turbulent flow when passing through the critical Reynolds number, and, thereby, to undesirable additional energy losses. One of the most effective ways to mitigate this phenomenon as much as possible is to use the Toms effect. This paradoxical effect, in a certain sense, is that very small additives of some high-molecular-weight compounds (instead of increasing the effective viscosity, as is the case of laminar flows) lead to a decrease in hydrodynamic resistance. The effect can be very significant, it is possible to reduce the drag by 70%. The fundamentals of this phenomenon and generalization of the early results of its studies are presented in classical reviews [98–100].
Obviously, the Toms effect is associated with the very nature of turbulence. In this regard, there are two approaches to explaining it and, hence, to a reasonable choice of technological solutions. The first of them is based on the understanding of turbulence as the formation of vortex motion. One of the pioneers of the theory of turbulence formulated this model in the following poetic form:
Big whirls make little whirls
Which feed on their velocity,
Little whirls have smaller ones
And so on to viscosity.
L.F.G. Richardson (1922)
If we take this approach, then the role of polymer additives is that having viscoelastic properties, they do not allow the decay of small vortices and thereby reduce energy loss.
The second approach to understanding the role of small additives is associated with ideas about the dominant role of the near-wall layer, which has a decisive influence on the drag. It is assumed that polymer additives laminate the flow in the near-wall layer (also suppressing eddies) and thereby reduce the flow resistance. A model of the structure of a turbulent flow, assuming the existence of a viscous wall layer and a transition region, was developed in [101, 102], which ultimately made it possible to obtain a generalized expression for the coefficient of friction in pipes of arbitrary diameter as the basis for the design of large-diameter pipeline systems [103].
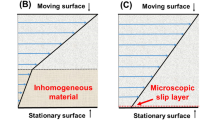
However, when considering the Toms effect, caution should be taken in scaling laboratory data to modeling flow conditions in real pipelines. For example, it was noted that the decrease in resistance under comparable conditions depends on the pipe diameter, which was interpreted in terms of slip (i.e., the role of wall resistance in the flow of a water–oil mixture was emphasized) [104].
One way or another, but the role of drag-reducing additives (DRA) and their promising technological significance is undeniable.
There are various estimates of the performance of the DRA used. Thus, the value of drag coefficient λ for the flow of a pure fluid can be compared with that for the same fluid with DRA at the same Reynolds number Re. A simple comparison method is to determine the drag reduction (DR) relative to the mass of fluid forced through a pipe in the absence, m0, and in the presence, mDR, of a drag-reducing agent. Then
The effect of polymeric additives on the drag during oil flow is exemplified in Fig. 11.
Dependences of drug reduction upon concentration of drug-reducing agents, available from different manufacturers, under identical hydrodynamic conditions in straight-run gasoline. Re = 6000 [105].
Another fundamental difficulty that remains is the effect of mechanically induced degradation of macromolecules during long-term deformation in real long pipelines (Fig. 12).
Change in drug reduction along the length of the pipeline of a 1000 mm diameter at different DRA concentrations (indicated on the curves) [105].
Finally, the way of introducing polymer additives into pipelines and dissolving them in oil is not only of great technical, but also utmost economic importance, since rather large quantities of raw materials are dealt with [105, 107].
As a rule, the Toms effect during oil flow is realized using polymers, predominantly poly-α-olefins with high molecular mass. However, this does not exclude the use of other additives, in particular, organic products of plant origin (e.g., see [108]).
Viscosity Reduction of Heavy Oil as a Rheology Problem
The high viscosity of heavy oil is the main problem, extremely complicating its production and transportation in existing pipeline systems. The heavy oil itself is close in its rheological properties to ordinary Newtonian fluids (see the example in Fig. 13), but the level of viscosity at low and room temperatures is unacceptably high.
Therefore, the search for technologically appropriate methods for regulating the rheological properties of heavy oil has been and remains the most important task of the oil industry, especially in connection with the ever-increasing demands on the volume of its production. The sometimes used method of reducing viscosity by heating [109], of course, leads to a decrease in viscosity, but, on the whole, it is hardly promising economically.
Considered below are the main solutions to this problem, offered to the industry at present.
Emulsification. Heavy crude oil in itself is an emulsion, since it consists of immiscible liquids (HCs and water) stabilized by other components present in the mixture [110], in particular, naphthenic acids and resins.
However, the technological challenge is to create an emulsion of the necessary structure, the viscosity of which would be significantly lower than the viscosity of crude oil. Nevertheless, it should immediately be noted that concentrated emulsions are always viscoplastic media in their rheology [111], so here we have to think not only about viscosity reduction, but also the existence and role of the yield stress, which was considered in detail in the section devoted to waxy oil. The difference, however, is that a solid-like structure with a sharply defined yield stress emerges during the wax crystallization, and yield stress in oil emulsions in water is often “smeared” over a rather wide range of stresses (Fig. 14), so, we should rather talk about a quasi-plastic non-Newtonian fluid. In this case, a decrease in viscosity in the region of high flow velocities by a factor of tens can be achieved.
Examples illustrating the change in the rheological properties of heavy oil (with a viscosity of 1700 mPa s) during the formation of emulsions of various compositions [112].
Emulsification of heavy oil is also used to reduce its viscosity and increase oil recovery, with the simultaneous use of surfactants and nanoparticles presumably playing a positive role [113, 114], since the presence of nanoparticles facilitates emulsion stabilization [115].
In attempts to reduce the viscosity of heavy and extraheavy oil, as well as bitumens of various origins, a huge number of different formulations of emulsifying systems were proposed. A fairly wide range of different surfactants and their combinations was considered in [112].
The technical problem is that any system of this type is specific for oil of a particular composition (origin), so there are no common recipes here: only a general physicochemical approach can be proposed. All substances that can be used to reduce the viscosity of heavy oils can be described by a common term viscosity modifiers (reducers), although they all with high probability play the role of emulsifiers. One of these substances, a multicomponent formulation composed of a number of specially synthesized polymers and Triton-X100 as a coemulsifier was described in [116]. It was claimed that there had been a significant decrease in viscosity of heavy oil of various origins (China, Canada, and Venezuela) tested using this formulation. It was proposed to use the same surfactant in combination with nanoparticles to reduce the viscosity of heavy oil from Oman [117].
One of the recently proposed amphiphilic polymers designed to reduce the viscosity of heavy oil and, apparently, also playing the role of a surface-active component, are oil-soluble copolymers based on 2-(acryloamide)octanesulfonic acid [118]. It is assumed that this compound actively destroys large aggregates formed by heavy fractions of oil, thereby leading to a decrease in viscosity.
It is possible that the experimental results described in [119], showing that the viscosity of heavy oil can be reduced several times by introducing small amounts of nanoparticles (10, 100, or 1000 mg/L) into the oil, also refer to the emulsification method, since such particles can create Pickering emulsions (when solid particles play the role of surfactants). A decrease in viscosity by introducing small amounts of nanoparticles was previously observed for polymer melts and was explained by a change in the melt flow mechanism due to the transition to laminar flow [120]. Probably, the nanoparticles introduced into oil destroy the aggregates formed by asphaltenes, thereby changing the colloidal structure of the mixture. This explanation does not contradict the idea that the stability of the destroyed asphaltene aggregates is due to the formation of interfacial layers, i.e., the formation of an emulsion stabilized by nanoparticles. At the same time, the use of nanoparticles as stabilizers of oil emulsions has negative consequences for the rheology of these systems, leading to the formation of a stronger structure with higher yield stresses, although apparent viscosity does decrease at high shear rates [121].
A general approach to the creation of multicomponent systems based on heavy oil with the aim of reducing its apparent viscosity is also realized in the case of gas (CO2) saturation of oil in the well, with the gas dissolving in both of the oil-constituting phases, the water and the oil [122]. The viscosity of such a composition is reduced tenfold in comparison with the initial water–oil emulsion. In physical meaning, a close effect is achieved in the case of supercritical water injection to the well (at a pressure of 25 MPa and a temperature of about 400°C) [123].
Experimental studies of a large number of oil batches (126 samples of both light and predominantly heavy oil) showed that the rheological properties of water-in-oil emulsions can vary over an extremely wide range, forming the “starry sky” in the plots, depending on oil composition and water content, so there are no universal recipes for this case [124].
Nonetheless, there are a fairly large number of empirical models for describing the non-Newtonian flow of specific formulations of oil emulsions. The best results are usually achieved with the use of power-law rheological equations in one or another modification. For example, quite satisfactory results were obtained using the following modified power model taking into account the pressure dependence of apparent viscosity [125]:
where \(\upsilon \) is the kinetic viscosity, K and n are the constants of the power model, P is the pressure, L is the pipe length, D is the pipe diameter, f(D) is the quadratic function of the diameter, and k is the coefficient accounting for the dependence of viscosity on pressure.
Deasphalting. Considering the presence of asphaltenes as the main factor determining the high viscosity values of heavy oil, one should take into account the possibility of reducing the viscosity by removing asphaltenes. This method of reducing viscosity was consistently considered in [19], where it was shown that the degree of viscosity reduction depends on the solvent chosen to remove asphaltenes. This relation is due to the fact that when using various reagents, groups of compounds of various structures, not the “asphaltenes” in general, are removed. According to the results of the cited study, the proportion of asphaltenes removed and, accordingly, the viscosity of the remaining oil are determined by the solubility parameter of the solvent used.
The results reported in [19] are represented in Fig. 15, which shows that the removal of asphaltenes can reduce the viscosity of oil by tens of times. However, the proportion of components removed from the crude oil appears to be very significant.
Dependence of the viscosity of heavy oil on the solvent used to remove asphaltenes [19].
This line of research also includes the use of mixtures of other solvents (triethanolamine and xylene) [126] or polymers and copolymers (esters of acrylic acid and benzyl acrylate) synthesized for the purpose, which made it possible to achieve an effect comparable to dilution of heavy oil with kerosene [127].
Structural studies have shown that a decrease solvent quality during the removal of asphaltenes leads to an increase in the concentration of components with a double bond and the number of heteroatoms per molecule with a constant number of carbon atoms [128]. In practice, it is preferable to use both blends of low-molecular-weight solvents and polymeric compounds. For example, the use of sulfonated polystyrene with various concentrations of sulfo groups proved to be effective [129].
In the practical application of deasphalting, it should be borne in mind that the separation of asphaltenes from crude oil using common solvents occurs rather slowly [130]. However, in the presence of inorganic compounds (minerals and other solid components) in heavy oil or bitumen, the separation of asphaltenes occurs in a heterogeneous mode, which leads to a sharp acceleration of the process [131]. The solvent injection rate is also important in the in-situ deasphalting of heavy oil, which determines the viscosity and, hence, the recovery of the oil fluid [132].
The negative role of asphaltenes can also be eliminated by the catalytic treatment of heavy oil in the presence of metal nanoparticles at elevated temperatures. This method leads to a significant decrease in viscosity [133, 134].
Dilution with low-viscosity solvents. Mixing heavy oil with low-viscosity liquids seems to be a fairly natural way to reduce viscosity to the norm specified by the technological requirements for pipeline transport. However, the choice of diluent and the establishment of the necessary proportions depend on the properties of heavy oil.
The theoretical basis for selecting the composition is the well-known rules for calculating the viscosity of a mixture. Thus, as long ago as 1933, Lederer proposed the following equation for calculating the viscosity of a mixture of two liquids, ηmix:
where η0 is the viscosity of the base liquid; ηd is the viscosity of the diluent; ω0 and ωd are the volume fractions of the main component and diluent, respectively; and α is the coefficient reflecting the nature of interaction of the liquids to be mixed. There are other options for calculating the viscosity of a mixture [135, 136], which were used for mixtures based on heavy oil and bitumen.
Various options for the selection of diluents are considered in [137]. In particular, it was proposed to use a mixture of naphtha with various solvents as an effective agent that reduces the viscosity of heavy oil. In this case, polar liquids are very effective, possibly due to the fact that they act not only as diluents, but also as active components that interact with asphaltenes and affect the structure they form.
In practice, the easiest option is to mix heavy and light oil, and the proportion of diluent depends on the viscosity of the heavy oil. For example, in the case of a not very high viscosity of heavy oil, the addition of 20% light oil was sufficient to reduce the viscosity of the mixture below the required limit of 0.4 Pa s [138]. This method of reducing the viscosity of heavy oil is quite acceptable if the sources of both types of oil are close to each other, or if there is a pipeline for supplying light oil to the well where heavy oil is produced. In a more general case, another diluent has to be looked for, and it is actually necessary that the diluent be a liquid compatible well with oil and fairly cheap.
Figure 16 shows the plot of viscosity at room temperature versus diluent concentration for the three available diluents—light oil (L), light gas oil (G), and spindle oil (S). As can be seen, it is possible to obtain a mixture with the required sufficiently low viscosity only in the case of using the solvent with the lowest viscosity. Therefore, to solve actual technological problems, it is desirable to combine two main factors affecting the viscosity, the diluent content and temperature T. Then such a combination of the required concentration φ* and temperature T can be chosen that would ensure a decrease in viscosity to a desired level. Figure 17 shows such a ratio for the three diluents listed above.
Relationship between temperature and the necessary diluent concentration φ* that provides the required viscosity level of (a) 0.2 or (b) 0.4 Pa s. Designations are the same as in Fig. 16 (data of M.P. Arinina).
The presented results illustrate the limitations of the blending method: in order to achieve the desired level of viscosity of the mixture at low temperatures, it is necessary to use a diluent with an exceptionally low viscosity and in rather large quantities, even in case of heavy oil with a relatively low viscosity.
In addition, the use of a large proportion of waxy oil as a diluent leads to the possibility of wax crystallization and the appearance of a yield point [112] with all the consequent technical problems discussed in the section on the rheology of waxy oils.
Oil–water core annular flow. The main hydraulic resistance during flow is associated with stresses on the pipe wall and energy losses in the near-wall layer. Therefore, it seems quite attractive to organize the flow of heavy oil in such a way that it is separated from the pipeline wall by an annular water layer (Fig. 18). Then heavy oil will move like a quasi-solid plug, and all energy losses will be caused by the flow of low-viscosity water in the annular gap [139]. The proportion of water in such a flow should be 10–30% [140].
The energy loss (i.e., the “pseudoviscosity” or pressure necessary to ensure a given productivity) for such a hydrodynamic scheme is calculated by the simple formula:
where R is the pipe radius; Rcore is the radius of the central core formed by the oil; and ηw and ηoil are the viscosities of water and oil, respectively.
Since ηw\( \ll \) ηoil, this formula clearly shows what is the gain in reducing pressure by forming the water annulus. Obviously, energy consumption will be much less than in the case of flow of high-viscosity oil through the pipe. The mathematical model of such a multiphase flow was considered in [141, 142]. The results obtained quite satisfactorily agree with the experimental data.
Certain doubts in the analysis of the proposed flow rheology concept arise in connection with possible stability disturbances [143], as a result of which the central core should “depart” to the channel wall. However, the experiments performed on a model unit showed that the initial form of the flow with an annular water layer is preserved even with sharp changes in the cross section of the pipe or with pipe turns [144].
In situ viscosity reduction. Currently, one of the most promising processes for the recovery of heavy oil is its in situ thermal treatment [145] leading to a decrease in viscosity to a level that meets the requirements of the existing technology. The process called Toe-to-Heel Air Injection (THAI) or its latest version THAI-CAPRI meets the current environmental requirements. It includes the catalytic cracking of oil fractions downhole [146–150], which leads to a decrease in the viscosity of the extracted product. Consideration of the process itself goes far beyond the scope of this publication, and it is mentioned here only as an indication of a possible and promising way to reduce the viscosity of heavy oil and bitumen.
CONCLUSIONS
The study of the rheological properties of oil is aimed at obtaining objective characteristics of the material either in the form of absolute indicators in terms of the selected model of its behavior (which is important for basic research and engineering calculations) or in the form of conventional standardized parameters (which is important for production control and applied assessment of specific types of products). Note that the results of measurements of rheological properties are correlated with the composition and structure of oil. In this regard, rheology is part of general materials science and does not differ in its global tasks, for example, from materials science of solids with measurement of their mechanical characteristics.
The value of the rheological measurements of oil is determined by the fact that they are associated with high-volume production, and the oil flow studied by rheological methods covers volumes of millions tons per year. In this case, the natural task is to optimize production, reduce technological costs, and improve product quality. These problems are solved by combining rheological measurements with physicochemical and structural studies.
The review of the current state of oil rheology research summarized the main lines of research aimed primarily at reducing viscosity and expanding the temperature range in which oil can be transported. A thorough analysis of the behavior of waxy oil during its flow and building adequate mathematical models make it possible to determine its transport characteristics and suggest ways to solve the engineering problems of calculating transport lines. The inevitable gradual transition from the conventional light oil to the production and transportation of heavy oil poses new challenges, and the search for ways to modify the rheological properties of oil is especially important in this connection.
The interest in these problems does not fade away, as evidenced by the flow of scientific and technical literature containing the results of the latest research. This review is an attempt to photograph, summarize, and analyze the current state of the art in the field of application of rheological methods in oil technology.
REFERENCES
A. Ya. Malkin, Fundamentals of Rheology (TsOP Professiya, St. Petersburg, 2018).
R. Martínez-Palou, M. de Lourdes Mosqueira, B. Zapata-Rendón et al., J. Pet. Sci. Eng. 75, 274 (2011).
A. Z. Driatskaya, M. A. Zhmykhova, and M. A. Mkhchian, A Handbook of USSR Oils, Suppl. vol.: Physicochemical Characterization of Oils, (Khimiya, Moscow, 1975).
A. Malkin, G. Rodionova, S. Sébastien, et al., Energy Fuels 30, 9322 (2016).
C. F. Conaway, The Petroleum Industry: A Nomenclature Guide (Pennwell, Tulsa, 1999).
A. D. Muñoz, J. Ancheyta, and L. C. Castañeda, Energy Fuels 30, 8850 (2016).
P. V. Ramírez-González, Energy Fuels 30, 7094 (2016).
E. A. Rops and L. R. Lines, in Proceedings of GeoConvention-2016: Optimizing Resources.
S. O. Ilyin, M. P. Arinina, M. Yu. Polyakova, et al., Fuel 186, 157 (2016).
A. Hart, J. Pet. Explor. Prod. Technol. 4, 327 (2014).
A. M. McKenna, Energy Fuels 27, 1246 (2013).
O. C. Mullins and E. Y. Sheu, Asphaltenes: Fundamentals and Applications (Plenum, New York, 1995).
O. C. Mullins, Annu. Rev. Anal. Chem. 4, 393 (2011).
C. Beal, Trans. AIME 165, 94 (1946).
H. D. Beggs and J. R. Robinson, J. Pet. Technol. 27, 1140(1975).
O. Glaso, J. Pet. Technol. 32, 785 (1980).
E. M. Mansour, S. M. Desouky, M. El Aily, and M. E. Helmi, Fuel 212, 405 (2018).
J. Modaresghazani, R. G. Moorea, S. A. Mehta, et al., Fuel 227, 6 (2019).
S. O. Ilyin, M. P. Arinina, M. Yu. Polyakova, et al., J. Pet. Sci. Eng. 147, 211 (2016).
T. Monger-McClure, J. Tackett, and L. Merrill, SPE Prod. Facil. 14, 4 (1999).
A. Belati and J. Cajaiba, Fuel 220, 264 (2018).
B. A. Tarcha, B. Forte, E. J. Soares, and R. L. Thompson, Rheol. Acta 54, 479 (2015).
B. Jia and J. Zhang, Ind. Eng. Chem. Res. 51, 10977 (2012).
M. Geri, R. Venkatesan, K. Sambath, and G. H. McKinley, J. Rheol. 61, 427 (2017).
J. A. L. da Silva and J. A. Coutinho, Rheol Acta 43, 433 (2004).
F. L. Paiva, F. H Marchesini., M. A. Calado, and A. Galliez, Energy Fuels 31, 6862 (2017).
C. Barbato, B. Nogueira, M. Khalil, et al., Energy Fuels 28, 1717 (2014).
A. Japper-Jaafar, T. Bhaskoro, L. L. Sean, et al., J. Non-Newton. Fluid Mech. 218, 71 (2015).
A. Ya. Malkin and S. N. Khadjiev, Pet. Chem. 56, 541 (2016).
GOST (State Standard) 20287-91: Petroleum Products: Methods of Test for Flow Point and Pour Point (Standartinform, Moscow, 2006); ASTM D5853-95; ISO 3016.
V. F. Nikolaev, A. V. Egorov, M. A. Vasin, and I. V. Nikolaev, Zavod. Lab. 78, 312 (2012).
D. W. Jennings and K. Weispfennig, Energy Fuels 19, 1376 (2005).
J. Bryan, A. Kantzas, and C. Bellehumeur, in Proceedings of SPE Annual Technical Conference and Exhibition, San Antonio, Texas, Sep. 29–Oct. 2, 2002, Paper 89070.
J. Ruwoldt, M. Kurniawan, and H.-J. Oschmann, J. Pet. Sci. Eng. 165, 114 (2018).
P. Sivakumar, A. Sircar, B. Deka, et al., J. Pet. Sci. Eng. 164, 24 (2008).
Y. M. Ganeeva, T. N. Yusupova, and G. V. Romanov, Pet. Sci. 13, 737 (2016).
G. Chena, J. Lina, W. Hua, et al., Fuel 218, 213 (2018).
D. Molina, E. Ariza, and J. C. Poveda, Energy Fuels 31, 133 (2017).
E. Arizo, A. Chaves-Guerrero, and D. Molina, Energy Fuels 32, 6557 (2018).
Y. Li, S. H. Han, Y. Lu, and J. Zhang, Energy Fuels 32, 1491 (2018).
A. Kh. Kuptsov and T. V. Arbuzova, Pet. Chem. 51, 203 (2011).
J. S. Lim, S. F. Wong, M. C. Law, et al., J. Appl. Sci. 15, 167 (2015).
S. F. Wong, M. C. Law, Y. Samyudia, and S. S. Dol, Chem. Eng. Trans. 45, 1411 (2015).
A. Omer and R. Pal, Ind. Eng. Chem. Res. 52, 9099 (2013).
T. S. T. Ariffin, E. Yahya, and H. Husin, Procedia Eng. 148, 1149 (2016).
S. F. Wong, S. S. Dol, S. K. Wee, and H. B. Chua, J. Pet. Sci. Eng. 165, 58 (2018).
A. A. Umar, I. B. M. Saaid, and A. A. Sulaimon, J. Pet. Sci. Eng. 165, 673 (2018).
T. T. Khidr, Part. Sci. Technol. 25, 671 (2007).
Y. Wu, G. Ni, F. Yang, et al., Energy Fuels 26, 995 (2012).
M. R. Patel, S. Chitte, and D. Bharambe, Egypt. J. Phys. 26, 895 (2017).
A. M. Al-Sabagh, M. R. N. El-Din, R. E. Morsi, and M. Z. Elsabee, J. Pet. Sci. Eng. 65, 139 (2009).
R. A. El-Ghazawy, A. M. Atta, and Kh. Kabel, J. Pet. Sci. Eng. 122, 411 (2014).
R. K. Farag, Int. J. Polym. Mater. 57, 189 (2008).
J. B. Taraneh, G. Rahmatollah, and A. Hassan, Fuel Process. Technol. 89, 973 (2008).
A. L. C. Machado, E. F. Lucas, and G. Gonzalez, J. Pet. Sci. Eng. 32, 159 (2001).
B. Yao, Ch. Chuanxian Li, F. Yang, et al., Energy Fuels 32, 5834 (2018).
C. He, Y. Ding, J. Chen, et al., Fuel 167, 40 (2016).
M. Kurniawan, S. Subramanian, J. Norman, and K. Paso, Energy Fuels 32, 5857 (2018).
E. Marie, Y. Chevalier, F. Eydoux, et al., J. Colloid Interface Sci. 290, 406 (2005).
F. Yang, Y. Zhao, J. Sjoblom, et al., J. Dispersion Sci. Technol. 36, 213 (2015).
A. M. Al-Sabagh, T. T. Khidr, H. Y. Moustafa, et al., J. Dispersion Sci. Technol. 38, 1055 (2016).
F. Yang, K. Paso, J. Norrman, et al., Energy Fuels 29, 1368 (2015).
M. Majid, D. Mitra, and D. Bahram, J. Mol. Liq. 238, 326 (2017).
K. Cao, X. Wei, B. Li, et al., Energy Fuels 27, 640 (2013).
Z. Zhao, Y. Xue, G. Xu, et al., Fuel 193, 65 (2017).
S. A. Mahmoud, T. T. Khidr, and F. M. Ghuiba, Part. Sci. Technol. 24, 1115 (2007).
T. T. Khidr, M. M. Doheim, O. A. A. and EI-Shamy, Part. Sci. Technol. 33, 1619 (2015).
H. S. EI-Sheshtawy and T. T. Khidr, Part. Sci. Technol. 34, 147 (2016).
R. M. Abd, A. H. Nour, and A. Z. Sulaiman, Int. J. Chem. Eng. Appl. 5, 204 (2014).
J. van Santvoort and M. Golombok, Energy Fuels 30, 9226 (2016).
N. Kumar and A. Mandal, Energy Fuels 32, 6452 (2018).
C. Gang, Y. Bai, J. Zhang, et al., Pet. Sci. Technol. 34, 1285 (2016).
J. Taheri-Shakib, A. Shekarifard, and H. Naderi, in Proceedings of the 8th International Conference and Exhibition, 9–12 April 2018, St. Petersburg, Russia. https://doi.org/10.3997/2214-4609.201800144
A. Agi, R. Radzuan Junina, and A. S. Chong, J. Pet. Sci. Eng. 166, 577 (2018).
R. M. Webber, J. Rheol. 43, 911 (1999).
P. Singh, H. S. Fogler, and N. Nagarajan, J. Rheol. 43, 1427 (1999).
Ph. Coussot, Q. D. Nguyen, H. T. Huynh, and B. Bonn, Phys. Rev. Lett. 88, 175571 (2002).
A. Ya. Malkin, Thermochim. Acta 624, 82 (2016).
R. Mendes, G. Vinay, G. Ovarlez, and Ph. Coussot, J. Rheol. 59, 703 (2015).
R. Mendes, G. Vinay, and Ph. Coussot, Energy Fuels 31, 395 (2017).
H. Liu, Y. Lu, and J. Zhang, J. Rheol. 62, 527 (2018).
R. de Souza-Mandes and R. L. Thomspon, Rheol. Acta 52, 673 (2013).
Ch. J. Dimitriou and G. H. McKinley, Soft Matter. 10, 6619 (2014).
Ch. van der Geest, C. B. Guersoni, D. Merino-Garcia, and A. C. Bannwart, Rheol Acta 54, 545 (2015).
R. Mendes, G. Vinay, G. Ovarlez, and Ph. Coussot, J. Rheol. 59, 703 (2015).
A. Ahmadpour, K. Sadeghy, and S.-R. Maddah-Sadatieh, J. Non-Newton. Fluid Mech. 205, 18 (2014).
L. Kumar, K. Paso, and J. Sjöblom, J. Non-Newton. Fluid Mech. 223, 9 (2015).
G. T. Chala, Sh. A. Sulaiman, and A. Japper-Jaafar, J. Non-Newton. Fluid Mech. 251, 69 (2018).
Sh. Zheng, M. Saidoun, Th. Palermo, et al., Energy Fuels 31, 5011 (2017).
H. Seyyedbagheri and B. Mirzayi, Energy Fuels 31, 8061 (2017).
D. A. Phillips, I. N. Forsdyke, I. R. McCracken, et al., J. Pet. Sci. Eng. 77, 237 (2011).
D. Kolotova, K. Bricka, G. Simonsen, et al., Energy Fuels 31, 7673 (2017).
S. R. Derkach, D. S. Kolotova, G. Simonsen, et al., Energy Fuels 32, 2197 (2018).
J. W. Lachance, E. D. Sloan, and C. A. Koh, Chem. Eng. Sci. 63, 3942 (2008).
M. Cha, S. Baek, J. F. Morris, and J. W. Lee, Chem. Asian J. 9, 261 (2014).
A. K. Y. Raman, D. Venkataraman, S. Bhagwat, et al., Colloid Surf., A 506, 607 (2016).
A. Ahuja, A. Iqbal, M. Iqbal, et al. Energy Fuels 32, 5877 (2018).
J. L. Lumley, Annu. Rev. Fluid Mech. 11, 367 (1969).
S. Virk, AIChE J. 21, 625 (1975).
C. M. White and M. G. Mungal, Annu Rev. Fluid Mech. 40, 235 (2008).
G. Brethouwer, P. Schlatter, Y. Duguet, et al., Phys. Rev. Lett. 112, 144502 (2014).
S. Salehi, M. Raisee, and M. J. Cervantes, J. Appl. Fluid Mech. 10, 1029 (2017).
X. F. Loyseau, G. Verdin, and L. D. Brown, J. Pet. Sci. Eng. 162, 1 (2018).
A. Abubakar, Y. Al-Wahaibi, T. Al-Wahaibi, et al., J. Pet. Sci. Eng. 162, 143 (2018).
G. V. Nesyn, V. P. Shibaev, R. Z. Sunagatullin, and A. Ya. Malkin, Nauka Tekhnol. Truboprov. Transporta Nefti Nefteprod. 8, 309 (2008).
Yu. V. Lisin, S. L. Semin, and F. S. Zverev, Nauka Tekhnol. Truboprov. Transporta Nefti Nefteprod. 3, 6 (2013).
G. V. Nesyn, R. Z. Sunagatullin, V. P. Shibaev, and A. Ya. Malkin, J. Pet. Sci. Eng. 161, 715. 2018.
E. C. Coelho, K. C. O. Barbosa, J. Edson, et al., Rheol. Acta 55, 983 (2016).
A. Shah, R. Fishwick, J. Wood, et al., Energy Environ. Sci. 3, 700 (2010).
N. M. Zadymova, Z. N. Skvortsova, V. Yu. Traskin, et al., Colloid J. 78, 735 (2016).
R. Foudazi, S. Qavi, I. Masalova, and A. Ya. Malkin, Adv. Colloid Interface Sci. 220, 78. 2015.
A. Ya. Malkin, K. V. Zuev, M. P. Arinina, and V. G. Kulichikhin, Energy Fuels 32, 11991 (2018).
N. Nizamidin, U. P. Weerasooriya, and G. A. Pope, Energy Fuels 29, 7065 (2015).
H. Pei, Zh. Zhan Shu, G. Zhang, et al., J. Pet. Sci. Eng. 163, 476 (2018).
T. Sharma, G. S. Kumar, and J. S. Sangwai, Ind. Eng. Chem. Res. 54, 1576 (2015).
Y. Yang, J. Guo, Z. W. Cheng, et al., Energy Fuels 31, 1159 (2017).
T. Al-Wahaibi, Y. Al-Wahaibi, A.-A. R. Al-Hashmi, et al., Pet. Sci. 12, 170. 2015.
J. Mao, J. Liu, Y. Peng, et al., J. Energy Fuels 32, 119 (2018).
E. A. Taborda, A. Camilo, C. A. Franco, et al., Energy Fuels 31, 1329 (2017).
V. G. Kulichikhin, L. A. Tsamalashvili, E. P. Plotnikova, et al., Polym. Sci., Ser. A. 45, 564 (2003).
A. Ya. Malkin, M. V. Mironova, S. O. Ilyin, J. Pet. Sci. Eng. 157, 117 (2017).
R. Hu, J. M. Trusler, and J. P. Crawshaw, Energy Fuels 31, 3399 (2017).
Q. Zhao, L. Guo, Z. Huang, et al., Energy Fuels 32, 1685. 2018.
M. C. K. de Oliveira, L. R. O. Miranda, A. B. M. de Carvalho, and D. F. S. Mirands, Energy Fuels 32, 2749 (2018).
J. Guo, Y. Yang, D. Zhang, et al., J. Pet. Sci. Eng. 160, 12 (2018).
O. Akinyemi, J. D. Udonne, V. E. Efeovbokhan, and A. A. Ayoola, J. Appl. Res. Technol. 14, 195 (2016).
L. Zhu, Y. Wang, Sh. Wang, et al., J. Pet. Sci. Eng. 163, 37 (2019).
M. Witt, M. Godejohann, S. Oltmanns, et al., Energy Fuels 32, 2653 (2018).
C. P. P. Mazzeo, F. A. Stedille, C. R. E. Mansur, et al., Energy Fuels 32, 1087 (2018).
Yu. V. Larichev and O. N. Martyanov, J. Pet. Sci. Eng. 165, 575 (2017).
W. Chaisoontornyotina, J. Zhang, S. Ngb, and M. P. Hoepfner, Energy Fuels 32, 7458. 2018.
Ch.-Y. Sie, B. Nguyen, M. Verlaan, et al., Energy Fuels 32, 360 (2018).
H. Patel, S. Shah, R. Ahmed, and S. Ucan, J. Pet. Sci. Eng. 167, 819 (2018).
H. Li, C. Kexin, J. Linga, et al., J. Pet. Sci. Eng. 170, 374 (2018).
G. Centeno, G. Sanchez-Reyna, J. A. Munoz, and N. Cardona, Fuel 90, 3561 (2011).
W. R. Shu, SPE J. 24, 277 (1984).
P. Gateau, I. Henaut, L. Barre, and J. F. Argillier, Oil Gas Sci, Technol. Rev. IFP 59, 503 (2001).
M. T. Ghannam, S. W. Hasan, B. Abu-Jdayil, and N. Esmail, J. Pet. Sci. Eng. 81, 122 (2012).
P. Poesio, D. Strazza, and G. Sotgia, Chem. Eng. Sci. 64, 1136 (2009).
A. Shmueli, T. E. Unander, and O. J. Nydal, in Proceedings of Offshore Technology Conference, October 27−29, 2014, Rio de Janeiro (OTC Brazil, Rio de Janeiro, 2015), Document ID OTC-26176-MS.
J. J. Wylde, D. Leinweber, D. Low, et al., in Proceedings of the World Heavy Oil Congress, Aberdeen, 2012.
A. Brito, N. Guzmán, L. Luis Rojas-Solórzano, and T. Zambrano, J. Pet. Sci. Eng. 170, 772. 2018.
D. D. Joseph, R. Bai, C. Mata, et al., J. Fluid Mech. 386, 127 (1999).
B. Meña, in Proceedings of the 12th Annual European Rheology Conference, April 17–20, 2018, Sorrento, Italy, Paper PG-2.
A. Hart, A. Shah, G. Leeke, et al., Ind. Eng. Chem. Res. 52, 15394 (2013).
M. Greaves, T. X. Xia, and A. T. Turta, J. Can. Pet. Technol. 47, 65 (2008).
A. Shah, R. Fishwick, J. Wood, et al., Energy Environ Sci. 3, 700 (2010).
Petrobank 2014: Petrobank and Touchstone to Combine to Create a High Growth, Fully Capitalized Oil Company (Petrobank, Calgary, Alberta). http:www.touchstoneexploration.com/files/5128.PBG-2014-arch6.pdf. Accessed May 9, 2016.
M. R. Ado, M. Greaves, and S. Rigby, Energy Fuels 31, 1276 (2017).
M. R. Ado, M. Greaves, and S. Rigby, J. Pet. Sci. Technol. 166, 94 (2019).
ACKNOWLEDGMENT
This work was carried out within the State Program of the Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Malkin, A.Y. Oil as an Object of Rheology (Review). Pet. Chem. 59, 1092–1107 (2019). https://doi.org/10.1134/S0965544119100062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544119100062