Abstract
Cerebral small vessel disease (cSVD) is the leading cause of vascular cognitive impairments and dementia, cerebral hemorrhages and lacunar strokes, as well as the most common form of asymptomatic vascular brain lesion. Major forms of cSVD are age- and arterial hypertension (AH)-associated arteriolosclerosis and cerebral amyloid angiopathy. The etiologies and the underlying mechanisms of disease development and progression remain unclear for a substantial group of cSVD types. Significant difficulties in the study of this pathology are explained by technical limitations in assessing smallest vessels in vivo. A modified correlation between MRI equivalents and their morphological manifestations in cSVD to use them subsequently as a surrogate marker of lesions in small vessels has allowed clinicians to establish disease progression regularities and the association of the latter with clinical symptoms. This review presents the results of studies showing the clinical significance and role of the leading MRI features in the assessment of disease progression, including white matter hyperintensity (WMH, formerly known as leukoaraiosis), lacunes, enlarged perivascular spaces, and microbleeds. The recognition of MRI features as diagnostic criteria for cSVD was specified by international experts in the Standards for Reporting Vascular Changes on Neuroimaging (the STRIVE criteria). Despite the enormous importance of this standardization for the improvement of concepts about the significance of different factors in the development and understanding of heterogeneity of cSVD forms, this categorization cannot provide for the prediction of the disease course in a particular patient and assess the treatment efficacy in short- and medium-term prospects. One of the approaches to solution was based on the use of diffusion methodologies for assessing a microstructural lesion in the visually unaltered brain matter. The obtained consistent association of the expressiveness of microstructural alterations with clinical impairments substantiates the expediency of multimodal MRI studies aimed to evaluate the pathophysiological mechanisms of disease progression, beginning from the subclinical brain lesion stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Scientific publications in the English language apply the terminology “cerebral small vessel disease” (cSVD) exclusively to describe clinical (including cognitive impairment and dementia), neuroimaging, and morphological manifestations caused by the lesion of perforating cerebral arterioles, capillaries, and venules, which lead to impairments in the brain white matter and nuclei [1]. In Russia, this pathology is considered within a broader context of dyscirculatory encephalopathy. cSVD is currently among the priority problems for the health care systems of developed countries, judging by its participation in invalidation and mortality [1–3]. CSVD has been recognized as the leading cause responsible for vascular cognitive impairments and dementia [4, 5], intracerebral hemorrhages [6], a fifth of ischemic strokes [7], and the most common asymptomatic vascular brain lesions [5, 8], as well as a risk factor for Alzheimer’s disease [9]. The most frequently occurring cSVD forms are age- and arterial hypertension (AH)-induced arteriolosclerosis and cerebral amyloid angiopathy [1, 5, 8]. However, researchers admit that both the etiology and the underlying development mechanisms in a significant number of cSVDs remain not well understood so far [3, 5], and that it is impossible to predict the reversibility of brain impairment even in cases with fully controlled hypertensive microangiopathy [10]. The main complexity in cSVD studies is explained by technical limitations of small vessel imaging in vivo. Essential breakthroughs in the understanding of the pathology became possible owing to the modified correlation between the neuroimaging equivalents and morphological manifestations of cSVD and their use as surrogate markers of small vasculature impairment and disease progression. The accumulation of evidence confirming associations between neuroimaging features and clinical manifestations and invalidation of patients served as a foundation for systematizing the ideas and applying the MRI criteria clinically significant in cSVD as diagnostic, using STandards for ReportIng Vascular Changes on nEuroimaging (STRIVE) [11]. The criteria include recently occurring small subcortical infarcts, lacunes, white matter hyperintensities (WMHs) (formerly, leukoaraiosis), enlarged perivascular spaces, and microbleeds, etc. [11]. This review presents data on the MRI features on which numerous confirmations of their association with disease progression have been obtained, including their neuroimaging characteristics and the clinical significance of the feature expansion, as well as the confirmed risk factors for their development.
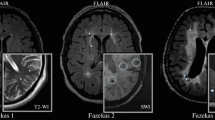
White matter hyperintensities (WMHs, or formerly leukoaraiosis) are MP signal zones of increased intensity on T2- and proton density-weighted fluid-attenuated inversion recovery (FLAIR) images [11, 12]. The WMH focal shapes are rather variable, including periventricular caps or strips, multiple pinpoint-sized or larger foci, partially or fully confluent, commonly, bilateral and symmetrical [13].
The expansion and expressiveness of WMHs are assessed in the periventricular, deep, and subcortical white matter. To assess the expressiveness of white matter lesions, clinicians use different visual scales and volumetric methods. Among the most popular is the Fazekas scale with three lesion stages, namely, Fazekas I, II, and III, respectively [14]. Other visual assessment tools include the Rotterdam Scan Study (RSS) [15], the Scheltens [16], Wahlund [17], and Longstreth [18] scales, and the Prins scale for rating WMH changes in dynamics [19].
Comparisons between WMHs on postmortem MRIs and the histological changes in the brain confirmed the association between their expressiveness and the expansion of changes [20]. The myelin paleness is associated with periventricular WMHs, loosened fibers, venular tortuosity, and, more frequently, with the absence of arteriolosclerosis and the loss of the ependyma integrity with gliosis of different expressivity degrees. Deep WMH was characterized in individual foci by the absence of ischemic changes, loss of myelin, atrophy of neuropil surrounding arterioles with hyalinosis and perivenous alterations; in early confluent foci, by perivascular myelin thinning, mild or moderate fiber losses, and different severities of gliosis; and in confluent foci, by uneven sites of incomplete parenchymal necrosis with transition to true infarctions [20].
Clinical significance. WMHs have long been considered as a neuroimaging phenomenon associated with normal aging of the brain. Both population-based cohort and clinical studies have later shown that WMH intensity expansion over time depends not only on aging. The established WMH expansion rate varies from 0.1 to 2.2 mL/year, differing by more than 20 times between groups [21–24].
Numerous prospective MRI-based studies have proven the significance of WMHs in the development of clinical manifestations associated with cerebral small vessel lesions. It has been shown that the presence of WMHs is accompanied in elderly people by an increased risk of dementia and stroke [25], progression of cognitive impairments in patients with both neurodegenerative and vascular diseases [26, 27], invalidation [28] and depression [29]. We should note that the absolute majority of studies included the senior age group, whereas data on the middle-aged group (45–60 years) were more limited. For example, the Northern Manhattan (NOMAS) [30] and American Religious Identification Survey (ARIS) [31] population-based cohort studies have shown an increased risk of stroke in a group of middle-aged persons with asymptomatic lacunar foci and WMHs, and these results coincided with the data obtained in the senior patient category. At the same time, the ABC study based on a cohort of 320 people aged below 60 years has shown no significant associations, in contrast to the older group, between WMHs and impairments in cognitive performance [32].
The literature contains solid reports confirming the influence of genetic factors on the WMH progression. There were descriptions of monogenic forms of the disease, and a high level of leukoaraiosis hereditability was shown in twin-based studies [33], whereas the genome-wide association studies (GWASs) allowed researchers to identify the locus associated with an increased risk of WMH development [34–36]. Some studies have recently shown that epigenetic deregulation, including DNA methylation alteration and deregulation of microRNA expression, is also significant for WMH formation and progression [37].
A large number of studies have confirmed the association of WMH expressiveness with the duration, profile, and manageability of AH as the leading cardiovascular risk factor [25, 28, 38, 39]. WMH progression was more clearly expressed in patients with untreated uncontrolled AH, compared to the treated patients with the same diagnosis [10]. We have previously shown the association between the WMH expressiveness and the severity of AH in asymptomatic first-ever diagnosed patients during the indiscriminate screening of an open population of working-age persons [41].
Other factors influencing WMH progression include diabetes mellitus, smoking at the time of assessment, and background WMH expressivity [19, 38, 42]. The Austrian Stroke Prevention Study has established that the volumetric growth rate was 1.3 mL/year in persons with confluent WMH foci, whereas changes practically did not grow in patients with pinpoint-sized foci [43]. Similar data were obtained in the Redboud University Nijmegen Diffusion Tensor and Magnetic Resonance Imaging Cohort (RUN DMC) study, for example, the probability of WMH progression was higher, if WMH was recorded as moderate and severe during the background assessment, whereas no progression of foci has been identified over a 9-year observational period in patients with mild WMH [44]. Differences in WMH progression regularities may most probably depend on the degree of their background expressivity. The expansion of focal and early confluent WMH was found to progress from the frontal to parietal brain regions and from the subcortical to deep white matter [40], whereas confluent WMH progression was associated with the transformation of the visually unaltered WMH penumbra into visual WMH [41, 45].
The studies have identified the association between risk factor significance and age. According to a large multifocal study comprising 2699 patients with stroke, an increased cholesterol level was an important risk factor for WMH appearance in old age patients with AH, whereas age in itself was an important risk factor in elderly patients without AH [46]. Similar data were obtained by the Rotterdam study, for example, a more expressed WMH progression was recorded in the group of more elderly persons irrespective of AH [15].
Lacunes (lacunar strokes) of vascular etiology are rounded or ovoid fluid-filled cavities with diameters from 3 to 15 mm, corresponding to an earlier occurred acute small deep cerebral infarct or a microbleed into the basin of one perforating artery. The signal of lacunar strokes is analogous to the cerebrospinal fluid under T2 and T1 modalities, i.e., hyper- and hypointensive, respectively; under the FLAIR modality, lacunes have a hypointensive MP signal (analogous to the cerebrospinal fluid) with a hyperintensive ring in the periphery [11].
The lacunar frequency rate reaches 9.5% per year, significantly differing between clinical and population-based studies [47]. For example, the annual frequency of lacunar detection in the large population-based Age, Gene/Environment Susceptibility Study (AGES-Reykjavik) constituted 0.8%, and the corresponding figures in the Rotterdam Scan Study and the Cardiovascular Health Study were represented by 3.5 and 2.9%, respectively. At the same time, according to the clinical Leukoaraiosis and Disability (LADIS) and Scan studies, the frequency of lacunar detection was equal to 5.8 and 9.5% per year and was most probably associated with both the expressiveness of clinical symptoms and the age of patients under observation.
The predictors of lacunar detection in dynamics include WMH expressiveness, the presence of lacunes during the baseline assessment, a stroke in anamnesis, atrial fibrillations, carotid artery atherosclerosis, and the presence of vascular risk factors, such as arterial hypertension and hypercholesterolemia [48, 49]. The newly detected lacunes are predominantly localized in the cerebral regions closely located to existing WMHs or those partially overlaying the latter [50].
The lacunar syndromes with their characteristic neurological symptoms develop when lacunes are located in the projection of conductors significant for clinical symptoms. Among lacunar syndromes, the absolute majority of cases are represented by pure motor, pure sensitive, and ataxic hemiparesis. The development of lacunes beyond the projection of significant conductors is commonly unaccompanied by clinical symptoms in patients with early cSVD. However, as the LADIS 2001–2011 [51], NOMAS [52], Rotterdam Scan [53], and Cardiovascular Health [48] Studies have shown, the risk of strokes, dementia, gait disorders, and developing pseudobulbar and pelvic impairments increases with the number of silent lacunes growing (NOMAS [52], Rotterdam Scan Study [53], Cardiovascular Health [48], and LADIS [51]).
Microinfarcts are ischemic foci with sizes of 50–400 μm to 3 mm, which were localized in the cortical grey and subcortical matter. Their number may reach hundreds and thousands per elderly person [18, 54]. They may be diagnosed under microscopy [55] and on a high-resolution 7T-MRI, corresponding to lacunes in their characteristics. The visualization of strokes on an MRI is limited to the sizes of 1–3 mm, and, therefore, MRI detection constitutes 0.5% of strokes detected microscopically [56, 57]. The current technical complexities in in vivo imaging of microinfarcts limit the use of this symptom as a clinical marker of cSVD progression. At the same time, their presence confirmed by the microscopy data is recognized as a reliable neuropathological symptom of vascular dementia [58].
Cerebral microbleeds correspond, in a majority of cases, to small areas of hemosiderin accumulation in macrophages. Microbleeds are identified as hypointensive rounded foci with sizes from 2 to 5 mm, and, rarely, up to 10-mm foci on “gradient echo” MP-sequences sensitive to paramagnetics (hemosiderin), including T2-GRE, SWI sequences, and those undetected under standard MRI modalities [11, 59]. They are located at the boundary of the cortex and the subcortical white matter, in the cortex, the deep white matter of the hemispheres, in the stem, and the cerebellum. The diagnoses of cerebral amyloid angiopathy [60] in case of their lobar location and sporadic nonamyloid, cSVD in case of their deep location [61] should be considered possible.
Population-based and clinical studies annually detect 2.9–3.5 and 2.2–31.2% of cerebral microbleeds, respectively [62–64]. The studies underlined the association of their growth with age. According to the population-based RSS data, the annual detection rate for microbleeds was 7.6% in 60–69-year olds, 15.6% in persons aged 70–79 years, and 18.6% in patients over 80 years [62]. Their highest detection rate (up to 41.8 per year) was recorded in patients with intracerebral hematomas and cerebral amyloid angiopathy.
The population-based Rotterdam study [65] showed the association of deep microbleeds with vascular risk factors, such as AH and smoking, whereas lobar microbleeds were shown to be associated with the risk of developing cerebral amyloid angiopathy, the apolipoprotein E epsilon 4 (APOE ε4) genotype [66]. Among other factors in favor of microbleed expansion are their number during the baseline assessment, the presence of lacunes, WMH expressiveness, and the identified APOE genotype.
Perivascular spaces (Virchow–Robin Spaces) are liquor-filled expansions surrounding vessels. Perivascular spaces may be linear in shape, if the scanning sections are parallel to the course of vessels and circular or ovoid if the sections are perpendicular to the course of vessels. The perivascular spaces in norm frequently become expanded in normal aging. The Virchow–Robin Spaces contain cerebrospinal liquor, and therefore, their signal is of increased intensity on T2-weighted imaging (WI) and FLAIR and decreased in the T1 sequence. They differ from lacunes in the absence of hyperintensive signal in their periphery in the FLAIR sequence and, generally, in smaller sizes. Perivascular spaces are usually localized in the semioval center, subcortical formations, and the hippocampus. This state is called état criblé in the cases when the expansion of these spaces is expressed [11, 13].
The differences detected between healthy subjects and patients with cSVD in the significance of enlarged perivascular spaces were as follows: no cognitive dysfunctions were present in this case in healthy subjects [69], whereas the presence of cognitive impairments was associated with age and cognitive losses in patients with cSVD [70, 71]. The interest in the role of perivascular spaces in cSVD was mainly caused in the last years by the correlation of significance of the recently discovered glymphatic system in the development of cognitive impairments belonging to this system. Their expansion is considered as one possible sign of stasis in the interstitial fluid with brain drainage dysfunction [72].
Diffusion-Weighted MRI Methodologies
The absence of a direct association between WMH expressiveness and cognitive impairments [67] in a significant portion of cSVD cases, which may be explained by heterogeneity of pathological processes underlying this phenomenon [20], motivated the search for sensitive indicators of the microstructural brain impairments. The diffusion-weighted (DW-MRI) methodologies with assessing different characteristics of free (extracellular) water diffusion in the brain matter and, correspondingly, the maintenance of its microstructural integrity, allow researchers to approach to the explanation of individually specific clinical manifestations of the disease and, possibly, predict its course under dynamic observation. The main indicator of a DW-MRI is the measured apparent diffusion coefficient (ADC, or mean diffusivity). The diffusion-tensor MRI, a modification of this method, offers estimated indicators, such as fractional anisotropy, axial and radial diffusion, to give one a possibility to determine not only the value, but also directionality (anisotropy) in the diffusion of water molecules. The lower significance of fractional anisotropy and, correspondingly, a high mean diffusion coefficient reflect a great loss to the microstructure. The axial and radial diffusion are used as the markers of neuronal impairments, which, according to experimental data, are associated, respectively, with the involvement of axon and myelin [73, 74]. The results of some accomplished studies dedicated to the correction of association between microstructural alterations in the brain of patients with cSVD and clinical manifestations of the disease have recently been published. An increase in ADC was diagnosed in the externally unaltered matter as a result of memory losses, disorders in controlling brain functions and the speed of psychic processes, but irrespective of vascular risk factors and the volume of a white matter lesion [67]. The association has also been found between an increase in ADC in the hippocampus, thalamus, cingulate gyrus, and hook-like bundle and subclinical depression, alarmism, and memory difficulties in patients with the first-ever diagnosed asymptotic AH and cSVD-specific MRI alterations [41]. It has also been found that fractional anisotropy and medium diffusion are associated in cSVD with AH severity, as well as fractional anisotropy and radial diffusion are associated with cognitive and gait disorders [75, 76]. The predictive ability of diffusion-weighted methodologies has also been shown in relation to the appearance of WMH under dynamic observation in the visually unaltered white matter with decreased fractional anisotropy and increased diffusion [77].
CONCLUSIONS
Thus, the current numerous evidences of association between the leading MRI features of cSVD and its clinical manifestations allow us to recognize the expediency of diagnosing this pathology only with its neuroimaging confirmation. Diagnosing cSVD by MRI must become for clinicians a foundation for correcting the form of the disease, its possible risk factors, and prediction. It should be recognized that the established regularities in the progression of the main MRI features at the group level are not reproduced at the individual level, generally demonstrating non-correspondence between the expressiveness of WMHs and cognitive impairments. In addition, the disease progression rates obviously depend on different cSVD forms, but this issue is not debated in the literature. These contradictions were partially resolved with the start of using the diffusion-weighted methodologies for assessing the structural brain lesions. However, this approach is currently limited to the confirmation of association between microstructural and clinical impairments. Taking into account the fact of a high social significance of the disease and population aging potentially increasing the percentage of cSVD patients, it is very important to conduct prospective studies, starting from the stage of subclinical brain lesions, using the MRI sequences aimed to assess different components of the pathological process. Considering that the therapeutic successes in the management of arterial hypertension, as the leading cSVD risk factor, have not led to a reduction in the healthcare burden generated by this pathology, it is necessary to consider different potential risk factors and their association with the disease progression.
REFERENCES
Pantoni, L., Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges, Lancet Neurol., 2010, vol. 9, no. 7, p. 689. https://doi.org/10.1016/S1474-4422(10)70104-6
Pasi, M., van Uden, I.W., Tuladhar, A.M., et al., White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: clinical consequences, Stroke, 2016, vol. 47, no. 6, p. 1679. https://doi.org/10.1161/STROKEAHA.115.012065
Wardlaw, J.M., Smith, C., and Dichgans, M., Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging, Lancet Neurol., 2013, vol. 12, no. 5, p. 483. https://doi.org/10.1016/S1474-4422(13)70060-7
Gorelick, P.B., Scuteri, A., Black, S.E., et al., Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association, Stroke, 2011, vol. 42, p. 2672. https://doi.org/10.1161/STR.0b013e3182299496
Charidimou, A., Pantoni, L., and Love, S., The concept of sporadic cerebral small vessel disease: a road map on key definitions and current concepts, Int. J. Stroke, 2016, vol. 11, no. 1, p. 6. https://doi.org/10.1177/1747493015607485
Qureshi, A.I., Mendelow, A.D., and Hanley, D.F., Intracerebral haemorrhage, Lancet, 2009, vol. 373, no. 9675, p. 1632. https://doi.org/10.1016/S0140-6736(09)60371-8
Sudlow, C.L. and Warlow, C.P., Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration, Stroke, 1997, vol. 28, p. 491.
Biessels, G.J., Diagnosis and treatment of vascular damage in dementia, Biochim. Biophys. Acta, 2016, vol. 1862, no. 5, p. 869. https://doi.org/10.1016/j.bbadis.2015.11.009
Smallwood, A., Oulhaj, A., Joachim, C., et al., Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort, Neuropathol. Appl. Neurobiol., 2012, vol. 38, p. 337. https://doi.org/10.1111/j.1365-2990.2011.01221.x
Verhaaren, B.F., Vernooij, M.W., de Boer, R., et al., High blood pressure and cerebral white matter lesion progression in the general population, Hypertension, 2013, vol. 61, p. 1354. https://doi.org/10.1161/HYPERTENSIONAHA.111.00430
Wardlaw, J.M., Smith, E.E., Biessels, G.J., et al., Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration, Lancet Neurol., 2013, vol. 12, no. 8, p. 822. https://doi.org/10.1016/S1474-4422(13)70124-8
Raina, A., Zhao, X. Grove, M.L., et al., Cerebral white matter hyperintensities on MRI and acceleration of epigenetic aging: the atherosclerosis risk in communities’ study, Clin. Epigenet., 2017, vol. 14, no. 9, p. 21. https://doi.org/10.1186/s13148-016-0302-6
Barkhofa, F. and Scheltensb, P., Imaging of white matter lesions, Cerebrovasc. Dis., 2002, vol. 13. suppl. 2, p. 21. https://doi.org/10.1159/000049146
Fazekas, F., Chawluk, J.B., Alavi, A., et al., MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging, Am. J. Roentgenol., 1987, vol. 149, no. 2, p. 351. https://doi.org/10.2214/ajr.149.2.351
de Leeuw, F.E., de Groot, J.C., Achten, E., et al., Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study: The Rotterdam Scan Study, J. Neurol. Neurosurg. Psychiatry, 2001, vol. 70, p. 9.
Scheltens, P., Barkhof, F., Leys, D., et al., A semiquantitative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging, J. Neurol. Sci., 1993, vol. 114, no. 1, p. 7.
Wahlund, L.O., Agartz, I., Almqvist, O., et al., The brain in healthy aged individuals: MR imaging, Radiology, 1990, vol. 174, no. 3, part 1, p. 675. https://doi.org/10.1148/radiology.174.3.2305048
Longstreth, W.T. Jr., Sonnen, J.A., Koepsell, T.D., et al., Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases, Alzheimer Dis. Assoc. Disord., 2009, vol. 23, p. 291. https://doi.org/10.1097/WAD.0b013e318199fc7a
Prins, N.D., van Straaten, E.C., van Dijk, E.J., et al., Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics, Neurology, 2004, vol. 62, no. 9, p. 1533.
Schmidt, R., Schmidt, H., Haybaeck, J., et al., Heterogeneity in age-related white matter changes, Acta Neuropathol., 2011, vol. 122, p. 171. https://doi.org/10.1007/s00401-011-0851-x
Dufouil, C., Chalmers, J., Coskun, O., et al., Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection against Recurrent Stroke Study) Magnetic Resonance Imaging substudy, Circulation, 2005, vol. 112, no. 11, p. 1644. https://doi.org/10.1161/CIRCULATIONAHA.104.501163
Gottesman, R.F., Coresh, J., Catellier, D.J., et al., Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study, Stroke, 2010, vol. 41, no. 1, p. 3. https://doi.org/10.1161/STROKEAHA.109.566992
Maillard, P., Crivello, F., Dufouil, C., et al., Longitudinal follow-up of individual white matter hyperintensities in a large cohort of elderly, Neuroradiology, 2009, vol. 51, p. 209. https://doi.org/10.1007/s00234-008-0489-0
Kloppenborg, R.P., Nederkoorn, P.J., Grool, A.M., et al., Cerebral small-vessel disease and progression of brain atrophy: the SMART-MR study, Neurology, 2012, vol. 79, p. 2029. https://doi.org/10.1212/WNL.0b013e3182749f02
Debette, S. and Markus, H.S., The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis, BMJ, 2010, vol. 341, p. c3666. https://doi.org/10.1136/bmj.c3666
Wardlaw, J.M., Valdés Hernández, M.C., and Muñoz-Maniega, S, What are white matter hyperintensities made of? Relevance to vascular cognitive impairment, J Am Heart Assoc., 2015, vol. 4, no. 6, p. 001140. https://doi.org/10.1161/JAHA.114.001140
Raman, M.R., Kantarci, K., Murray, M.E., et al., Imaging markers of cerebrovascular pathologies: pathophysiology, clinical presentation, and risk factors, Alzheimers Dement. (Amsterdam), 2016, vol. 5, p. 5. https://doi.org/10.1016/j.dadm.2016.12.006
LADIS Study Group, 2001—2011: a decade of the LADIS (LeukoaraiosisAndDISability) study: what have we learned about white matter changes and small-vessel disease? Cerebrovasc. Dis., 2011, vol. 32, no. 6, p. 577. https://doi.org/10.1159/000334498
Herrmann, L.L., Le Masurier, M., and Ebmeier, K.P., White matter hyperintensities in late life depression: a systematic review, J. Neurol. Neurosurg. Psychiatry, 2008, vol. 79, p. 619. https://doi.org/10.1136/jnnp.2007.124651
Wright, C.B., Dong, C., Perez, E.J., et al., Subclinical cerebrovascular disease increases the risk of incident stroke and mortality: The Northern Manhattan Study, J. Am. Heart Assoc., 2017, vol. 6, no. 9. https://doi.org/10.1161/JAHA.116.004069
Windham, B.G., Deere, B., Griswold, M.E., et al., Small brain lesions and incident stroke and mortality: a cohort study, Ann. Int. Med., 2015, vol. 163, no. 1, p. 22. https://doi.org/10.7326/M14-2057
Schretlen, D.J., Testa, S.M., Winicki, J.M., et al., Frequency and bases of abnormal performance by healthy adults on neuropsychological testing, J. Int. Neuropsychol. Soc., 2008, vol. 14, no. 3, p. 436. https://doi.org/10.1017/S1355617708080387
Carmelli, D., DeCarli, C., Swan, G.E., et al., Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins, Stroke, 1998, vol. 29, no. 6, p. 1177.
Verhaaren, B.F., de Boer, R., Vernooij, M.W., et al., Replication study of chr17q25 with cerebral white matter lesion volume, Stroke, 2011, vol. 42, no. 11, p. 3297. https://doi.org/10.1161/STROKEAHA.111.623090
Adib-Samii, P., Rost, N., Traylor, M., et al., 17q25 Locus is associated with white matter hyperintensity volume in ischemic stroke, but not with lacunar stroke status, Stroke, 2013, vol. 44, no. 6, p. 1609. https://doi.org/10.1161/STROKEAHA.113.679936
Tabara, Y., Igase, M., Okada, Y., et al., Association of Chr17q25 with cerebral white matter hyperintensities and cognitive impairment: the J-SHIPP study, Eur. J. Neurol., 2013, vol. 20, no. 5, p. 860. https://doi.org/10.1111/j.1468-1331.2012.03879.x
Lin, Q., Huang, W.Q., and Tzeng, C.M., Genetic associations of leukoaraiosis indicate pathophysiological mechanisms in white matter lesions etiology, Rev. Neurosci., 2015, vol. 26, no. 3, p. 343. https://doi.org/10.1515/revneuro-2014-0082
de Leeuw, F.E., de Groot, J.C., Oudkerk, M., et al., Hypertension and cerebral white matter lesions in a prospective cohort study, Brain, 2002, vol. 125, part 4, p. 765.
Dufouil, C., de Kersaint-Gilly, A., Besancon, V., et al., Longitudinal study on blood pressure and white matter hyperintensities: the EVA MRI cohort, Neurology, 2001, vol. 56, no. 7, p. 921.
Dobrynina, LA., Gnedovskaya, E.V. Sergeeva, A.N., et al., Subclinical cerebral manifestations and changes of brain associated with newly diagnosed asymptomatic arterial hypertension, Ann. Klin. Eksp. Nevrol., 2016, vol. 10, no. 3, p. 26.
Dobrynina, L.A., Gnedovskaya, E.V., Sergeeva, A.N., et al., Changes in the MRI brain picture associated with newly diagnosed asymptomatic arterial hypertension, Ann. Klin. Eksp. Nevrol., 2016, vol. 10, no. 3, p. 33.
Schmidt, R., Fazekas, F., Enzinger, C., et al., Risk factors and progression of small vessel disease-related cerebral abnormalities, J. Neural Transm., Suppl., 2002, vol. 62, p. 47.
Schmidt, R., Enzinger, C., Ropele, S., et al., Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study, Lancet, 2003, vol. 361, p. 2046.
van Leijsen, E.M.C., van Uden, I.W.M., Ghafoo-rian, M., et al., The rise and fall of cerebral small vessel disease—the RUN DMC study, Eur. Stroke J., 2016.
Maillard, P., Fletcher, E., Lockhar, S.N., et al., White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain, Stroke, 2014, vol. 45, no. 6, p. 1721. https://doi.org/10.1161/STROKEAHA.113.004084
Ryu, W.S., Woo, S.H., Schellingerhout, D., et al., Grading and interpretation of white matter hyperintensities using statistical maps, Stroke, 2014, vol. 45, p. 3567. https://doi.org/10.1161/STROKEAHA.114.006662
van Leijsen, E.M.C., de Leeuw, F.E., and Tuladhar, A.M., Disease progression and regression in sporadic small vessel disease—insights from neuroimaging, Clin. Sci., 2017, vol. 131, no. 12, p. 1191. https://doi.org/10.1042/CS20160384
Longstreth, W.T., Jr., Dulberg, C., Manolio, T.A., et al., Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study, Stroke, 2002, vol. 33, no. 10, p. 2376.
Gouw, A.A., van der Flier, W.M., Pantoni, L., et al., On the etiology of incident brain lacunes: longitudinal observations from the LADIS study, Stroke, 2008, vol. 39, no. 11, p. 3083. https://doi.org/10.1161/STROKEAHA.108.521807
Duering, M., Csanadi, E., Gesierich, B., et al., Incident lacunes preferentially localize to the edge of white matter hyperintensities: insights into the pathophysiology of cerebral small vessel disease, Brain, 2013, vol. 136, part 9, p. 2717. https://doi.org/10.1093/brain/awt184
Jokinen, H., Gouw, A.A., Madureira, S., et al., Incident lacunes influence cognitive decline: the LADIS study, Neurology, 2011, vol. 76, no. 22, p. 1872. https://doi.org/10.1212/WNL.0b013e31821d752f
Wright, C.B., Festa, J.R., Paik, M.C., et al., White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility, Stroke, 2008, vol. 39, no. 3, p. 800. https://doi.org/10.1161/STROKEAHA.107.484147
van Dijk, E.J., Prins, N.D., Vrooman, H.A., et al., Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study, Stroke, 2008, vol. 39, no. 10, p. 2712. https://doi.org/10.1161/STROKEAHA.107.513176
Schneider, J.A., Aggarwal, N.T., Barnes, L., et al., The neuropathology of older persons with and without dementia from community versus clinic cohorts, J. Alzheimers Dis., 2009, vol. 18, no. 3, p. 691. https://doi.org/10.3233/JAD-2009-1227
Brundel, M., de Bresser, J., van Dillen, J.J., et al., Cerebral microinfarcts: a systematic review of neuropathological studies, J. Cereb. Blood Flow Metab., 2012, vol. 32, no. 3, p. 425. https://doi.org/10.1038/jcbfm.2011.200
van Veluw, S.J., Zwanenburg, J.J., Engelen-Lee, J., et al., In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI, J. Cereb. Blood Flow Metab., 2013, vol. 33, no. 3, p. 322. https://doi.org/10.1038/jcbfm.2012.196
Auriel, E., Edlow, B.L., Reijmer, Y.D., et al., Microinfarct disruption of white matter structure: a longitudinal diffusion tensor analysis, Neurology, 2014, vol. 83, no. 8, p. 182. https://doi.org/10.1212/WNL.0000000000000579
Deramecourt, V., Slade, J.Y., Oakley, A.E., et al., Staging and natural history of cerebrovascular pathology in dementia, Neurology, 2012, vol. 78, no. 14, p. 1043. https://doi.org/10.1212/WNL.0b013e31824e8e7f
Patel, B. and Markus, H.S., Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker, Int. J. Stroke, 2011, vol. 6, no. 1, p. 47. https://doi.org/10.1111/j.1747-4949.2010.00552.x
Knudsen, K.A., Rosand, J., Karluk, D., et al., Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria, Neurology, 2001, vol. 56, no. 4, p. 537.
Iadecola, C., The pathobiology of vascular dementia, Neuron, 2013, vol. 80, no. 4, p. 844. https://doi.org/10.1016/j.neuron.2013.10.008
Poels, M.M., Ikram, M.A., van der Lugt, A., et al., Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study, Stroke, 2011, vol. 42, no. 3, p. 656. https://doi.org/10.1161/STROKEAHA.110.607184
Lee, S.H., Lee, S.T., Kim, B.J., et al., Dynamic temporal change of cerebral microbleeds: long-term follow-up MRI study, PloS One, 2011, vol. 6, no. 10. e2593. https://doi.org/10.1371/journal.pone.0025930
Akoudad, S., Ikram, M.A., Koudstaal, P.J., et al., Cerebral microbleeds are associated with the progression of ischemic vascular lesions, Cerebrovasc. Dis., 2014, vol. 37, no. 5, p. 382. https://doi.org/10.1159/000362590
Vernooij, M.W., van der Lugt, A., Ikram, M.A., et al., Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study, Neurology, 2008, vol. 70, no. 14, p. 1208. https://doi.org/10.1212/01.wnl.0000307750.41970.d9
Kim, M., Bae, H.J., Lee, J., et al., APOE epsilon2/epsilon4 polymorphism and cerebral microbleeds on gradient-echo MRI, Neurology, 2005, vol. 65, no. 9, p. 1474. https://doi.org/10.1212/01.wnl.0000183311.48144.7f
Schmidt, R., Ropele, S., Ferro, J., et al., Diffusion-weighted imaging and cognition in the leukoariosis and disability in the elderly study, Stroke, 2010, vol. 41, no. 5, p. e402. https://doi.org/10.1161/STROKEAHA.109.576629
Goos, J.D., Henneman, W.J., Sluimer, J.D., et al., Incidence of cerebral microbleeds: a longitudinal study in a memory clinic population, Neurology, 2010, vol. 74, no. 24, p. 1954. https://doi.org/10.1212/WNL.0b013e3181e396ea
MacLullich, A.M., Wardlaw, J.M. Ferguson, K.J., et al., Enlarged perivascular spaces are associated with cognitive function in healthy elderly men, J. Neurol. Neurosurg. Psychiatry, 2004, vol. 75, no. 11, p. 1519. https://doi.org/10.1136/jnnp.2003.030858
van Swieten, J.C., Hout, J.H., van Ketel, B.A., et al., Periventricular lesions in the white matter on magnetic resonance imaging in the elderly: a morphometric correlation with arteriolosclerosis and dilated perivascular spaces, Brain, 1991, vol. 114, p. 761.
Bokura, H., Kobayashi, S., and Yamaguchi, S., Distinguishing silent lacunar infarction from enlarged Virchow—Robin spaces: a magnetic resonance imaging and pathological study, J. Neurol., 1998, vol. 245, no. 2.
Mestre, H., Kostrikov, S., and Mehta, R.I., Perivascular spaces, glymphatic dysfunction, and small vessel disease, Clin. Sci. (London), 2017, vol. 131, no. 17, p. 2257. https://doi.org/10.1042/CS20160381
Song, S.K., Sun, S.W., Ramsbottom, M.J., et al., Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water, NeuroImage, 2002, vol. 17, no. 3, p. 1429.
Pasi, M., van Uden, I.W., Tuladhar, A.M., et al., White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease clinical consequences, Stroke, 2016, vol. 47, no. 6, p. 1679. https://doi.org/10.1161/STROKEAHA.115.012065
Hannesdottir, K., Nitkunan, A., Charlton, R.A., et al., Cognitive impairment and white matter damage in hypertension: a pilot study, Acta Neurol. Scand., 2009, vol. 119, no. 4, p. 261. https://doi.org/10.1111/j.1600-0404.2008.01098.x
Lawrence, A.J., Patel, B., Morris, R.G., et al., Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s cognition and neuroimaging in stroke (SCANS) study, PloS One, 2013, vol. 8, no. 4. e61014. https://doi.org/10.1371/journal.pone.0061014
de Groot, M., Verhaaren, B.F., de Boer, R., et al., Changes in normal-appearing white matter precede development of white matter lesions, Stroke, 2013, vol. 44, no. 4, p. 1037. https://doi.org/10.1161/STROKEAHA.112.680223
Funding
The study was not supported by any particular organization.
Conflict of interests. The authors declare the absence of a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Tarasyuk
Rights and permissions
About this article
Cite this article
Gnedovskaya, E.V., Dobrynina, L.A., Krotenkova, M.V. et al. MRI in the Assessment of Cerebral Small Vessel Disease. Hum Physiol 48, 938–945 (2022). https://doi.org/10.1134/S0362119722080023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722080023