Abstract—
Adaptive shifts in systemic hemodynamic parameters and energy consumption were evaluated in 15 healthy young men in response to a single procedure of passive hyperthermia (PH) and during a course of ten such procedures. PH procedures with a duration of 40 min were performed in an infrared body heating capsule at 65–80°C, with the head remaining outside the capsule. Heart rate, blood pressure, and SрО2 were analyzed. Oxygen and energy consumption were measured by indirect calorimetry. The sweating rate and the physiological strain index (PSI) were calculated. It was found that PH procedures were accompanied by an increase in energy consumption (in comparison with placebo procedures), but without a pronounced stress response of systemic hemodynamics. PSI values during PH corresponded to moderate heat stress. During the course, adaptive shifts occurred in the form of a decrease in energy consumption and an increase in the sweating rate. In the tenth procedure the relationship between the degree of increase in body temperature and the level of energy consumption was revealed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Adaptation to repetitive passive thermal influences (without a combination with physical or other loads) and acclimation can improve various body functions in health and disease [1–3]. As an example, in a systematic review [2], it was shown that sauna bathing has positive effects on both apparently healthy people and patients with diseases of the cardiovascular, respiratory, and immune systems; chronic pain syndrome; and depression. The neuroendocrine foundations of the influence of thermal procedures on physical performance in athletes and their rehabilitation after injuries are being studied [4, 5]. There are reasons for their use in order to increase resistance to infections, including viral ones [1].
Physiological mechanisms of changes in the dynamics of repeated thermal stimulation are associated with heat-induced activation of metabolism: increased oxygen and energy consumption, cardiorespiratory functions, and increased peripheral blood flow [6–9]. Anabolic processes are enhanced after the procedures and in the delay period [5, 10].
The widespread use of this relatively cheap and affordable method is limited by the lack of protocols for conducting procedures with a clear justification of the effectiveness and safety for different population groups [1]. In order to achieve adaptive changes, it is recommended to have at least six to seven procedures lasting at least than 30 min, with systemic hyperthermia, an increase in the body core temperature, being an important criterion for the effectiveness of the treatment [11, 12]. Water immersions, or a supply of moist air with a temperature of 33–42°C, or dry sauna baths (60–90°C) [5] are used as a means of heating the human body. Obviously, the dynamics of body temperature and cardiometabolic parameters may differ significantly under different regimens. In addition to the rate and amplitude of the rise in the body core temperature, the effect on peripheral thermoreceptors, as well as heating of the head/face, and inhalation of hot air, may be important [13–15].
Only a limited number of rather contradictory publications on the ratio of the amount of energy consumption (EC) in passive hyperthermia (PH) to the levels of heat stress indicators, including the dynamics of cardiovascular parameters and body temperature, are available in the literature. The amount of the energy consumed is often evaluated according to hemodynamic parameters by analogy with the assessment of the “pulse cost” of physical activity. As an example, according to the data in [16], in a single sauna bath, the dynamics of cardiovascular indicators corresponds to physical training of moderate intensity in the range of 60–100 W. However, the data on the correlation between EC/O2 consumption and heart rate (HR), blood pressure (BP), and body temperature during PH are rather contradictory. In [13], the body core temperature, skin temperature, systolic (SBP) and diastolic blood pressure (DBP) during dry sauna bathing had different dynamics. When several short sauna sessions are taken in succession, the degree and velocity of changes in the HR, BP, and oxygen consumption are also different [9].
A decrease in the heart rate increment, body temperature, an increase in the sweat rate, as well as the hypotensive effect, are considered to be typical changes in the process of long-term thermal adaptation [2, 6, 17]. Information on the dynamics of EC in the course of a heat procedure is scarce. Some authors claim that they did not reveal significant changes in cardiometabolic parameters, for example, in [8] even after 3 weeks of thermal adaptation. Others demonstrated shifts aimed at the formation of a more economical mode of functioning: a decrease in the resting core temperature and in the HR increment under stress; however, no difference in the amount of oxygen consumed at rest was noted [12, 18–20].
Differences in experimental data are associated with a large variability in both the methods of using PH (methods, duration and intensity of heating, body position during the procedure, duration of the course) and groups of subjects (age, sex, physical fitness, state of health, initial level of adaptation to thermal effects, etc.).
The purpose of this study was to assess adaptive shifts in systemic hemodynamic parameters and energy expenditure of healthy young men for a single procedure of intense heating, as well as in a course of ten PH procedures.
MATERIALS AND METHODS
The design of the work was a balanced longitudinal crossover study, which initially enrolled 23 relatively healthy young volunteers (men, mean age 20.1 ± 2.3 years, a baseline body weight of 71.4 ± 11.6 kg, and body length 178.3 ± 7.2 cm). All participants had an average level of daily physical activity assessed using the Russian version of the Global Physical Activity Questionnaire (GPAQ). Most of them had minimal preliminary experience of taking a sauna/steam bath: not regularly and no more than 1–2 times a month (eight volunteers reported taking a sauna two to three times a month).
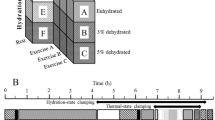
The experiment included two 2-week stages (Fig. 1), during which the subjects underwent ten pseudohyperthermic (placebo) and PH procedures, whose order was randomized: 12 subjects first underwent a placebo course followed by a PH course; 11 other participants started with PH procedures and then took a placebo course.
Hyperthermic/placebo procedures were performed in an Alpha Basic infrared heating capsule (Sybaritic Inc., Minnesota, United States). During the procedure, the subject lay in the ergonomic capsule for 40 min, while his head was outside and could be cooled with cool air by the fans installed at the head of the capsule. The temperature in the PH capsule was set in the range of 65–80°C based on the individual’s tolerance determined in the test procedure. During the placebo procedure, the comfort temperature was set at 24–25°C. Participants were instructed to have the sauna in the morning, at least 2 hours after a light breakfast, in a sufficiently hydrated state, but not drink water/other beverages 20–30 min before and during the entire procedure. It was recommended not to change their usual regime and not to engage in intense physical training during the entire observation period.
Registration of physiological parameters. In the dynamics of each procedure, the blood oxygen saturation (SpO2) values were monitored using an MD300 pulse oximeter (BCE Tech, China); heart rate was monitored by a Polar 610i chest belt (Finland). Body temperature was assessed at baseline, after 20 min and at the end of the procedure (AND DT-635 infrared thermometer, AND, Japan) taking into account the averaged data of two measurements in both axillary areas. The axillary temperature in this case reflects the body core temperature; since heating increases the efficiency of heat transfer between the core and the shell the gradient between them decreases.
Before and immediately after each PH procedure, the body weight was measured with an accuracy of 10 g with a Tanita BC-601 portable bioimpedance meter (Tanita, Japan); in addition, the subjects in the capsule bed had their SBP and DBP measured (AND UA-767 automatic tonometer, AND, Japan).
The physiological strain index (PSI) for a procedure was calculated using the formula: PSI = 5 (Ts – To) (39.5 – To)–1 + 5 (HRs – HRo) (180 – HRo)–1, where Ts and HRs are the maximum values of the subject’s body temperature and HR during heat stress, while To and HRo are the initial values of the subject’s body temperature and HR. The range of PSI variations was from 0 (no strain), 4–6, moderate stress, to 10 (maximum heat stress) [21]. The sweat rate (SR) was determined by the formula: SR = Δm/BSA, where Δm is the difference in body weight measured before and immediately after the procedure and BSA is the subject’s body surface area.
The EС of the subjects over 40 min of the first and tenth procedures was measured by indirect calorimetry using a Fitmate MED gas analyzer (COSMED, Italy). All participants in the study underwent preliminarily introductory testing; the gas analyzer was auto-calibrated before each measurement. The oxygen contents in exhaled air and pulmonary ventilation were recorded. Respiratory rate (RR, cycles/min), respiratory minute volume (RMV, L/min) were assessed; oxygen consumption (VO2, mL/min) and EС were calculated using standard metabolic formulas using a fixed respiratory quotient equal to 0.85. In the dynamics of the procedure, measurements were carried out twice for 15 min; the data were averaged and then recalculated for 40 min.
The results were statistically processed using Statistica 11.0 software. Data in the work are presented as the mean and the standard deviation M ± SD. The normality of the distribution was tested using the Kolmogorov–Smirnov test; the one-sample t-test and the Mann–Whitney test were used to assess the significance of within- and between-group differences in dynamics, respectively. The correlations between the parameter shifts in the dynamics of observations and their significance were assessed by nonparametric Spearman’s rank correlation coefficient with the interpretation of the coefficient values as strong (r ≥ 0.6), medium-strength (r = 0.59–0.40), and weak (r = 0.39–0.20). Differences were considered significant at p < 0.05.
RESULTS
The analysis included the results of a survey of 15 participants who completed the full cycle of procedures (5 participants dropped out due to low adherence to the study protocol and 3 left due to acute respiratory disease). All subjects tolerated the PH procedures satisfactorily.
By the end of the PH procedures, the body temperature increased by an average of 1.5–3.2°C, while in the first three procedures the temperature rise was in the range of 1.4–1.8°C and subsequently increased to 2.6–3.2°C (Fig. 2). The increase in temperature values, starting from the fourth procedure, occurred more intensively in the first 20 min from the beginning of the exposure; the changes then stabilized. By the end of the fourth and all subsequent procedures, the body temperature reached 39.0–39.4°С and was significantly higher than at the end of the first or second procedures.
The average values of body temperature (a) and hemoglobin saturation with oxygen (SpO2) of the subjects (b) in the dynamics of ten passive hyperthermia procedures: baseline (a), 20 min after the beginning (b), and at the end of the procedure (c). The abscissa shows the procedure number. The graph shows the means and standard deviation. Significant differences were noted (p < 0.05): (a) in relation to the temperature value at the corresponding stage in the first procedure; (b) in relation to the baseline value.
The SpO2 value practically did not change in the dynamics of the first PH procedure. Later, starting from the second procedure, significant blood desaturation was noted: SpO2 reached 94.8–95.5% (Fig. 2).
During all PH procedures, a typical hemodynamic response was observed in the form of a moderate heart rate increase and a SBP decrease without significant shifts in DBP (Fig. 3), as described previously in [4, 14].
The average values of systemic hemodynamic parameters in the dynamics of ten passive hyperthermia procedures: initially (a) and at the end of the procedure (b). (a) Heart rate (HR); (b) systolic blood pressure (SBP); (c) diastolic blood pressure (DBP). The abscissa shows the procedure number. The graph shows the means and standard deviation. Significant differences (p < 0.05) were noted in relation to the baseline value.
The values of the PSI integrative index, which reflect the degree of heat stress, were consistently higher in PH compared with simulation procedures (Fig. 4). In the course dynamics, the PSI values significantly increased by the fifth procedure (in relation to the values in the first one) and then stabilized, remaining at the level of moderate heat stress indicators (5–6 c.u.). SR in the dynamics of placebo procedures remained minimal, while during adaptation to PH, the SR values were expectedly higher (by 6–8 times on average) (Fig. 4). Starting from the eighth procedure, the SR values significantly exceeded the data obtained during the first one.
The average values in the dynamics of ten placebo (a) and passive hyperthermia procedures (b): (a) physiological strain index (PSI); (b) sweat rate. The abscissa shows the procedure number. The graph shows the mean and standard deviation. Significant differences (p < 0.05) were noted in relation to the value in the placebo procedure (asterisk) and in relation to the value in the first hyperthermia procedure (hash).
The VO2 and EC values during the PH procedures were higher by 36 and 29.7%, respectively, than in the corresponding placebo procedures (Table 1). At the same time, in the dynamics of the tenth procedure, the VO2 and EC indicators were recorded at a significantly lower level than in the first procedure. The activation of metabolism during hyperthermia was accompanied by the development of moderate hyperpnea (a significant increase in the RMV values in relation to the placebo data), whose degree decreased in the tenth PH procedure.
The VO2 increase in terms of an increase in body temperature by 1°С in the first procedure averaged 49.1 ± 57.5%/1°С, and the increase in heart rate was 32.7 ± 23.1 bpm/1°С in relation to the values of the corresponding placebo session. In the tenth PH procedure, the relative indicators were significantly lower than in the first procedure: the increase in VO2 was 10.9 ± 11.6%/1°C (p = 0.001), and the heart rate was only 13.2 ± 7.1 bpm/1°C (p = 0.05).
No significant correlations were found between the rise in body temperature and the dynamics of the analyzed metabolic parameters in the first PH procedure. During the tenth PH procedure, correlations were revealed between the increase in body temperature and the volume of consumed oxygen – r(ΔT–VO2) = 0.56, p = 0.02, as well as the level of energy consumption r(ΔT – EC) = 0.39, p = 0.07. No correlations were found between the shifts in hemodynamic parameters and the VO2 and EC levels.
DISCUSSION
As follows from the data, with the PH mode we used the adaptive “shifts” of hemodynamic and metabolic indicators for a single procedure and in the course dynamics were different. It should be noted that the work evaluated the thermophysiological effects of heat adaptation in the passive mode, i.e., without a combination with physical activity, which is more typical of heat acclimation [22]. In contrast to the latter, where the effects of exogenous and endogenous heat are combined, we focused on assessing the effects of exogenous intense (65–80°C) temperature stimuli without the heads of participants being heated.
A single passive heating procedure with a 1.4–1.8°C increase in the body temperature of the subjects was accompanied by an intensification of metabolic processes, which was the expected effect and was evidently due to both the triggering of neuroendocrine mechanisms and the direct effect of heat on tissues [12]. The activation of metabolic processes is also confirmed by moderate desaturation of blood with oxygen, which, in addition to the effect of increased tissue/blood temperature on hemoglobin dissociation, may be associated with the accumulation of metabolites and the development of relative acidosis [23, 24].
As judged by the dynamics of PSI values and hemodynamic parameters, the level of induced heat stress was moderate. The degree of heart rate increase during acute hyperthermia (in the range of 35–50%) is comparable to the increase in heart rate during physical exertion of moderate intensity. However, in contrast to the data in [16], where a significant increase in BP values was noted immediately after a 25-minute sauna bath procedure, the PH procedure in our study was accompanied by a significant decrease in SBP values. A similar hypotensive effect was noted in several other works on the study of urgent and long-term adaptive effects of sauna bathing [9, 17, 25]. The noted contradictions in relation to the heat-induced dynamics of blood pressure can be associated with differences in the protocols of the PH and dry sauna procedure. As an example, in [9], the authors believed that in the nature of cardiovascular responses to heat stress and the position of the body during PH (sitting/lying) is important. Heating of the head/face and inhalation of hot air are likely to play a significant role in the formation of the stress response [9, 15, 23]. Thus, cooling the face with a ventilator during whole-body passive heat stress changed the hemodynamics in the carotid artery basin [15].
For an integral assessment of cardiometabolic adaptive rearrangements influenced by various temperature stressors, the thermal cardioreactivity and Q10 were proposed, reflecting, respectively, the degree of changes in heart rate and oxygen consumption with a 1°C shift in the human body core temperature [3]. It has been shown that, on average, with an increase in core temperature by 1°C, the thermal reactivity index values are 25–40 beats/min, and Q10 increases reflecting a 7–8% VO2 increase in relation to rest values with significant interindividual variability [26, 27]. It is hypothesized that adaptive thermophysiological effects can manifest themselves in a decrease in the values of these indices; however, in a single work devoted to this problem, it was found that acclimation in heat chambers at a temperature of 25–45°С for 3 weeks did not lead to such changes [27]. We did not determine these indices, but the data calculated for a 1°C increase in body temperature in the initial procedure slightly exceed the results obtained in [3, 27], as well as the level of cardiac reactivity during 30-minute sauna procedures [17].
At the end of the course, during the tenth PH procedure, despite a more pronounced increase in body temperature, the VO2 and EC values were significantly lower than the baseline values, which indicates an adaptive decrease in heat-induced metabolic activity. Similar conclusions were made in [20], where a decrease in EC recorded at rest (but not during heat stress as in our study) after ten PH procedures was also noted. It is logical that the relative indicators of the degree of the VO2 and HR increase for each 1°C rise in body temperature decreased in contrast to the data obtained in [27]. At the same time, the level of energy expenditure and VO2 significantly correlated with the degree of increase in body temperature. One explanation for the above-mentioned adaptive shifts may be a decrease in the degree of heat-induced sympathetic activity, the level of adrenaline and cortisol, which, in particular, was noted in the examination of healthy volunteers after 7 days of adaptation to PH [28]. The increase in body temperature is possibly associated with intense vasodilation. This issue requires additional research.
We did not see any changes in the shifts in the hemodynamic parameter values in response to PH in the course of the procedures; each heat treatment was accompanied by approximately the same levels of tachycardia development and a decrease in SBP. The dynamics of PSI also testifies to stable hemodynamic responses to hyperthermia; its increase was noted in the range of moderate stress in the second part of the PH course, but mainly due to an increase in temperature, whereas the heart rate increase remained at the same level. On the other hand, there is evidence that during regular PH for 2 or more weeks, there is a decrease in both blood pressure and resting heart rate [2, 6, 17] and the degree of HR increase with repeated exercise [22]. According to [7], such dynamics is observed only in physically prepared people (athletes). Healthy untrained volunteers took part in our study. In addition, it is possible that the course of adaptation to PH was not long enough for the formation of stable adaptive hemodynamic rearrangements under heat stress.
An increase in SR confirms the development of adaptation of thermoregulatory mechanisms in the course of PH; during the tenth procedure, its values were on average 40% higher than in the first procedure, which may be associated with an improvement in blood supply to the skin and activation of peripheral thermoreceptors due to significant heating. In addition, the relationship between regulation of the sweating process and metabolism confirms the fact that during heat adaptation, virtually simultaneously with an increase in the sweat rate, the sweat composition changes, in particular, the lactate level decreases [29]. The putative cutaneous vasodilation in PH in the confined space of the capsule may be due to reflex mechanisms, direct exposure to heat, and the formation of humoral regulators. The main stimuli for their release are the shear stress of the endothelium and metabolites, including those formed in the sweat glands [7, 24]. Previously, it was shown that in the process of adaptation to PH, vascular reactivity improves, among other things due to endothelial function, which contributes to a decrease in blood pressure and an increase in peripheral blood flow [1, 7].
CONCLUSIONS
A single PH procedure in an infrared body heating capsule (without affecting the head) lasting 40 min was accompanied by an increase in metabolic rate by an average of 36% compared with the placebo procedure, the development of moderate heat stress, and a decrease in SBP. Conducting ten PH procedures in 2 weeks caused adaptive changes in thermoregulatory mechanisms, which are manifested by the EC increment decrease and the SR increase. We note that the shifts in the systemic hemodynamic parameters remained at the same level. Thus, when using the course of PH procedures in the above mode, healthy volunteers exhibited marked adaptive metabolic changes with no development of a significant stress response of systemic hemodynamics, which indicates the effectiveness and safety of the chosen technique. The results we obtained demonstrate the absence of a direct correlation between the level of energy consumption and indicators of systemic hemodynamics during hyperthermic procedures.
REFERENCES
Cohen, M., Turning up the heat on COVID-19: heat as a therapeutic intervention, F1000Research, 2020, vol. 9, p. 292.
Heathcote, S.L., Hassmén, P., Zhou, S., and Stevens, C.J., Passive heating: reviewing practical heat acclimation strategies for endurance athletes, Front. Physiol., 2018, vol. 9, p. 1851.
Ihsan, M., Périard, J.D., and Racinais, S., Integrating heat training in the rehabilitation toolbox for the injured athlete, Front. Physiol., 2019, vol. 10, p. 1488.
Glazachev, O.S., Kofler, W., Dudnik, E.N., et al., Impact of adaptation to passive hyperthermia on aerobic performance and cardio-respiratory endurance in amateur athletes, Hum. Physiol., 2020, vol. 46, no. 1, p. 66.
Hussain, J. and Cohen, M., Clinical effects of regular dry sauna bathing: a systematic review, Evidence-Based Complementary Altern. Med., 2018, vol. 2018, art. ID 1857413.
Brunt, V.E., Howard, M.J., Francisco, M.A., et al., Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans, J. Physiol., 2016, vol. 594, no. 18, p. 5329.
Gravel, H., Behzadi, P., Cardinal, S., et al., Acute vascular benefits of Finnish sauna bathing in patients with stable coronary artery disease, Can. J. Cardiol., 2021, vol. 37, no. 3, p. 493.
Kampmann, B. and Bröde, P., Heat acclimation does not modify Q10 and thermal cardiac reactivity, Front. Physiol., 2019, vol. 10, p. 1524.
Podstawski, R., Borysławski, K., Clark, C.C.T., et al., Correlations between repeated use of dry sauna for 4 × 10 min, physiological parameters, anthropometric features, and body composition in young sedentary and overweight men: health implications, Biomed. Res. Int., 2019, vol. 2019, art. ID 7535140.
Kim, K., Monroe, J.C., Gavin, T.P., and Roseguini, B.T., Skeletal muscle adaptations to heat therapy, J. Appl. Physiol., 2020, vol. 128, no. 6, p. 1635.
Gryka, D., Pilch, W.B., Czerwińska-Ledwig, O.M., et al., The influence of Finnish sauna treatments on the concentrations of nitric oxide, 3-nitrotyrosine and selected markers of oxidative status in training and non-training men, Int. J. Occup. Med. Environ. Health, 2020, vol. 33, no. 2, p. 173.
Pilch, W., Szyguła, Z., Klimek, A., et al., Changes in the lipid profile of blood serum in women taking sauna baths of various duration, Int. J. Occup. Med. Environ. Health, 2010, vol. 23, no. 2, p. 167.
Bakulin, V.S., Makarov, V.I., Bogomolova, M.M., and Panina, N.G., Physiological criteria for regulation of the level of hyperthermic effects of the sauna, Vestn. Volgograd. Gos. Med. Univ., 2007, vol. 3, no. 23, p. 6.
Glazachev, O.S., Kryzhanovskaya, S.Yu., Dud-nik, E.N., et al., Passive heat acclimation: influence on the subjective quality of life, anxiety, and the level of the brain-derived neurotrophic factor (BDNF), Ross. Fiziol. Zh. im. I.M. Sechenova, 2019, vol. 105, no. 5, p. 544.
Nakata, H., Namba, M., Kakigi, R., and Shibasaki, M., Effects of face/head and whole-body cooling during passive heat stress on human somatosensory processing, Am. J. Physiol.: Regul. Integr. Comp Physiol., 2017, vol. 312, no. 6, p. 996.
Ketelhut, S. and Ketelhut, R.G., The blood pressure and heart rate during sauna bath correspond to cardiac responses during submaximal dynamic exercise, Complementary Ther. Med., 2019, vol. 44, p. 218.
Li, Z., Jiang, W., Chen, Y., et al., Acute and short-term efficacy of sauna treatment on cardiovascular function: a meta-analysis, Eur. J. Cardiovasc. Nurs., 2020, vol. 20, no. 2, p. 96.
Mang, Z.A., Fennel, Z.I., Realzola, R.A., et al., Heat acclimation during low-intensity exercise increases V O2max and Hsp72, but not markers of mitochondrial biogenesis and oxidative phosphorylation in skeletal tissue, Exp. Physiol., 2021, vol. 106, no. 1. P. 290.
Pallubinsky, H., Phielix, E., Dautzenberg, B., et al., Passive exposure to heat improves glucose metabolism in overweight humans, Acta Physiol., 2020, vol. 229, no. 4, p. 1.
Pallubinsky, H., Schellen, L., Kingma, B.R.M., et al., Thermophysiological adaptations to passive mild heat acclimation, Temperature, 2017, vol. 4, no. 2, p. 176.
Moran, D.S., Shitzer, A., and Pandolf, K.B., A physiological strain index to evaluate heat stress, Am. J. Physiol.: Regul. Integr. Comp. Physiol., 1998, vol. 275, no. 1, p. R129.
Périard, J.D., Travers, G.J.S., Racinais, S., and Sawka, M.N., Cardiovascular adaptations supporting human exercise-heat acclimation, Auton. Neurosci., 2016, vol. 196, p. 52.
Zinchuk, V.V. and Zhadko, D.D., The effect of a sauna on blood oxygen transport and the prooxidant-antioxidant balance in untrained subjects, Hum. Physiol., 2012, vol. 38, no. 5, p. 548.
Litvitskii, P.F., Thermal dysbalance of the body: hyperthermia, hyperthermic reactions, heat stroke, and sunstroke, Vopr. Sovrem. Pediatrii, 2010, vol. 9, no. 1, p. 96.
Laukkanen, T., Kunutsor, S.K., Zaccardi, F., et al., Acute effects of sauna bathing on cardiovascular function, J. Hum. Hypertens., 2018, vol. 32, no. 2, p. 129.
Dubé, P.-A., Imbeau, D., Dubeau, D., and Auger, I., Worker heat stress prevention and work metabolism estimation: comparing two assessment methods of the heart rate thermal component, Ergonomics, 2019, vol. 62, no. 8, p. 1066.
Kampmann, B. and Bröde, P., Metabolic costs of physiological heat stress responses—Q10 coefficients relating oxygen consumption to body temperature, Extreme Physiol. Med., 2015, vol. 4, p. A103.
Tomiyama, C., Watanabe, M., Honma, T., et al., The effect of repetitive mild hyperthermia on body temperature, the autonomic nervous system, and innate and adaptive immunity, Biomed. Res., 2015, vol. 36, no. 2, p. 135.
Klous, L., De Ruiter, C., Alkemade, P., et al., Sweat rate and sweat composition during heat acclimation, J. Therm. Biol., 2020, vol. 93, p. 102697.
ACKNOWLEDGMENTS
We are grateful to all the volunteers who participating in the study, as well as Sybaritic Inc. (United States) for the Alfa Basic thermal capsules provided free of charge for hyperthermic procedures. The company and its representatives were not involved in the study design, data collection, analysis and interpretation, and preparation of the publication.
Funding
This work was in part supported by the Russian Foundation for Basic Research, project no. 19-013-00465 A “Direct and Crossover Effects of Adaptation to Systemic Hyperthermia: Influence on the Quality of Life, Neurohormonal and Psychophysiological Status of a Person.”
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the biomedical ethics principles formulated in the 1964 Helsinki Declaration and its later amendments and approved by the local bioethical committee of the Sechenov First Moscow State Medical University (Sechenov University) (Moscow).
Conflict of interests. The authors declare that they have no conflict of interest.
Informed consent. Each study participant provided a voluntary written informed consent signed by him after explaining to him the potential risks and benefits, as well as the nature of the upcoming study.
Additional information
Translated by E. Babchenko
Rights and permissions
About this article
Cite this article
Kryzhanovskaya, S.Y., Zapara, M.A., Dudnik, E.N. et al. Adaptive Changes in the Indicators of Systemic Hemodynamics and Energy Consumption by Young Men during Passive Hyperthermia Procedures. Hum Physiol 48, 161–169 (2022). https://doi.org/10.1134/S0362119722020104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722020104