Abstract
Purpose
This experiment was designed to quantify the independent and combined influences of hyperthermia and dehydration on effector control during rest and exercise.
Methods
To achieve that, whole-body hydration of healthy adults (N = 8) was manipulated into each of three states (euhydrated, 3% and 5% dehydrated), and then clamped within each of two thermal states (normothermia [mean body temperature: 36.1 °C] and moderate hyperthermia [mean body temperature: 38.2 °C]). Those treatment combinations provided six levels of physiological strain, with resting physiological data collected at each level. The effects of isothermal, thermally unclamped and incremental exercise were then investigated in normothermic individuals during each level of hydration.
Results
At rest, dehydration alone reduced urine flows by 83% (3% dehydrated) and 93% (5% dehydrated), while the reduction accompanying euhydrated hyperthermia was 86%. The sensitivities of renal water conservation to 3% dehydration (−21% mOsm−1 kg H2O−1) and moderate hyperthermia (−40% °C−1) were independent and powerful. Evidence was found for different renal mechanisms governing water conservation between those treatments. Cutaneous vasomotor and central cardiac responses were unresponsive to dehydration, but highly sensitive to passive thermal stress. Dehydration did not impair either whole-body or regional sweating during rest or exercise, and not even during incremental cycling to volitional exhaustion.
Conclusion
In all instances, the physiological impact of these thermal- and hydration-state stresses was independently expressed, with no evidence of interactive influences. Renal water-conservation was independently and powerfully modified, exposing possible between-treatment differences in sodium reabsorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans are constantly exchanging matter and energy with our surroundings. Even in basal states, physiological variables are rarely stable, necessitating data averaging to reduce those variations and to quantify the resulting dynamic equilibrium (Cannon 1929; Prosser 1964). During physical activity, those exchanges become more pronounced, particularly when homoeostasis is challenged. Such conditions move experimental participants progressively further away from steady-state conditions, and, in so doing, reduce the opportunity to gather mechanistic physiological information. For example, within the dehydration literature, which is often driven by pragmatic, rather than by mechanistic objectives, one frequently finds that investigators have combined exercise, thermal and dehydration stresses within a single experimental treatment, resulting in simultaneous disturbances of thermal and body-fluid homoeostasis (Adolph 1921; Strydom et al. 1966; Costill et al. 1976; Sawka et al. 1985; González-Alonso et al. 1995). Since those states co-exist in most applications (Sawka et al. 2015; Trangmar and González-Alonso 2019), such combinations of stimuli are not inappropriate, although they can make it difficult to identify causal relationships.
Whilst those stresses activate some common homoeostatic effectors (e.g., cutaneous vasomotor activity), they also induce stress-dependent responses (e.g., elevated alveolar ventilation [exercise], sudomotor activation [thermal] and endocrine responses [dehydration]). Moreover, some stresses can stimulate opposing influences, such as cutaneous vasodilatation during hyperthermia (Rowell et al. 1970; Hales et al. 1979) and vasoconstriction at the onset of exercise (Blair et al. 1961; Bevegård and Shepherd 1966). Indeed, cutaneous vasoconstriction can occur when exercise and whole-body heating are simultaneously imposed (Patterson et al. 1994; Shibasaki et al. 2005). Similarly, during heat stress, body fluids lost as sweat are principally of an extracellular origin, coming from the intravascular and interstitial spaces (Costill et al. 1976; Patterson et al. 2014). During exercise, fluid moves into the intracellular compartment of the active muscles, and is of an intravascular origin (Convertino et al. 1983; Maw et al. 1998). Not surprisingly, mechanistic interpretations can be difficult to unambiguously deduce when stresses are combined, leading to the possibility that another experimental approach may be required.
One solution involves stress separation, such that each stress is imposed in the absence of the others. That approach was partially adopted by González-Alonso et al. (1997), who endeavoured to separate the thermal and hydration stresses, although they used exercise to elevate body temperatures during every trial, thermal stability was only approximated and the control trials were actually mildly hyperthermic states (deep-body temperatures > 38 °C). Dynamic states can also present investigative difficulties, and a solution to that is to study physiological steady-states that are clamped so that the movement and exchange of matter and energy, whilst beyond basal levels, might be more stable. Thus, thermal clamping in animals (Jessen 1981; Mercer and Simon 1984; Gordon et al. 2004) and humans (Patterson et al. 1998; Cotter and Taylor 2005; Burdon et al. 2017) has become a well-established, if not a widely used, technique. On the other hand, only one group appears to have used hydration clamps in humans (van den Heuvel et al. 2017, 2020), although González-Alonso et al. (1997) attempted clamping in one trial.
In the experiment described herein, thermal and hydration clamping were used to explore the physiological impact of those stresses, firstly when independently manipulated at rest, since they may be individually problematic (González-Alonso 1998), and then under conditions in which those stresses were combined. Whilst those stresses are often simultaneously encountered, that is not always the case with chronically sedentary individuals, and dehydration can even occur in the presence of hypothermia. Therefore, to examine the physiological mechanisms activated by each stress, dehydration and hyperthermia were independently investigated. To explore the role of steady-state exercise in normothermic individuals, isothermal exercise was performed. The thermal interaction with exercise was then examined with the thermal clamp terminated, but with the hydration clamp sustained. Finally, incremental exercise was performed to volitional exhaustion in the absence of either clamp.
Methods
Participants
The design of this experiment, which was multi-disciplinary and involved six testing days (~ 9–10 h per day), required individuals who could satisfy several pre-requisites, including adequate visual acuity for cognitive tests (for details, see: van den Heuvel et al. 2017, 2020) and a somewhat high, whole-body sweat rate, so that dehydration (5% body-mass loss) could be achieved within ~ 3.5 h. From the 17 males initially recruited, nine were eliminated (see van den Heuvel et al. 2020), leaving eight individuals (mean age 25 years [standard deviation (SD) 7], height 1.79 m [SD 0.08], body mass 74.3 kg [SD 8.8]). None had a prior history of heat illness, all habitually performed endurance exercise and they were all experienced laboratory-based research participants who had previously undertaken fatiguing exercise in the heat. The resulting trials were conducted over a 12-month period, however, seasonal variations within participants were minimised by limiting the trials for each person to one season. Prior to commencing, participants provided written, informed consent to procedures approved by a Human Research Ethics Committee (University of Wollongong; HE09/373) in accordance with the regulations of the National Health and Medical Research Council (Australia), and in compliance with the Declaration of Helsinki.
Procedures
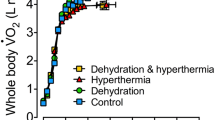
Procedural overview
This experiment relied on the premise that whole-body water losses could be reliably tracked from body-mass changes (Baker et al. 2009), and that, during caloric balance, mass losses would provide a valid index of hydration state over the short term (Cheuvront and Montain 2017). Therefore, participants were kept in caloric balance throughout the preparatory stages (short term) through the provision of pre-selected foods and isotonic drinks (see below). Based on those assumptions, subjects completed six experimental days, serving as their own controls, with at least 7 d between successive trials. On each test day, participants were passively and sequentially treated (whole-body water immersion) to elicit the required hydration- and thermal-state manipulations, yielding six treatment combinations (Fig. 1a), such that each of three clamped hydration states (euhydrated, 3% and 5% dehydrated [sweat induced body-mass reductions; intracellular dehydration or hypohydration]) was combined with each of the two clamped thermal states (normothermia and moderate hyperthermia [mean body temperature]). The physiological responses to those treatments were investigated during 30 min of seated rest in all six combinations (Fig. 1a; Trials A–F). When participants were normothermic, three consecutive exercise stages followed the rest period (Trials A–C). Therefore, every participant contributed to 15 data-collection blocks (Fig. 1a; six resting, plus nine exercising states). To the authors’ knowledge, that level of experimental control and complexity has not previously been used.
Experimental overview. a A model of the three experimental treatments (mean body temperature, hydration-state and physical-activity manipulations). Participants were studied in each of three, clamped hydration states (Y axis): euhydrated (open cubes), 3% dehydrated (cross-hatched cubes) and 5% dehydrated (horizontally lined cubes). Two thermal treatments were induced (X axis), and then clamped at each of two mean body temperature targets: 36.5 °C (normothermia: right side, grey backgrounds) and 38.5 °C (moderate hyperthermia: white backgrounds). The combination of those hydration and thermal states provided six unique trials (coded A–F). Finally, when normothermic (Trials A–C), subjects progressed through a series of four physical-activity levels (Z axis): rest; isothermal, steady-state exercise (A); thermally unclamped, steady-state exercise (B); and incremental exercise to volitional exhaustion (C). b The experimental timeline for this part of the overall experiment (~ 9 h) included two instrumentation phases (diagonally marked bars: A, B), two baseline data-collection periods (black bars: 1 and 2) and the experimental dehydration and thermal treatments (grey bars with wave patterns: 1 and 2). After leaving the climate chamber, subjects were transferred to, and then back from, a second laboratory (wheel-chair), in which cognitive function (van den Heuvel et al. 2017) and electroencephalographic (EEG) data were collected (van den Heuvel et al. 2020). Those periods are indicated by the grey bars (7–8 h). The respective hydration and thermal clamping durations (thick horizontal arrows), as well as the resting and exercising data-acquisition periods are indicated (four rightmost bars; 8.5–9.5 h)
The control condition was euhydrated normothermia (Trial A). Dehydration was induced using passive heating (Fig. 1b, treatment one: intermittent warm-water immersion; van den Heuvel et al. 2017, 2020), and by controlling fluid replacement. Each hydration state was defined on the basis of body-mass changes relative to the pre-experimental mass: euhydrated (normal body mass), 3% and 5% mass reductions. Once attained, those hydration targets were clamped using strictly controlled, isotonic-fluid administration. Two hours following the establishment of each hydration state, either of two, whole-body thermal states was passively induced (treatment two: continuous, whole-body water immersion; van den Heuvel et al. 2017, 2020): normothermia and moderate hyperthermia. In the normothermic trials (Trials A-C), exercise followed resting data collection, with thermal clamping continued to the end of the first (isothermal) exercise period, and with the hydration clamp terminated at the start of incremental exercise (Fig. 1b, exercise C).
Familiarisation and standardisation
Pre-experimental screening and familiarisation, as well as drinking and dietary prescriptions preceding testing, are described elsewhere (van den Heuvel et al. 2017, 2020). Subjects were familiarised with the cycling position (seated behind the ergometer), and completed a four-stage incremental protocol (YMCA cycle-ergometer protocol) to predict peak work rates (mean peak power 397 W) and to standardise the experimental exercise intensities. On each of the three mornings immediately preceding every trial (18 d in total for each person), subjects recorded their own post-void, preprandial body masses. Those data provided a reliable body-mass baseline (Cheuvront et al. 2004) against which dehydration could be estimated from mass changes accompanying thermal sweating. The impact of circadian variations was minimised by testing at the same time of day (within subjects). Over the 12 h preceding each trial, strenuous exercise, alcohol and caffeine were avoided, and eating and drinking instructions were provided (van den Heuvel et al. 2017, 2020).
On presentation, euhydration was verified (urine specific gravity). Once each dehydration target was reached, subjects were provided with controlled volumes of isotonic drinks (sodium chloride at ~ 40 mmol L−1; Merson et al. 2008). To sustain caloric balance, mass-dependent servings of pre-selected foods were provided at fixed intervals throughout the day, but preceded data collection by > 1 h. Finally, once each hydration target was achieved, an upright, seated posture was maintained during the preparatory, thermal treatment and experimental stages to minimise influences on compartmental body-fluid distributions (Harrison 1985; Maw et al. 1995). The exception to this was during body-mass measurements and urine collections. Blood samples were taken before those postural disturbances, with postural control enforced for at least 20 min prior to the next sample.
Experimental protocol
Every trial followed the same timeline (Fig. 1b), which consisted of two instrumentation stages, two experimental treatments (water immersions: hydration and thermal manipulations), two baseline data-collection periods, and four experimental stages (rest [all trials] plus three consecutive exercise blocks [Fig. 1a: Trials A–C only]). Participants arrived at 07:00 h, dressed in a swimming costume, were fitted with a deep-body temperature sensor (auditory canal) and heart rate monitor, and the first baseline data collection commenced. Individualised dehydration protocols followed (treatment one), during which sweat losses were either fully replaced (euhydrated), partially replaced with isotonic drinks (3% dehydration) or not replaced at all (5%). Those hydration targets were then clamped (for details, see van den Heuvel et al. 2020).
Participants then rested in an air-conditioned laboratory (20 °C, 2 h) to ensure recovery back to normothermia and whole-body fluid equilibration (Maw et al. 2000), although the fluid compartments would not be fully restored (Patterson et al. 2014). The second instrumentation now occurred, with additional deep-body temperature (oesophageal and rectal) and skin temperature sensors being positioned, followed by the second baseline period (Fig. 1b). The thermal treatment followed; water immersion (normothermia: 34–35 °C; or hyperthermia: 40–41 °C). Thus, two water immersions occurred in every trial, thereby ensuring that body-fluid redistributions, which accompany hydrostatic loading (Stocks et al. 2004), did not introduce measurement artefacts.
Subjects were immediately transferred (5-m distance) to an adjacent, thermally equilibrated climate chamber (6.0 m long, 3.6 m wide, 2.4 m high), regulated at either 25 °C (normothermia) or 48 °C (hyperthermia). They were rapidly dressed in a water-perfusion garment and insulated clothing. The former was used to clamp each thermal state; the latter minimised heat exchange. At that time (~ 7 h), each person was transferred (wheelchair: < 10 min; Fig. 1b) to another air-conditioned laboratory (20 °C), in which treatment-induced changes in cognitive function (van den Heuvel et al. 2017) and neurophysiological responses were investigated (van den Heuvel et al. 2020). During that time (~ 45 min), the hydration and thermal clamps were retained using isotonic drinks, with heated water being continuously pumped through the perfusion garment. The water temperatures were both trial- and subject-specific, to account for variations in heat loss. After completing those measurements (seated posture), participants were transported back to the climate chamber (wheelchair), the temperature of which was still regulated (25 °C or 48 °C). The insulation and perfusion garments were removed, shoes were donned and subjects sat behind a cycle ergometer (Excaliber Sport, Lode Cycle Ergometer, Lode B.V., Groningen, The Netherlands).
Following seated rest (20 min), which ensured the attainment of a thermal, hydration-state and posture-specific steady state, as well as providing an opportunity to check and adjust the thermal clamp, 10 min of resting data collection commenced (Fig. 1b; ~ 8.5 h). The hyperthermic trials then ended (Fig. 1a; Trials D–F), while the normothermic trials (A-C) continued with three consecutive exercise periods. That progression commenced immediately, with the chamber rapidly cooled to 15 °C (~ 1.0 kPa water vapour pressure). Subjects started steady-state, isothermal and semi-recumbent cycling (20 min at 40% of predicted peak power; Fig. 1b; exercise A). The aim was to investigate the impact of exercise per se, and then to isolate the effects of exercise-induced heat storage from those related to changes in hydration state. To achieve that, the climatic conditions were set so that heat production was matched by heat loss (i.e., cool air, light work rate and a continuous airflow over the subject [industrial fan: 4 m s−1]), thereby allowing normothermia to be sustained. Extensive pilot testing was used to identify the most effective conditions to support such isothermal exercise. Data were collected during the last 10 min of exercise, after which thermal clamping ended (Fig. 1b).
At the commencement of the second (thermally unclamped) exercise block, the chamber was reheated to 25 °C (~ 1.6 kPa water vapour pressure) and the fan was turned off. Participants rested (10 min), and then recommenced steady-state cycling, which consisted of 20 min at 40% of their predicted peak power. Data were collected over the last 10 min, with the hydration clamp maintained (Fig. 1b; exercise B). Finally, and without stopping, subjects progressed into an incremental (ramp) forcing function, with the work rate increased at 10 W min−1, and with verbal encouragement provided, until volitional exhaustion (Fig. 1b; exercise C). Data were recorded throughout incremental exercise and immediately thereafter. Times to volitional failure varied from 8.1 to 20.1 min, averaging 14.6 min (standard deviation [SD] 3.2). Trials finished with supervised recovery and isotonic-fluid consumption equal to the body-mass reduction. Subjects left when rehydrated and after deep-body temperatures had decreased to < 37.5 °C. Additional rehydration fluids were taken home.
These combinations of experimental treatments and clamping facilitated the isolation of the independent thermal (Trial D) and hydration-state influences (Trials B and C) from their combined physiological impact during rest (Trials E and F). Similarly, during exercise, the effects of exercise per se (isothermal exercise) were differentiated from the relatively small thermal influences (isothermal versus thermally unclamped exercise). Finally, the impact of two physiologically significant dehydration states was isolated from the exercise effect (Trial A versus Trials B and C).
Manipulating and clamping whole-body hydration states
Details relating to changing and then clamping the whole-body hydration states are contained elsewhere (van den Heuvel et al. 2020). In brief, three hydration states were induced (Fig. 1a; euhydrated [Trials A and D], and 3% [Trials B and E] and 5% dehydrated [Trials C and F]), using an intermittent immersion protocol unique to each participant (water temperature 39–41 °C; 5° head-toe declined litter with an electronic winch), with body-mass changes tracked. Those protocols were replicated in every trial, with sweat losses replaced as described above. Accordingly, the only difference among those treatments was the fluid-replacement volume, with condition-specific volumes consumed every 15–20 min (< 300 mL isotonic fluid). Across participants, the respective fluid-replacement volumes for Trials A–F were 0.89 L (SD 0.18), 0.22 L (SD 0.06), 0.05 L (SD 0.03), 2.71 L (SD 0.18), 1.95 L (SD 0.35) and 2.06 L (SD 0.34).
Manipulating and clamping mean body temperature
Procedural details for the thermal treatments and clamping are described in our companion manuscript (van den Heuvel et al. 2020). Briefly, those treatments were achieved using seated, head-out immersion (electronic winch) within either normothermic water (34–35 °C) or hyperthermic water (40–41 °C), with the target mean body temperature being 36.5 °C and 38.5 °C (respectively). Immediately following immersion, subjects entered an adjacent chamber, regulated at either 25 °C (normothermia; water vapour pressure 1.58 kPa) or 48 °C (moderate hyperthermia; water vapour pressure 2.23 kPa), and donned a water-perfusion garment (Cool Tubesuit, Med-Eng, Ottawa, Canada and pump: Delta Wing, Med-Eng, Ottawa, Canada; 325 mL min−1) and thermally equilibrated, insulated clothing (thermal protective ensemble). Those ensembles were used to clamp mean body temperature during the neurophysiological data-collection periods, as well as during transportation to and from that laboratory (Fig. 1b).
For the hyperthermic clamp, water pumped through the suit was initially heated to 50 °C (portable water bath: DC210, Med-Eng, Canada; water-bath pump and thermostat: GD120, Grant Instruments, U.K.; 17 mL.min−1), as the water-cooled en route to the skin, and participants immersed both feet in 40 °C water (water bath: Type VFP, Grant instruments, U.K.). Thereafter, adjustments to heat storage were made by changing the water flow and by intermittently removing the feet from the bath. To standardise the experimental conditions, subjects also wore the perfusion suit during non-physiological testing in the normothermic trials, but the water was not heated, the pump was not activated and the feet were not immersed. Finally, upon returning to the climate chamber, additional preparation was completed before commencing resting data collection. Thermal clamping during the hyperthermic trials was assisted using a substantial radiant-heat source (~ 1500 W; three halogen heaters: Thorn Lighting, Wheterill Park, NSW, Australia). During normothermic, isothermal exercise, the ambient conditions were modified and the fan was activated (as described above).
Physiological measurements
Indices of hydration state
Variations in hydration state were estimated using surrogate measures; body mass, urine specific gravity and osmolality (osmotic concentration per unit mass of water), serum osmolality and plasma-volume changes. In the first instance, body mass was recorded over the 3 days preceding every trial (± 0.05 kg: A&D, Model UC-321, CA, U.S.A.). On each experimental day, body mass was measured on five occasions; during both baseline periods, at the start and end of the rest period, and as soon as possible after the incremental exercise ended (Fig. 1b; ± 0.02 kg: A&D, Model Fw-150 k, CA, U.S.A.). In all instances, body mass was measured before urine samples were collected to differentiate between the transcutaneous and renal water losses.
Urine specimens were collected as mid-stream samples and transferred into sterile containers (Sarstedt Australia, Pty. Ltd., Ingle Farm, SA, Australia). Samples were obtained on presentation (baseline one), during baseline two, at the start of the rest period and at the conclusion of incremental exercise (four urine specimens). The specific gravity of each specimen was immediately determined (Clinical refractometer, Model 140, Shibuya Optical, Tokyo, Japan), with samples also transferred to Eppendorf tubes and stored at −80 °C. Those samples were thawed and osmolality determined once the experiment concluded (freezing-point depression; Model 3250 Advanced Osmometer, Advanced Instruments Inc., Norwood, MA, U.S.A.). The measurement variation was 0.2%.
Venous blood samples were collected during both baselines, during rest (but following 20 min of seated rest; ~ 8.5 h), during the final 10 min of exercise periods A and B, and at volitional exhaustion (Fig. 1b). The first sample was drawn from an antecubital vein (21-gauge butterfly needle). Subsequent samples were drawn through an indwelling catheter (20-gauge) positioned in the other antecubital vein, and kept patent using saline flushes. Blood samples were collected into blood-gas tubes (lithium-heparin 50 I.U. heparin mL−1 of blood) and serum vacutainers (Sarstedt Australia Pty. Ltd., Ingle Farm, SA, Australia).
The former samples were used to provide indirect estimates of plasma-volume changes from whole-blood haematocrit and haemoglobin concentration (Strauss et al. 1951; Dill and Costill 1974). Postural changes (Edwards and Harrison 1984; Harrison 1985; Maw et al. 1995) and whole-body water immersion (Gordon et al. 2003; Stocks et al. 2004) can significantly modify body-fluid distributions, and can independently invalidate indirect volume estimates. Therefore, those effects were minimised by standardising the immersion durations and ensuring that at least 20 min of postural stability (seated upright) preceded every blood collection. Samples were immediately analysed, using a portable blood-gas analyser (ABL77, Radiometer Pacific, Melbourne, Australia). Changes in plasma volume were transformed to absolute volumes using the prediction of Pearson et al. (1995). Assumptions concerning the stability of the F-cell ratio were previously validated against radionuclide dilution methods during postural, thermal and exercise manipulations (Maw et al. 1996).
The serum vacutainers were gently rocked to mix the blood with the serum gel, stored vertically while clotting (~ 30 min), then centrifuged (10 min at 20 °C and 2000g), with serum extracted, transferred to Eppendorf tubes and stored at −80 °C. When the experiment concluded, samples were thawed over ice, with serum osmolality determined in duplicate using freezing-point osmometry. If between-sample differences > 2 mOsm kg H2O−1 were found, a third sample was analysed with the two closer values averaged. Assays for each individual were completed on one day (calibration standard: 290 mOsm kg H2O−1), with osmometer repeatability and linearity verified before and after every analysis block. Those procedures resulted in measurement variations of 0.2%.
Body-tissue temperatures
Three indices of deep-body temperature were used. Auditory canal temperature was measured during all preparatory and experimental phases. That index was taken from within the external auditory meatus using an ear-moulded plug, with a protruding thermistor (Edale Instruments Ltd. Cambridge, U.K.). During the treatment stages, those temperatures were used only to monitor changes in the thermal state of each participant, and were not recorded until after baseline two (Fig. 1b). In the second instrumentation stage, an oesophageal thermistor (Edale Instruments Ltd. Cambridge, U.K.) was inserted transnasally and positioned behind the heart (after Mekjavic and Rempel 1990). A rectal thermistor (YSI type 401, Yellow Springs Instruments Co. Ltd., Yellow Springs, OH. U.S.A.) was positioned 10 cm beyond the anal sphincter (Lee et al. 2010). To estimate temperature changes throughout the deep-body tissues, those three deep-body temperatures were averaged (unweighted) to provide a closer approximation of the overall deep-body temperature (Taylor et al. 2014). However, one participant was unable to tolerate the oesophageal probe, so his average deep-body temperature was the simple average of his auditory canal and rectal temperatures.
Mean skin temperature was approximated using an area-weighted summation of eight local temperatures (Hardy and DuBois 1938; forehead, scapula, upper chest, lateral mid-upper arm, dorsal mid-forearm, dorsal hand, anterior mid-thigh and mid-calf). Thermistors were attached with a single layer of waterproof tape (Type EU, Yellow Springs Instruments Co. Ltd., Yellow Springs, OH. U.S.A.). Mean skin temperature was added to the average deep-body temperature using condition-specific weighting coefficients (80% deep-body [normothermia] or 90% deep-body [hyperthermia]; Vallerand et al. 1992), to provide an index of mean body temperature. Temperatures were sampled at 15-s intervals (1206 Series Squirrel, Grant Instruments Pty. Ltd., Cambridge, U.K.), with thermistors calibrated across relevant physiological ranges (stirred water bath) against a certified reference thermometer (± 0.05 °C; Dobros total immersion thermometer, Dobbie Instruments, Sydney, NSW, Australia).
Thermoeffector measurements
Whole-body sweat losses were estimated from mass changes, corrected for fluid intake and urine production, but not for metabolic or respiratory losses. Local sweat rates were measured from four sites (central forehead, chest [central pectoralis], central scapula and dorsal forearm) using ventilated sweat capsules (3.16 cm2) glued to the skin (Collodion U.S.P., Mavidon Medical Products, U.S.A.). Pre-capsular airflows were independently regulated, with relative humidity maintained at 12% by passing room air through an enclosed container holding a saturated lithium-chloride solution. Post-capsular humidities were independently measured (capacitance hygrometers), as were pre- and post-capsular air temperatures (thermistors), with all components forming parts of a sweat-monitor system (Clinical Engineering Solutions, NSW, Australia). Those data were sampled at 1 Hz (DAS1602, Keithley Instruments, Inc., Cleveland, U.S.A.), and used to compute local sweat rates. Hygrometer calibration preceded experimentation (saturated salt-solution standards). Since the sweat capsules were attached during the second instrumentation stage (Fig. 1b), they were continually ventilated (portable air pump) to prevent either water from entering or moisture from accumulating within each capsule. However, during the hyperthermic trials, donning and doffing the perfusion garment and insulated clothing dislodged some capsules. Therefore, during the resting trials, only forehead sweating data are reported. Since capsules were not dislodged during the normothermic trials, all of those data are reported during exercise. Finally, the possibility is noted that the two water immersions, although separated by > 120 min and terminating > 70 min before data collection, might have elicited suppression of the local sweat responses (hidromeiosis). That possibility was neither expected nor evaluated, since the experiment was based on a repeated-measures design, during which subjects acted as their own controls.
Cutaneous (forearm) vasomotor activity was evaluated using both venous-occlusion plethysmography (mL 100 mL tissue−1 min−1) and laser-Doppler flowmetry (arbitrary units). It was assumed the former would reflect blood flows within the cutaneous vasculature (Edholm et al. 1956; Detry et al. 1972). Simultaneously measured skin blood flows were used to provide a comprehensive assessment of vasomotor activity. Both values were normalised to changes in mean arterial pressure, with data expressed in conductance units.
A mercury-in-silastic strain gauge was positioned at the widest girth of the left forearm, with pressure cuffs around the arm and wrist. The distal cuff was inflated to 180 mm Hg prior to measurement, and to minimise measurement artefacts created by variations in hand blood flow. The upper cuff was intermittently inflated to 50 mm Hg (8 s inflated, 12 s deflated; EC 4 Plethysmograph with AG 101 Cuff inflator air source, D.E. Hokanson, Inc., Bellevue, U.S.A.). That cyclical pattern was repeated over 2-min sampling blocks at the end of the rest period, at the end of each steady-state exercise stage and immediately following incremental exercise. In the last instance, data collection followed the cessation of exercise. Laser-Doppler data were simultaneously collected over the same durations from the mid-ulnar, right dorsal forearm (TSI Laserflo BPM2 with a P-435 laser fibre optic probe, Vasamedics Inc., St. Paul, U.S.A.; 37 internal refresh rate ~ 7 Hz). That site provides a representative index of forearm cutaneous blood flow (Cotter et al. 1993). The integrity of the fibre optic probe, and the electrical zero of the system were checked prior to every trial. Both sets of data were collected at 20 Hz (Computer Boards Inc., PPIOA18, Mansfield, OH, U.S.A.).
Other physiological indices
Systolic and diastolic blood pressures were measured from the right brachial artery using an automated monitor (Omron M6 Comfort, Omron Healthcare Co. Ltd., Kyoto, Japan) during both baseline periods, during rest and steady-state exercise, and following volitional exhaustion. Mean arterial pressure was calculated as one-third systolic pressure plus two-thirds diastolic pressure. Heart rates were monitored continuously (15-s intervals; Polar S610i, Polar Electro Oy, Finland).
Sodium and chloride concentrations were obtained from both whole-blood (baseline specimens) and plasma samples (all other data-collection periods), and were quantified during the measurement of haematocrit and haemoglobin concentrations (ABL77, Radiometer Pacific, Melbourne, Australia). For the plasma concentrations, lithium-heparin vacutainers were used during blood sampling (15 I.U. heparin mL−1 of blood). Those samples were gently rocked, stored on ice until the end of each trial, then centrifuged for 10 min (2000g at 20 °C), with plasma extracted and immediately analysed (ABL77, Radiometer Pacific, Melbourne, Australia). Thus, electrolyte concentrations were determined using whole-blood for both baselines, with plasma samples used elsewhere. Plasma electrolyte contents were calculated as the product of each concentration and the corresponding (absolute) plasma volume.
Data analysis
This study was based on a repeated-measures design, with subjects participating in all trials (Fig. 1a, Trials A–F). Analytical and biological variabilities were determined for several key variables, with the former coefficients of variation determined from measurements performed in duplicate (serum and urine osmolality) and triplicate (baseline body mass). Biological variability was evaluated from the intra-individual coefficients of variation (CVI), and used to assess the within-subject, between-trial baseline variability.
To determine the relative impact (at rest) of dehydration and moderate hyperthermia, response sensitivities to those treatments were determined for 12 physiological variables, using a two-step process that would facilitate between-variable, sensitivity comparisons. Step one: Individual raw data were expressed as percentages of the average, variable-specific functional range of each variable, as observed at rest across the six experimental conditions. Those ranges were the difference between the lowest trial means (generally Trial A or zero) and the mean of the trial in which the largest deviation was observed. Step two: Those percentages were then scaled to the corresponding, treatment-induced changes in serum osmolality (Trials B and C: hydration sensitivity) and mean body temperature (Trial D: thermal sensitivity), as observed within each participant, and multiplied by 100 to provide sensitivities that ranged between −100 (treatment-induced reductions) and 100 (elevations) for an osmolality change of 1 mOsm kg H2O−1, or a temperature change of 1 °C. Since serum osmolality and mean body temperature are homoeostatically regulated, then this analysis provided an evaluation of the relative impact of those regulatory processes on those 12 physiological variables.
Following those calculations, the independent effect of dehydration was determined using data from Trials B and C (normothermia with 3% and 5% dehydration), while the independent thermal impact was derived from Trial D (moderate hyperthermia and euhydrated). Since those sensitivity indices were scaled to different variables, statistical comparisons were not performed. To evaluate the impact of the combined (hydration plus thermal) treatment, resting data from Trials E and F were compared with the corresponding values obtained from Trials A and D. The independent physiological impact of exercise was determined from comparisons of data obtained during isothermal and thermally unclamped exercise. Finally, the independent influences of dehydration during exercise were evaluated by comparing the between-trial, but within-exercise stage, physiological responses.
Least-squares, best-fit linear regression analyses were performed to evaluate the relationships between local sweat rates and serum osmolality. Differences between time-dependent data were identified using two-way, repeated-measures Analyses of Variance, followed by Tukey’s HSD post hoc tests to isolate sources of significance differences. Intermittently recorded data were analysed using one-way, repeated-measures Analyses of Variance, and Tukey’s HSD post hoc procedure. Alpha was set at the 0.05 level for each analysis, with data reported as means and standard errors of the means (±), or as standard deviations (SD) for describing data distributions. For non-significant differences, post hoc power analyses were performed to evaluate the strength of the experimental design, with a statistical power > 0.8 (beta < 0.2) interpreted to signify an adequately powered design (Cohen 1988).
Results
Basal states, experimental treatments and the veracity of the clamps
Data from the first baseline period are contained within Table 1 of van den Heuvel et al. (2020). In addition to body-mass stability over the two months of experimentation, those data demonstrate that subjects consistently presented in uniform states of whole-body hydration (urine specific gravity [CVI 0.4%]; P > 0.05), deep-body temperature (auditory canal [CVI 0.6%]; P > 0.05) and central cardiovascular function (heart rate [CVI 9.9%] and mean arterial pressure [CVI 4.6%]; both P > 0.05). Additional baseline data are contained in Table 1 of this manuscript. Collectively, those data are consistent with normal, healthy and euhydrated individuals (Walker et al. 1990; Institute of Medicine 2005; Taylor et al. 2014), confirming that the standardisation procedures yielded consistent body-fluid states before the experimental treatments commenced. It was therefore assumed the hydration- and thermal-state manipulations were superimposed upon states of normal physiological function, such that the treatment effects could be attributed to those treatments, and not to abnormal basal states.
The dehydration treatment yielded uniform body-mass changes, and, when expressed relative to each person’s baseline mass and averaged across participants and thermal states, those changes represented 0.4% (euhydrated), 3.1% and 5.0% body-mass reductions. Within each thermal state, the absolute mass changes differed significantly (Table 2; all P < 0.05), but differences within each hydration state were not significant (all P > 0.05). That is, the hydration states were successfully isolated. Fluid losses during dehydration were primarily due to eccrine sweating, which necessitated intermittent, isotonic fluid consumption to sustain the hydration clamp, and ensure body-mass stability (Tables 1 and 2; all P > 0.05).
Similarly, within each hydration state, significant differences were observed for the average deep-body, mean skin and mean body temperatures between the normothermic and hyperthermic states (Table 2; all P < 0.05). The corresponding, site-specific deep-body temperatures, as well as the mean skin temperatures, are provided in Supplementary Table 1. Across the normothermic trials, mean body temperatures averaged 36.1 °C prior to the resting data collection. During moderate hyperthermia, it was 38.2 °C (P < 0.05). Those temperatures were averaged over the final 10 min of rest, and differ slightly from the values reported in our companion paper (van den Heuvel et al. 2020), which were determined during the neurophysiological data collection (Fig. 1b). Finally, thermal clamping was successful, and mean body temperatures did not vary significantly during periods of clamping within any trial (Fig. 2 and Table 3; P > 0.05). Consequently, markedly different, yet clamped, hydration and thermal states were obtained, which were essential for differentiating between the independent and combined effects of those stresses.
Representative tissue temperatures during, and following, the thermal treatments, during the preparatory and transfer stages before and after the cognitive-function and electroencephalographic (EEG) data collection (van den Heuvel et al. 2020), and during the resting data-collection period (Fig. 1b). Average deep-body temperature is the unweighted average of three deep-body surrogates (oesophageal, auditory canal and rectal), mean skin temperature is an area-weighted average of eight skin temperatures and mean body temperature is a condition-specific weighting of the mean skin temperatures with an unweighted average of three deep-body temperatures (see “Methods”). Thermal treatments involved whole-body water immersion to establish either normothermia or moderate hyperthermia. Mean body temperatures were then clamped before commencing the neurophysiological tests, with those clamps sustained during resting data collection (Trials D-F; Fig. 1a), and until after the isothermal exercise period (Trials A–C; Fig. 1a [exercise A])
Physiological influences of altered body temperatures and hydration states at rest
With the exception of urine production, body-water losses were negligible during normothermic rest (Trial A; Table 2). In the dehydrated, normothermic trials (B and C), urine flows were reduced by 83% and 93% (respectively) relative to the Trial A (both P < 0.05). Euhydrated hyperthermia (Trial D) was accompanied by an 86% reduction in urine production (P < 0.05). That renal compensation occurred over the 4 h following treatment one (Fig. 1b), and mostly during the first 3% of the overall fluid loss. When dehydrated and hyperthermic (Trials E and F), > 98% reductions were observed (Table 2). However, a significant interaction was not observed between the hydration and thermal states (P > 0.05; power > 0.8), which implies independent and powerful influences, although a significant additional reduction in urine flow did occur relative to Trial D (P < 0.05; Table 2).
When normothermic and 3% dehydrated (Trial B), the average sensitivity of renal water conservation to changes in serum osmolality was -21% mOsm−1 kg H2O−1 (Table 4). When 5% dehydrated (Trial C), urine flow was modified by -6% mOsm−1 kg H2O−1. The thermal sensitivity of urine production in Trial D (euhydrated, moderate hyperthermia) was −40% °C−1 (Table 4). In Trials B and D, resting urine flows were reduced to just 3.4 and 3.8 mL min−1 (respectively; Table 2; P < 0.05). Urine was significantly more concentrated in Trials B and C (Table 2; P < 0.05), with the sensitivity of that process being inversely related to water conservation (Table 4). However, that pattern was not evident during hyperthermia (Trial D), with a negligible urine-osmolality sensitivity to changes in mean body temperature (Table 4). This implies a different mechanism for reducing urine production between the hydration and thermal treatments.
Moderate hyperthermia produced a dramatic and indirect elevation in heart rate (Fig. 3a; Trial D), although when normothermic, neither level of dehydration elicited a significant chronotropic response (Trials B and C; P > 0.05; power > 0.8). Even when hyperthermic, the added strain accompanying 3% and 5% dehydration had minimal additional impact (Trials E and F; P > 0.05; power > 0.8). Whilst heart rates were certainly elevated in Trials E and F, > 75% of that elevation was due to the thermal stimulus (Trial D; P < 0.05), with differences between those trials being non-significant (P > 0.05; power > 0.8), and a significant interaction between those treatments was not observed (P > 0.05; power > 0.8). Indeed, the chronotropic sensitivity to heating (Trial D) was more pronounced than its sensitivity to changes in serum osmolality (Table 4; Trial B). Therefore, dehydration and moderate hyperthermia did not behave additively, but independently, with only the latter being expressed in a statistically and physiologically meaningful manner.
Cardiovascular and sudomotor responses during the final 10 min of seated rest, with participants studied in six separate trials, as indicated by the letters on each bar (Trials A-F; N = 8). In each of two clamped thermal states (normothermia [grey bars] and moderate hyperthermia [black bars]), three levels of whole-body hydration were investigated: euhydrated (0%), and 3% and 5% dehydrated. Data are means with standard errors of the means. The horizontal lines on each graph correspond to the means for Trials A (solid) and D (dashed) to facilitate an immediate evaluation of the independent hydration (Trials B and C) and thermal (Trial D) effects, as well as their combined impact (Trials E and F). Significant differences are indicated by the symbols (P < 0.05): * thermoneutral versus hyperthermia within the same hydration state; † euhydrated versus 5% dehydrated in the same thermal state
The cutaneous vasculature is highly thermosensitive, with both cutaneous and forearm vascular conductances revealing exquisite sensitivities to whole-body heating (Trial D; Fig. 3c; Table 4), but not to increased serum osmolality (Trials B and C; Table 4). This is shown in Fig. 3c, with forearm vascular conductance in Trials D-F being more than five-fold greater than observed in the control state (Trial A; all P < 0.05). The hydration-induced conductance changes were not significant in either thermal state (P > 0.05; power > 0.8), and there was no treatment interaction (P > 0.05; power > 0.8).
Resting blood-pressure regulation was not challenged until the most stressful combination of conditions (Fig. 3b). When moderately hyperthermic, mean arterial pressure decreased in a step-wise manner from Trials D to F, although only Trial F differed significantly from Trial D (P < 0.05). None of the other comparisons was significant (P > 0.05; power > 0.8), nor were there significant treatment interactions (P > 0.05; power > 0.8). Since systemic vasomotor activity defends blood pressure, then that linkage is reflected in the thermal sensitivity of those variables (Table 4). Nevertheless, over the functional blood-pressure range, mean arterial pressure displayed the highest sensitivity to changes in serum osmolality (Table 4).
Both whole-body and forehead sweat secretion were highly thermosensitive (Table 4), with significant forehead sweating observed in the hyperthermic trials (Fig. 3d; all P < 0.05). However, significant sudomotor changes were not evident when the hydration state was modified (Trials E and F; all P > 0.05; power > 0.8), and hydration- by thermal-state interactions did not occur (P > 0.05; power > 0.8).
Dehydration significantly reduced the resting plasma volume relative to Trial A (control), with those changes averaging −8.9% (Trial B) and −11.5% (Trial C) during normothermia (Table 2). When euhydrated and moderately hyperthermic (Trial D), a change of −13.9% was observed relative to the control trial (P < 0.05). With each level of added strain (Trials E and F), the plasma volume contracted further, although differences between the 3% (−20.9%) and 5% dehydration levels (−21.9%) were not significant (P > 0.05; power > 0.8). There were no significant interactions between those treatments (P > 0.05; power > 0.8), with the plasma volume being moderately sensitive to the thermal treatment (Table 4).
The total sodium and chloride contents of the plasma were not affected by either experimental treatment (Table 2; all P > 0.05 and power > 0.8), nor were there significant interactions (all P > 0.05 and power > 0.8). However, the electrolyte concentrations were modified due to variations in plasma volume, with significant sodium and chloride concentration changes accompanying 5% dehydration in both thermal states (Table 2; both P < 0.05). No significant interactions were present (P > 0.05; power > 0.8). Moderate hyperthermia-induced non-significant changes in electrolyte concentrations (P > 0.05; power > 0.8), as reflected within the sensitivity indices (Table 4), whilst 5% dehydration increased both concentrations (P < 0.05). Those changes were reflected within the serum osmolality, which was dominated by the dehydration effect (Table 2; P < 0.05), with hyperthermia producing a non-significant reduction (P > 0.05; power > 0.8). There was no treatment interaction (P > 0.05; power > 0.8). Collectively, those changes verified that dehydration had indeed elicited a state of hypertonic hypovolaemia.
Physiological influences of changes in hydration state during exercise
Isothermal exercise
Steady-state, isothermal exercise was not accompanied by changes in mean body temperature, relative to the resting state (P > 0.05). When averaged over Trials A–C, mean body temperature was 36.0 °C at the end of the rest period (SD 0.3), and was unchanged (36.0 °C SD 0.4) during isothermal exercise. Notwithstanding, the corresponding oesophageal temperatures were 37.1 °C (SD 0.3) and 37.6 °C (SD 0.4; P < 0.05), with increases in the auditory canal (0.2 °C) and rectal temperatures (0.3 °C). Therefore, exercise was isothermal only when considered from a mean-body perspective.
The heart rate was significantly elevated when euhydrated (Trial A), and was 52 beats min−1 higher than the corresponding resting phase (Fig. 4a; P < 0.05). That was just 7 beats min−1 greater than during euhydrated, hyperthermic rest (Trial D). Thus, the exercise and thermal stresses imposed equivalent central cardiovascular strain, but for different reasons. Each level of dehydration added to that strain in a stepwise manner, and each by another 12 beats min−1 (± 4) relative to Trials A (3% dehydration) and B (5% dehydration), although only the latter difference was statistically significant (P < 0.05). Significant interactions between exercise and dehydration were absent (P > 0.05; power > 0.8). Mean arterial pressure was also significantly elevated during isothermal exercise (Fig. 4b; P < 0.05). Dehydration-induced influences were not significant (P > 0.05; power > 0.8), as were the exercise by hydration-state interactions (P > 0.05; power > 0.8).
Heart rate (a), mean arterial pressure (b) and plasma-volume responses (c) averaged over the final 10 min of two, steady-state exercise blocks (isothermal and thermally unclamped exercise [20 min each]), and at volitional exhaustion. Those exercise periods occurred consecutively, but with a 10-min rest following isothermal exercise. Data are means (with standard errors of the means) from three trials (N = 8), all of which commenced with subjects in a normothermic state (euhydrated [Trial A, black bars], 3% dehydrated [Trial B], 5% dehydrated [Trial C]). Three horizontal lines are shown on each graph. They correspond to the mean resting values for Trials A (normothermic euhydration (Fig. 3); solid) and D (hyperthermic euhydration (Fig. 3); dashed), and to the isothermal exercise block of Trial A (dotted). Significant differences are indicated by the symbols (P < 0.05): * different from the corresponding resting data in Fig. 3; § euhydrated versus both 3% and 5% dehydrated; † euhydrated versus 5% dehydrated; ‡ isothermal versus incremental exercise; and ¶ thermally unclamped versus incremental exercise
Neither forearm nor cutaneous vasculature conductance was significantly elevated above the corresponding resting stage of any trial (Fig. 5; all P > 0.05; power > 0.8). Moreover, exercise-induced conductances remained well below values accompanying resting hyperthermia (Fig. 5). Dehydration did not exert a significant influence on either variable (all P > 0.05; power > 0.8), and there was no evidence of treatment interactions (all P > 0.05; power > 0.8). Non-thermal sweating seemed to exist at all sites during isothermal exercise (Fig. 6; all P < 0.05), although the deep-body temperatures did increase. Local sweating was not influenced by hydration state (P > 0.05; power > 0.8), and interaction effects were absent (P > 0.05; power > 0.8). Whole-body sweat losses could only be determined intermittently, due to enforced postural control. When averaged across the three exercise periods, those sweat losses were uninfluenced by dehydration (P > 0.05; power > 0.8): Trial A 0.55 L h−1 (± 0.08), Trial B 0.38 L h−1 (± 0.05) and Trial C 0.56 L h−1 (± 0.22).
Forearm (a) and cutaneous vascular conductance (b) during three consecutive exercise periods; the final 10 min of steady-state isothermal and thermally unclamped exercise, and the end of incremental exercise. Data are means (with standard errors of the means) from three trials (N = 8); euhydrated normothermia (Trial A; black bars), 3% dehydrated normothermia (Trial B) and 5% dehydrated normothermia (Trial C). On each graph are three horizontal lines that correspond to the mean resting values for Trials A (normothermic euhydration (Fig. 3); solid) and D (hyperthermic euhydration (Fig. 3); dashed), and to the isothermal exercise block of Trial A (dotted). There were no significant differences across any trial for either dependent variable (P > 0.05)
Local sudomotor responses (forehead (a), scapula (b), chest (c) and forearm (d)) over the final 10 min of isothermal and thermally unclamped (steady-state) exercise, and for the minute preceding volitional exhaustion during incremental exercise. Those exercise periods occurred consecutively, with a 10-min rest following isothermal exercise. Data are means (with standard errors of the means) from three trials (N = 8), each of which commenced with subjects in a normothermic state (euhydrated [Trial A, black bars], 3% dehydrated [Trial B], 5% dehydrated [Trial C]). The dotted horizontal lines represent the means of the isothermal exercise period of Trial A. Significant differences are indicated by the symbols (P < 0.05): ‡ isothermal versus thermally unclamped or incremental exercise; and ¶ thermally unclamped versus incremental exercise
Plasma volume was significantly reduced during all isothermal exercise periods (Fig. 4c), relative to both baseline and resting values (Tables 1 and 2; all P < 0.01). During euhydrated normothermia (Trial A), exercise per se produced a volume change of −275 mL (± 102 [−9.6%]). When dehydration was overlaid, greater reductions were observed relative to the same phase of Trial A (P < 0.05), with a further 283 mL (± 94 [−11.2%] for Trial B) and 327 mL of fluid lost from the plasma (± 100 [−12.5%] for Trial C). Relative to the resting stage of Trial A, those volume changes were now -558 mL (Trial B) and -615 mL (Trial C), with just over 50% being attributable to exercise alone. Relative to the resting phases of Trials B and C, the within-trial volume contractions were −211 mL (± 59) and -223 mL (± 50; respectively). There was, however, no treatment interaction (P > 0.05; power > 0.8).
Those plasma changes were reflected in the serum osmolality elevations, which averaged 273 mOsm kg H2O−1 (± 7; Trial A), 286 mOsm kg H2O−1 (± 2; Trial B) and 290 mOsm kg H2O−1 (± 2; Trial C). The difference between Trials A and C was significant (P < 0.05). Similarly, the respective plasma sodium and chloride concentrations differed significantly between Trials A and C (P < 0.05 for both electrolytes): 139 mmol L−1 (± 1) and 107 mmol L−1 (± 1) for Trial A, 141 mmol L−1 (± 1) and 110 mmol L−1 (± 1) for Trial B and 143 mmol L−1 (± <1) and 111 mmol L−1 (± 1) for Trial C.
Exercise without thermal clamping
When the thermal clamp was released, the average elevation in the mean body and oesophageal temperatures across the three trials was 0.6 °C (± 0.1; P < 0.05). Whilst that was sufficient to activate strong sudomotor responses (Fig. 6), it did not constitute a significant thermal load. A non-significant chronotropic response was evident in Trial A (Fig. 4a; P > 0.05; power > 0.8), while independent hydration influences were apparent in Trials B and C (Fig. 4a), with the latter differing significantly from the euhydrated trial (P < 0.05). Significant exercise and dehydration interactions were not present (P > 0.05; power > 0.8). Mean arterial pressure remained stable, with neither the dehydration-induced changes (P > 0.05; power > 0.8), nor the interaction effects being significant (P > 0.05; power > 0.8).
Forearm and cutaneous vasculature conductance changes were non-significant (all P > 0.05; power > 0.8), with neither the dehydration main effects nor exercise by hydration-state interactions being significant (all P > 0.05; power > 0.8). On the other hand, local sweat rates were evident at all locations (Fig. 6), although significant elevations were only observed at the forehead and scapula (P < 0.05), with three- to four-fold increases relative to isothermal exercise. Those responses were of an entirely thermal origin, with dehydration having no independent influence on local sweat secretion (all P > 0.05; power > 0.8).
A partial restoration of the plasma volume seemed to occur (Fig. 4c), as none of the trial-specific volumes, though still contracted, was significantly reduced relative to the corresponding rest period (all P > 0.05; power > 0.8). However, in Trials B and C, the plasma volume remained significantly smaller than the same exercise phase of Trial A (Fig. 4c; P < 0.05). Those data were matched by increases in serum osmolality, which averaged 277 mOsm kg H2O−1 (± 2; Trial A), 285 mOsm kg H2O−1 (± 2; Trial B) and 290 mOsm kg H2O−1 (± 2; Trial C), with the differences between Trials A and C being significant (P < 0.05). Similarly, the respective plasma sodium and chloride concentrations remained stable, relative to isothermal exercise (all P > 0.05; power > 0.8): 139 mmol L−1 (± 1) and 108 mmol L−1 (± 1; Trial A); 141 mmol L−1 (± 1) and 110 mmol L−1 (± 1; Trial B) and 143 mmol L−1 (± 1) and 111 mmol L−1 (± 1; Trial C). Differences between the euhydrated and 5% dehydrated states were significant for both electrolytes (Trial A versus C; both P < 0.05), but no other comparisons were significant (P > 0.05; power > 0.8).
Unclamped incremental exercise
At the termination of incremental exercise, oesophageal temperature, when averaged across the final minute of Trials A-C, was 38.4 °C (± 0.2; range: 37.5-39.6 °C); an elevation of 1.3 °C (± 0.1) relative to the resting phases. The corresponding averages across the three deep-body indices and the three mean body temperatures were: 38.3 °C (± 0.1) and 36.9 °C (± 0.2; respectively). Times to volitional exhaustion changed inversely with dehydration level (16.54 min [± 0.99; Trial A], 14.86 min [± 1.00; Trial B], 12.51 min [± 1.07; Trial C; all P < 0.05]), as did the peak work rates (324.5 W [± 13.7; Trial A], 307.5 W [± 14.4; Trial B], 284.3 W [± 11.8; Trial C; all P < 0.05]).
Peak heart rates were consistent across the three trials (Fig. 4a), as expected from well-motivated individuals, and between-trial differences and treatment interactions were absent (all P > 0.05; power > 0.8). Conversely, mean arterial pressures (Fig. 4b) measured immediately after exhaustion, declined, and were no longer significantly higher than during the corresponding rest periods (Fig. 3b; all P > 0.05; power > 0.8). Those pressures were also significantly lower than the two preceding exercise periods (P < 0.05). Between-trial differences in forearm and cutaneous vascular conductances were not significant (Fig. 5; P > 0.05; power > 0.8), nor were significant interactions evident (P > 0.05; power > 0.8).
Local sweat secretion increased significantly at three sites (scapula, chest and forearm) during the last minute of incremental exercise (Fig. 6; all P < 0.05). However, the forehead was already sweating at near-maximal levels during the thermally unclamped exercise stage (P > 0.05; power > 0.8). The apparently lower sweating at the scapula and chest when 5% dehydrated (Trial C; Figs. 6b, c) were not significant relative to Trials A or B (P > 0.05; power > 0.8). Indeed, there was no indication of a significant hydration influence on local sweating (P > 0.05; power > 0.8).
Plasma volume was significantly reduced following incremental exercise, relative to the resting stage of each trial, averaging −15.5% across the three trials (Fig. 4c; P < 0.05). Differences between the euhydrated state and both dehydration levels were significant (P < 0.05). Serum osmolality averaged 287 mOsm kg H2O−1 in Trial A (± 4), and then 293 mOsm kg H2O−1 (± 2; Trial B) and 292 mOsm kg H2O−1 (± 4; Trial C), with the only significant difference being between Trials A and C (P < 0.05). To evaluate the possibility that serum osmolality and sudomotor activity were mechanistically linked, as suggested by Sawka et al. (1985), changes in local sweat rates were regressed on osmolality changes, yielding very weak correlations (forehead r2 = 0.07, chest r2 = 0.26, scapula r2 = 0.02 and forearm r2 = 0.07), with only forehead sweating decreasing as osmolality increased. The respective plasma sodium and chloride concentrations were unaffected by incremental, relative to isothermal exercise: 140 mmol L−1 (± 1) and 108 mmol L−1 (± 1; Trial A); 141 mmol L−1 (± 1) and 109 mmol L−1 (± 1; Trial B) and 143 mmol L−1 (± 1) and 111 mmol L−1 (± 1; Trial C). Between-trial differences were not significant (all P > 0.05; power > 0.8), but differences between the euhydrated and 5% dehydrated states were (Trial A versus C; P < 0.05).
Discussion
Each of the six trials for this experiment was undertaken by euhydrated, healthy adults who were uniformly manipulated into the required levels of hydration and thermal stress, that were then clamped to provide markedly different, yet stable, physiological states. The unique aspects of this experiment included the initial separation of those stresses, firstly at rest, and then during exercise, followed by their combined application. The experimental design was sufficiently powered to detect treatment effects (beta < 0.2), should such effects have existed. Whilst much is known concerning the physiological impact of hyperthermia and dehydration (hypohydration), evidence from the current experiment has extended our mechanistic understanding in several ways. Firstly, the separate thermal and dehydration sensitivities obtained at rest, revealed clearer homoeostatic relationships between those stresses and the regulated-variable and controlled-organ (effector) responses. Secondly, both stresses exerted a powerful, but different mechanistic control over renal water conservation. Thirdly, whilst the thermoeffectors were very sensitive to passive thermal stress, the cutaneous vascular and central cardiac responses were generally unresponsive to dehydration, which, during exercise, did not affect either whole-body or regional sweat rates. Finally, it appeared that at least half of plasma-volume reduction seen during combined cycling and dehydration can be attributed to the impact of exercise alone.
Thermal- and hydration-dependent sensitivities were derived during rest for three groups of physiological responses (Table 4; note some reduced sample sizes). To assess the impact of each stress on the controlled tissues and organs (the effectors), response sensitivities were determined for renal water conservation (urine production), cutaneous vasomotion, sudomotion and heart rate. Treatment sensitivities were then derived to reveal the physiological consequences of those altered effector functions (urine osmolality, and plasma sodium and chloride concentrations). Finally, since those effectors support other homoeostatic mechanisms, then the sensitivities of stress-induced changes within mean arterial pressure and plasma volume were also determined.
Within the thermal sensitivity cluster (Table 4), physiological responses fell into three classes. Whilst those classifications are somewhat arbitrary, some variables were more powerfully influenced by the thermal stress. With due regard to the limitations of such analyses, and sample-size reductions for some variables, the most sensitive class was dominated by the control of the following physiological effectors; cutaneous and forearm vascular conductance, whole-body and forehead sweat secretion, heart rate and urine production. Intermediate sensitivities were found for blood-pressure and plasma-volume regulation. The insensitive variables included the composition and osmolality of the plasma and urine, with those sensitivities falling within the “noise” range. On the other hand, dehydration, as reflected in serum osmolality changes (hydration sensitivity), had a moderately powerful influence on just one of the physiological effectors investigated (the kidney [water conservation]), with urine osmolality and production being mechanistically linked (Table 4). Dehydration was strongly associated with blood-pressure regulation, although it is independently regulated. No intermediate class existed within that sensitivity cluster, with a negligible impact evident for the central cardiac, cutaneous vasomotor and the plasma-volume and plasma-composition responses.
These thermal and dehydration stresses independently modified water conservation, which is achieved by regulating the intravascular, extracellular fluid volume (Gauer et al. 1970). Due to the relatively free movement of sodium, chloride and water across semi-permeable membranes, that process results in the regulation of total body water (Nose et al. 1988a, b). The blood volume, systemic blood pressure and osmolality regulatory limbs of that process have unique sensory pathways, but they share the control over some tissues and organs. During dehydration, the reduction in blood volume, primarily from its extracellular component, and the simultaneous elevation in its osmolality, trigger renal and vascular compensatory processes, which also support blood-pressure regulation. Consequently, reduced renal blood flow, glomerular filtration and urine production occur when heat, dehydration and exercise are combined (Adolph 1921; Smith et al. 1952; Kachadorian and Johnson 1970; Hales 1973; Melin et al. 2001). The current changes are in accordance with those observations.
Another novel feature of this experiment is a quantification of the separate sensitivity indices for renal water conservation during the independent thermal and dehydration treatments (Table 4). It appears that, under resting conditions, a 1 °C change in mean body temperature, on its own (Trial D: -40% °C−1), exerted at least as powerful an effect on water conservation as a dehydration-induced rise in serum osmolality of 2 mOsm kg H2O−1 (Trial B: -21% mOsm−1 kg H2O−1). Whilst that similarity could indicate activation of a common control loop, it is more probable that those treatments activated different loops, with evidence of a possible ceiling effect (i.e., minimal further change during 5% dehydration). Consequently, urine was more concentrated following both treatments (Table 2), with that effect being significantly greater when subjects were dehydrated, regardless of their thermal state, and presumably as a consequence of both carotid body and central osmoreceptor integration (da Silva 2019). When dehydrated, urine-osmolality sensitivity was equal and opposite to that of urine production (Table 4), as anticipated.
Large dehydration-induced reductions in urine volume are well established (Adolph 1947; Lee 1964; Costill et al. 1976), and are initiated via osmoreceptor activation, which reduces renal blood flow and glomerular filtration. Those effects are mediated through the renin–angiotensin–aldosterone axis, direct sympathetic activity and the release of antidiuretic hormone (Verney 1947; Andreoli et al. 2000; Sparks et al. 2014; Zimmerman et al. 2017). However, due to an obligatory excretory role, urine production does not cease, resulting in non-linear reductions (Lee 1964), as reflected in a much-reduced sensitivity in Trial C (5% dehydration; Table 4).
Hyperthermia (Trial D) was accompanied by an equally effective reduction in urine production (Table 2), as described previously (Smith et al. 1952; Kanter 1960; Melin et al. 2001), but not with a reciprocal elevation in urine-osmolality sensitivity (Table 4). Presumably, that resulted from a heat-induced redistribution of the central blood volume to the periphery to facilitate heat dissipation (Rowell et al. 1970; Hales et al. 1979). Indeed, Eisman and Rowell (1977) reported changes in renal blood flow of -23.7% °C−1 (deep-body temperature; baboons), with Hales (1973; sheep) and Cham and Badoer (2008; rats) describing similar changes. Those responses are also hormonally mediated (renin-angiotensin; Eisman and Rowell 1977), and involve the hypothalamic, paraventricular nucleus (Cham and Badoer 2008). What is different between the independent hydration (Trials B and C) and thermal treatments (Trial D) was the retention of solutes that contribute to urine osmolality. During hyperthermia (Trial D), urine osmolality at the end of the rest period increased only 2.8-fold relative to the control trial (A; Table 2), but when participants were dehydrated and normothermic, 8.4-fold (Trial B; 3%) and 10.2-fold increments (Trial C; 5%) were observed. Thus, changes in urine osmolality appeared to be insensitive to moderate hyperthermia (Table 4). Presumably, renal, as well as a more generalised central vasoconstriction, was produced via the combined actions of angiotensin and antidiuretic hormone, with that difference being expressed through sodium reabsorption, and its accompanying chloride ions. Accordingly, it is hypothesised that the impact of hyperthermia on aldosterone secretion was more powerful than the effect of 3% dehydration, as urine production was equivalent in Trials B and D (Table 2).
When heat storage moves beyond thermoneutrality, thermoeffectors are activated (Giaja 1938; Hardy and Soderstrom 1938; Taylor and Gordon 2019). Thus, passive heating induced highly sensitive cutaneous vasomotor and sudomotor responses during rest (Trial D), yet the former was insensitive to dehydration during both normothermia and moderate hyperthermia (Fig. 3c, Table 4). That insensitivity indicates that, for resting hyperthermic individuals with water deficits up to 5%, elevations in cutaneous vasomotor activity can be wholly assigned to the independent thermal impact (heat dissipation), with no evidence of dehydration modulation. Since others have described elevated limb blood flow at rest during dehydration (Pearson et al. 2013), then the elevation they described must have been restricted to the intramuscular vascular beds, and was possibly associated with the preceding exercise used during that experiment.
During steady-state and incremental exercise across three levels of hydration, cutaneous perfusion remained equivalent to that observed during normothermic, euhydrated rest (Fig. 5). Indeed, perfusion changes were minimal and non-significant (P > 0.05). What is most apparent was that, relative to the vasodilatation observed during hyperthermic rest (Trial D, average deep-body temperature 38.1 °C), the exercise-associated changes in conductance were much smaller (Fig. 5), presumably due to greater muscle perfusion. Even at the end of maximal exercise, that elevation only attained 60% of the difference in conductance between the control (Trial A) and hyperthermic resting trials (Trial D), yet the average deep-body temperatures were equivalent to Trial D (Trial A 38.2 °C, Trial B 38.3 °C, Trial C 38.3 °C). Moreover, the apparent step-wise decline in conductance following incremental exercise was not supported statistically.
When resting, there was a powerful chronotropic effect of hyperthermia (Fig. 3a; Trial D), with a thermal sensitivity (42% °C−1; Table 4) matching that observed for forearm vascular conductance (46% °C−1). Those variables, along with cutaneous vascular conductance (52% °C−1), are mechanistically linked, and indicate that thermally driven increases in skin blood flow were not fully compensated by a redistribution of the central blood volume, and required a greater cardiac output to sustain mean arterial pressure. However, a linkage between the resting heart rate and changes in hydration-induced serum osmolality was not evident, either when normothermic (Table 4; Trials B and C) or when hyperthermic (Fig. 3a; Trials E and F), and treatment interactions were not observed. Whilst resting blood-pressure regulation was unaffected by dehydration during normothermia (Fig. 3b), when hyperthermic, the added strain of a 5% water deficit significantly reduced blood pressure. That reduction was not centrally compensated (Fig. 3a), although it appeared not to influence either vascular conductance or sweating (Figs. 3c, d). During exercise, between-trial differences in heart rate were only significant between the two hydration extremes (Fig. 4a), and were presumably associated with reductions in ventricular filling pressure and stroke volume (González-Alonso et al. 2000).
During isothermal exercise in the control trial (Trial A), heart rates were elevated by a very similar magnitude (52 beats min−1; Fig. 4a) to those observed at rest when euhydrated and moderately hyperthermic (45 beats min−1; Fig. 3a). Thus, the (passive) thermal and exercise stresses imposed equivalent central cardiovascular strain, which increased in a step-wise manner during both isothermal and thermally unclamped exercise with each level of dehydration (Fig. 4a), although only the difference between Trials A and C was significant. Those changes (12 beats min−1) matched values reported by Costill et al. (1976) for similar body-fluid losses during cycling. At the end of incremental exercise, saturation of the chronotropic response occurred. Under those conditions, it is suggested that cardiovascular insufficiency led to a reduced exercise tolerance (Taylor 2015), brought on by reductions in blood volume and ventricular filling (González-Alonso et al. 2000).
Whole-body and forehead sweat rates were highly sensitive to moderate hyperthermia (Table 4 and Fig. 3d). During isothermal exercise, sweating was minimal (Fig. 6), with powerful thermal influences observed thereafter, but with no evidence for direct or interactive hydration effects (Fig. 6). The same outcome was evident for whole-body sweating. Those observations appear inconsistent with the long-standing assumption that dehydration impairs sweating (Hertzman and Ferguson 1960; Greenleaf and Castle 1971; Fortney et al. 1984; Sawka et al. 1985; Montain et al. 1995), although contradictory evidence has long existed (Ladell 1955; Senay and Christensen 1965; Strydom et al. 1966; Claremont et al. 1976; Kamijo et al. 2005). Clearly, that truism must hold in extreme states, but its universal application lacks unequivocal support. The true test of that hypothesis is whether or not sweating is impaired at the same level of thermal strain and autonomic drive. Evidence from Sawka et al. (1985) seemed to satisfy that requirement, although there was extensive between-trial overlap of the whole-body sweat rates, and isothermal states were neither sought nor obtained.
On the other hand, in the current resting trials, moderate hyperthermia was clamped and thermal strain matched. Under those conditions, neither level of dehydration, which were established and clamped ~ 2 h before the thermal exposure (Fig. 1b), significantly modified forehead sweating (Fig. 3d). Furthermore, during the exercise periods that followed, the average deep-body, mean skin and mean body temperatures were equivalent across all trials (Table 3). Therefore, in our hands, significant dehydration-dependent sudomotor differences were not evident, either across the four local sites or on a whole-body basis (Fig. 6), and not even at volitional exhaustion.
When changes in both sudomotor activity and serum osmolality were simultaneously analysed, evidence for a causal linkage was not revealed. Sawka et al. (1985) reported a similar non-significant outcome, although they observed reduced whole-body sweating with a stronger correlation (r2 = 0.58). That group subsequently demonstrated a dependency of the sudomotor thresholds and sensitivities on plasma osmolality (Montain et al. 1995). Therefore, under conditions involving multiple co-existing, and more powerful stresses that deplete the body-fluid reserves, hydration-dependent reductions in sudomotor activity may occur.
Thermal sweating without fluid replacement affects all body-fluid compartments (Kozlowski and Saltin 1964; Morimoto et al. 1981; Maw et al. 1998; Patterson et al. 2004, 2014), with fluid initially mobilised from the interstitial space, then from the plasma (Sato 1977) and eventually the intracellular compartment (Maw et al. 1998). Since eccrine sweat is hypotonic to plasma (Sato 1977), extended sweating reduces the plasma volume whilst simultaneously elevating its osmolality (Nose et al. 1988b), resulting in hypertonic hypovolaemia (Table 2). The two dehydration treatments modified the normothermic, resting plasma volume by −8.9% (Trial B) and −11.5% (Trial C) relative to the control state (Trial A). Moderate hyperthermia (Trial D) independently reduced the plasma volume of these euhydrated participants (−13.9% relative to Trial A), with that impact being elevated when dehydration was superimposed, now with changes of −20.9% (3% dehydrated) and −21.9% (5% dehydrated), although a significant interaction was not detected.
When exercise is performed, other fluid shifts occur (Maw et al. 1998), with elevations in the intravascular hydrostatic pressure and intramuscular tonicity now causing fluid movement into the active skeletal muscles (Sejersted et al. 1986; Björnberg 1990). During upright exercise, that effect is intensity-dependent (Miles et al. 1983), although in the current experiment, with the exception of the incremental forcing function, the absolute work rates were matched across trials. That haemoconcentration effect was evident in Trial A, where isothermal (normothermic) exercise significantly reduced the plasma volume below its resting level (−9.6%; Fig. 4c). The 3% and 5% dehydration treatments elicited significantly greater volume reductions, although about 50% of those changes can be attributed to the exercise per se (Maw et al. 1998), with those effects remaining during both the thermally unclamped and incremental exercise stages.
Conclusion
Unique combinations of experimental treatments and clamping have enabled the isolation of independent thermal, hydration and exercise influences from their combined physiological impact in both resting and exercising individuals. At rest, the sensitivity of the renal water-conservation mechanism in seated individuals to thermal and dehydration stimuli was both independent and similarly powerful, but a possible difference in the control of sodium reabsorption was identified. Whilst the thermoeffectors were very sensitive to passive thermal stress at rest, the cutaneous vasomotor and central cardiac responses were unresponsive to dehydration. In all instances, the impact of those stresses was independently expressed, and there was no evidence of any interactive influences. The exercise-related outcomes are applicable to normothermic, but significantly dehydrated people who might commence light-moderate physical activity in temperate conditions (40 min), followed by high-intensity exercise (15 min). Under those conditions, dehydration did not impair either whole-body or regional sweating. Finally, it appeared that at least half of plasma-volume reduction seen during combined exercise and dehydration can be attributed to the impact of exercise alone. These novel observations require verification under conditions that would induce significantly greater physiological strain. Whilst stress isolation and clamping do not necessarily reflect applied scenarios, they are important design considerations for the exploration of mechanistic physiology. Moreover, as students of both mechanistic and applied physiology, we must appreciate the need for experimental control when seeking either practical solutions or knowledge expansion.
Abbreviations
- SD:
-
Standard deviation
- CVI :
-
Intra-individual coefficient of variation
References
Adolph EF (1921) The regulation of the water content of the human organism. J Physiol 55:114–132
Adolph EF (1947) Physiology in man in the desert. Interscience, New York
Andreoli, T.E., Reeves, W.B., and Bichet, D.G. (2000). Endocrine control of water balance. Comprehensive Physiology, Supplement 22: Handbook of Physiology, The Endocrine System, Endocrine Regulation of Water and Electrolyte Balance: 530–569
Baker LB, Lang JA, Kenney WL (2009) Change in body mass accurately and reliably predicts change in body water after endurance exercise. Eur J Appl Physiol 105:959–967
Bevegård BS, Shepherd JT (1966) Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. J Appl Physiol 21:123–132
Björnberg J (1990) Forces involved in transcapillary fluid movement in exercising cat skeletal muscle. Acta Physiol Scand 140:221–236
Blair DA, Glover WE, Roddie IC (1961) Vasomotor responses in the human arm during leg exercise. Circ Res 9:264–274
Burdon CA, Tagami K, Park J, Caldwell JN, Taylor NAS (2017) Indirect hand and forearm vasomotion: regional variations in cutaneous thermosensitivity during normothermia and mild hyperthermia. J Therm Biol 65:95–104
Cannon WB (1929) Organisation for physiological homeostasis. Physiol Rev 9:399–431
Cham JL, Badoer E (2008) Hypothalamic paraventricular nucleus is critical for renal vasoconstriction elicited by elevations in body temperature. Am J Physiol 273:F309–F315
Cheuvront SN, Montain SJ (2017) Myths and methodologies: making sense of exercise mass and water balance. Exp Physiol 102:1047–1053
Cheuvront SN, Carter R, Montain SJ, Sawka MN (2004) Daily body mass variability and stability in active men undergoing exercise-heat stress. Int J Sport Nutr Exerc Metab 14:532–540
Claremont AD, Costill DL, Fink W, Van Handel P (1976) Heat tolerance following diuretic induced dehydration. Med Sci Sports 8:239–243
Convertino VA, Keil LC, Greenleaf JE (1983) Plasma volume, renin, and vasopressin responses to graded exercise after training. J Appl Physiol 54:508–514
Costill DL, Coté R, Fink W (1976) Muscle water and electrolytes following varied levels of dehydration in man. J Appl Physiol 40:6–11
Cotter JD, Taylor NAS (2005) The distribution of cutaneous sudomotor and alliesthesial thermosensitivity in mildly heat-stressed humans: an open-loop approach. J Physiol 565:335–345
Cotter JD, Mark AJ, Regan JM, Taylor NAS (1993) Optimal sites for the measurement of human skin blood flow using laser Doppler velocimetry. Proceedings of the Australian Physiological and Pharmacological Society 24:184P
da Silva EF, Bassi M, Menani JV, Colombari DSA, Zoccal DB, Pedrino GR, Colombari E (2019) Carotid bodies contribute to sympathoexcitation induced by acute salt overload. Exp Physiol 104:15–27
Detry JM, Brengelmann GL, Rowell LB, Wyss C (1972) Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol 32:506–511
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Edholm OG, Fox RH, Macpherson RK (1956) The effect of body heating on the circulation in skin and muscle. J Physiol 134:612–619
Edwards RJ, Harrison MH (1984) Intravascular volume and protein responses to running exercise. Med Sci Sports Exerc 16:247–255
Eisman MM, Rowell LB (1977) Renal vascular response to heat stress in baboons - role of renin-angiotensin. J Appl Physiol 43:739–746
Fortney SM, Wenger CB, Bove JR, Nadel ER (1984) Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol 57:1688–1695
Gauer OH, Henry JP, Behn C (1970) The regulation of extracellular fluid volume. Annu Rev Physiol 32:547–595
Giaja J (1938) Homéothermie et thermorégulation I - L’homothermie. Hermann & Cie, Paris
González-Alonso J (1998) Separate and combined influences of dehydration and hyperthermia on cardiovascular responses to exercise. Int J Sports Med 19:S111–S114
González-Alonso J, Mora-Rodríguez R, Below PR, Coyle EF (1995) Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. J Appl Physiol 79:1487–1496
González-Alonso J, Mora-Rodríguez R, Below PR, Coyle EF (1997) Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol 82:1229–1236
González-Alonso J, Mora-Rodríguez R, Coyle EF (2000) Stroke volume during exercise: interaction of environment and hydration. Am J Physiol 278:H321–H330
Gordon CJ, Fogarty AL, Greenleaf JE, Taylor NAS, Stocks JM (2003) Direct and indirect methods for determining plasma volume during thermoneutral and cold-water immersion. Eur J Appl Physiol 89:471–474
Gordon CJ, Haley CD, McLennan PL, Tipton MJ, Mekjavic IB, Taylor NAS (2004) An open-loop model for investigating mammalian thermosensitivity. J Therm Biol 29:703–707
Greenleaf JE, Castle BL (1971) Exercise temperature regulation in man during hypohydration and hyperhydration. J Appl Physiol 30:847–853
Hales JRS (1973) Effects of exposure to hot environments on the regional distribution of blood flow and on cardiorespiratory function in sheep. Pflügers Archiv 344:133–148
Hales JRS, Rowell LB, King RB (1979) Regional distribution of blood flow in awake heat-stressed baboons. Am J Physiol 273:H705–H712
Hardy JD, DuBois EF (1938) Basal metabolism, radiation, convection, and vaporization at temperatures of 22 to 35°C. J Nutr 15:477–497
Hardy JD, Soderstrom GF (1938) Heat loss from the nude body and peripheral blood flow at temperatures of 22°C. to 33°C. J Nutr 16:493–510
Harrison MH (1985) Effects of thermal stress and exercise on blood volume in humans. Physiol Rev 65:149–209
Hertzman AB, Ferguson ID (1960) Failure in temperature regulation during progressive dehydration. United States Armed Forces Med J 11:542–560
Institute of Medicine. (2005). Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. National Academies Press, Washington, DC, U.S.A
Jessen C (1981) Independent clamps of peripheral and central temperatures and their effects on heat production in the goat. J Physiol 311:11–22
Kachadorian WA, Johnson RE (1970) Renal responses to various rates of exercise. J Appl Physiol 28:748–752
Kamijo Y, Okumoto T, Takeno Y, Okazaki K, Inaki M, Masuki S, Nose H (2005) Transient cutaneous vasodilatation and hypotension after drinking in dehydrated and exercising men. J Physiol 568:689–698
Kanter GS (1960) Glomerular filtration and renal plasma flow during hyperthermia. Am J Physiol 198:1044–1048
Kozlowski S, Saltin B (1964) Effect of sweat loss on body fluids. J Appl Physiol 19:1119–1124
Ladell WSS (1955) The effects of water and salt intake upon the performance of men working in hot and humid environments. J Physiol 127:11–46
Lee DHK (1964) Terrestrial animals in dry heat: man in the desert. In: Dill DB, Adolph EF, Wilber CG (eds) Handbook of Physiology, Section 4: Adaptation to the Environment. American Physiological Society, Washington, pp 551–582
Maw GJ, Mackenzie IL, Taylor NAS (1995) Redistribution of body fluids during postural manipulations. Acta Physiol Scand 155:157–163
Maw GJ, Mackenzie IL, Comer DAM, Taylor NAS (1996) Vascular fluid volumes during postural, thermal and exercise stress: methodological comparisons. In: Shapiro Y, Moran DS, Epstein Y (eds) Environmental Ergonomics: Recent Progress and New Frontiers. Freund Publishing House, London, pp 413–416
Maw GJ, Mackenzie IL, Taylor NAS (1998) Human body-fluid distribution during exercise in hot, temperate and cool environments. Acta Physiol Scand 163:297–304
Maw GJ, Mackenzie IL, Taylor NAS (2000) Can skin temperature manipulation, with minimal core temperature change, influence plasma volume in resting humans? Eur J Appl Physiol 81:159–162
Melin B, Koulmann N, Jimenez C, Savourey G, Launay JC, Cottet-Emard JM, Pequignot JM, Allevard AM, Gharib C (2001) Comparison of passive heat or exercise-induced dehydration on renal water and electrolyte excretion: the hormonal involvement. Eur J Appl Physiol 85:250–258
Mercer JB, Simon E (1984) A comparison between total body thermosensitivity and local thermosensitivity in mammals and birds. Pflügers Archiv 400:228–234
Merson SJ, Maughan RJ, Shirreffs SM (2008) Rehydration with drinks differing in sodium concentration and recovery from moderate exercise-induced hypohydration in man. Eur J Appl Physiol 103:585–594
Miles DS, Sawka MN, Glaser RM, Petrofsky JS (1983) Plasma volume shifts during progressive arm and leg exercise. J Appl Physiol 54:491–495
Montain SJ, Latzka WA, Sawka MN (1995) Control of thermoregulatory sweating is altered by hydration level and exercise intensity. J Appl Physiol 79:1434–1439
Morimoto T, Miki K, Nose H, Tanaka Y, Yamada S (1981) Transvascular fluid shift after blood volume and modification in relation to compliances of the total vascular bed and interstitial fluid space. Jpn J Physiol 31:869–878
Nose H, Mack GW, Shi XR, Nadel ER (1988a) Shift in body fluid compartments after dehydration in humans. J Appl Physiol 65:318–324
Nose H, Mack GW, Shi XR, Nadel ER (1988b) Role of osmolality and plasma volume. J Appl Physiol 65:325–331
Patterson MJ, Warlters D, Taylor NAS (1994) Attenuation of the cutaneous blood flow response during combined exercise and heat stress. Eur J Appl Physiol 69:367–369
Patterson MJ, Cotter JD, Taylor NAS (1998) Human sudomotor responses to heating and cooling upper body skin surfaces: differential cutaneous thermal sensitivity. Acta Physiol Scand 163:289–296
Patterson MJ, Stocks JM, Taylor NAS (2004) Sustained and generalised extracellular fluid expansion following heat acclimation. J Physiol 559:327–334
Patterson MJ, Stocks JM, Taylor NAS (2014) Whole-body fluid distribution in humans during dehydration and recovery, before and after humid-heat acclimation induced using controlled hyperthermia. Acta Physiol 210:899–912
Pearson TC, Guthrie DL, Simpson J, Chinn S, Barosi G, Ferrant A, Lewis SM, Najean Y (1995) Interpretation of measured red cell mass and plasma volume in adults: expert panel on radionuclides of the international council for standardization in haematology. Br J Haematol 89:748–756
Pearson J, Kalsi KK, Stöhr EJ, Low DA, Barker H, Ali L, González-Alonso J (2013) Haemodynamic responses to dehydration in the resting and exercising human leg. Eur J Appl Physiol 113:1499–1509
Prosser, C.L. (1964). Perspectives of adaptation: theoretical aspects. In: Dill, D.B., and Adolph, E.F. (Editors). Handbook of physiology. Section 4. Adaptation to the environment. American Physiological Society, Washington, D.C. Pp. 11-25
Rowell LB, Brengelmann GL, Blackmon JR, Murray JA (1970) Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol 28:415–420
Sato K (1977) The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol 79:51–131
Sawka MN, Young AJ, Francesconi RP, Muza SR, Pandolf KB (1985) Thermoregulatory and blood responses during exercise at graded hypohydration levels. J Appl Physiol 59:1394–1401
Sawka MN, Cheuvront SN, Kenefick RW (2015) Hypohydration and human performance: impact of environment and physiological mechanisms. Sports Med 45:S51–S60
Sejersted OM, Vøllestad NK, Medbø JI (1986) Muscle fluid and electrolyte balance during and following exercise. Acta Physiol Scand 556:119–127
Senay LC, Christensen ML (1965) Cutaneous circulation during dehydration and heat stress. J Appl Physiol 20:278–282
Shibasaki M, Secher NH, Johnson JM, Crandall CG (2005) Central command and the cutaneous vascular response to isometric exercise in heated humans. J Physiol 565:667–673
Smith JH, Robinson S, Pearcy M (1952) Renal responses to exercise, heat and dehydration. J Appl Physiol 4:659–665
Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM (2014) Classical renin-angiotensin system in kidney physiology. Comprehensive Physiol 4:1201–1228
Stocks JM, Patterson MJ, Hyde DE, Jenkins AB, Mittleman KD, Taylor NAS (2004) Effects of immersion water temperature on whole-body fluid distribution in humans. Acta Physiol Scand 182:3–10
Strauss MB, Davis RK, Rosenbaum JD, Rossmeisl EC (1951) “Water diuresis” produced during recumbency by intravenous infusion of isotonic saline solution. J Clin Invest 30:862–868
Strydom NB, Wyndham CH, van Graan CH, Holdsworth LD, Morrison JF (1966) The influence of water restriction on the performance of men during a prolonged march. S Afr Med J 40:539–544
Taylor NAS (2015) Overwhelming physiological regulation through personal protection. J Strength Cond Res 29:S111–S118
Taylor NAS, Gordon CJ (2019) The origin, significance and plasticity of the thermoeffector thresholds: extrapolation between humans and laboratory rodents. J Therm Biol 85:102397
Taylor NAS, Tipton MJ, Kenny GP (2014) Considerations for the measurement of core, skin and mean body temperatures. J Therm Biol 46:72–101
Trangmar SJ, González-Alonso J (2019) Heat, hydration and the human brain, heart and skeletal muscles. Sports Med 49:S69–S85
Vallerand AL, Savourey G, Hanniquet A, Bittel JHM (1992) How should body heat storage be determined in humans: by thermometry or calorimetry? Eur J Appl Physiol 65:286–294
van den Heuvel AMJ, Haberley BJ, Hoyle DJR, Taylor NAS, Croft RJ (2017) The independent influences of heat strain and dehydration upon cognition. Eur J Appl Physiol 117:1025–1037
van den Heuvel AMJ, Haberley BJ, Hoyle DJR, Taylor NAS, Croft RJ (2020) Hyperthermia, but not dehydration, alters electrical activity of the brain. Eur J Appl Physiol. https://doi.org/10.1007/s00421-020-04492-5
Verney, E.B. (1947). The antidiuretic hormone and the factors which determine its release. Proceedings of the Royal Society of London. Series B, Biological Sciences. 135:25-106
Walker HK, Hall WD, Hurst JW (eds) (1990) Clinical methods: The history, physical, and laboratory examinations, 3rd edn. Butterworths, Boston
Zimmerman CA, Leib DE, Knight ZA (2017) Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci 18:459–469
Acknowledgements
The participants within this investigation performed above and beyond all reasonable expectations, and the authors are very appreciative of their commitment and generosity. AMJvdH held an Australian Post-Graduate Award from the University of Wollongong (Australia) throughout this investigation. NAST was supported during the writing of this manuscript by the Brain Pool Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and Information, Communication and Technology (Grant number: 2019H1D3A2A01061171). No other funding was provided by agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AMJvdH, NAST and RJC designed and planned this research, and were involved in data analysis and all phases of manuscript preparation. AMJvdH ran the experiments and was also responsible for data collection. BJH and DJRH were essential participants in subject preparation, data collection, laboratory operations and manuscript preparation. GEP assisted with pilot testing, experimental design, data collection and analysis, and manuscript writing. All authors read and approved the final version of the manuscript and its submission for publication, and agree to be accountable for this work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship have been listed as authors.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Communicated by George Havenith.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van den Heuvel, A.M.J., Haberley, B.J., Hoyle, D.J.R. et al. Hyperthermia and dehydration: their independent and combined influences on physiological function during rest and exercise. Eur J Appl Physiol 120, 2813–2834 (2020). https://doi.org/10.1007/s00421-020-04493-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04493-4