Abstract
Investigations on the coactivation of antagonist muscles was for the first time carried out in combination with noninvasive electrical stimulation of spinal cord. Transcutaneous electrical stimulation (TES) of the dorsal roots of spinal cord was performed in healthy volunteers during their walk on a treadmill, purposefully activating the motor pools of flexor muscles during the swing phase and those of extensor muscles during the stance phase. This type of stimulation altered the kinematics of gait. The muscle coactivation index (CI) values obtained for the swing and stance phases during walking including stimulation were compared with the values obtained during walking without stimulation. It has been shown that TES increases the CI values for the thigh muscles by ~10% and those for the ankle muscles by ~5%. This stimulation also alters the correlation between the CI values for the thigh and ankle muscles, depending on the gait cycle phases. With TES applied during the stance phase, a significant difference was recorded between the CI values for the thigh and ankle muscles during the stance phase, but this difference was absent during walking without stimulation. With TES used during the swing phase, no difference was recorded between the CI values for the ankle muscles during the swing phase and the CI values for the ankle muscles during the stance phase, whereas the indicated difference was present during walking without stimulation, i.e., during the swing phase, the ankle joint was recorded in a not fully extended position left in it from the stance phase. If the walking was accompanied by alternating stimulation of the flexor motor pools during the swing phase and the extensor motor pools during the stance phase, the stiffness of the ankle joint during the swing phase was the same as during the stance phase. Therefore, both the value and the direction of the indicated changes in CI depend on the spatiotemporal parameters of stimulation and correlate with the gait alterations caused by the targeted stimulation of the dorsal roots of spinal cord.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Muscle coactivation is a synchronous contraction of antagonist muscles generating pulling force at the same joint. The index of muscle coactivation (CI) is usually calculated per gait cycle. The concept of coactivation is discussed within the biomechanics of movement and in terms of participation of different brain structures in the locomotion control. An increase in coactivation is associated with an increase in joint stiffness; and it is now accepted that coactivation is controlled at the supraspinal level [1]. A number of studies have demonstrated that targeted rehabilitation measures within the therapy of patients with motor impairments contribute to reductions in the CI values for muscles providing movements [1]. For example, a significant decrease in the CI values for the thigh and ankle muscles and a significant increase in the range of movements for the joints of lower limbs were recorded in children with cerebral palsy after a combined course of transcutaneous electrical stimulation (TES) of the spinal cord and locomotor mechanotherapy [2].
We have reports on investigations carried out in healthy volunteers during the development of “neuroprosthesis,” a device and technology to support the walking of post-stroke patients. The application of TES to the spinal cord roots provided the targeted spatiotemporal activation of motor pools, including flexor muscles during the swing phase and extensor muscles during the stance phase [3]. The application of TES to flexors during walking on a treadmill helped to raise the foot significantly higher over the support surface, while TES of extensors reduced the stance phase length and increased the frequency of steps.
The purpose of this study was to determine, using the TES method, the effect of targeted spatiotemporal activation of motor pools on the coactivation of antagonist muscles during a steady walk by the targeted impact of these muscles during the stance and swing phases.
METHODS
The study participants were represented by a group of healthy volunteers (n = 6). All subjects were male (mean age, ~25 years). The investigations were conducted at the Research Institute for the Problems of Sports and Physical Culture, Velikie Luki State Academy of Physical Culture and Sports (VLSAPhC). During the investigations, the subjects had to walk on a treadmill (h/p/cosmos gaitway®) at a speed of ~1.5 km/h. After a 15-s walk, the experimenters initiated TES of the spinal right dorsal root associated with the motor pool of extensor muscles (activation of extensors) during the right foot stance phase, but this stimulation was stopped after 15 s, and the experimenters initiated TES of the spinal right dorsal root associated with the motor pool of flexor muscles (activation of flexors) during the right foot swing phase, and after 15 s, the alternating TES of extensors and flexors was initiated during the stance and swing phases of the right foot, respectively (Fig. 1). The investigations were repeated in the same order, stimulating the roots on the left side, depending on the gait phases in the left lower limb.
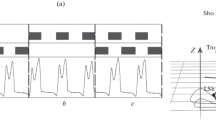
Principle of spatiotemporal transcutaneous stimulation of the spinal cord in the neuroprosthesis. (a) The scheme for the positions of cathodes and the succession of TES in time. White circles, inactive cathodes; grey circles, cathodes active during the swing (Th11–12) and stance phase (L1–2) of the ipsilateral foot; the dashed line, the axial line of the spine; Th11–12 and L1–2, vertebrae. (b) The TES algorithm on the example of the last 15 s of the investigation and the principle of the EMG analysis on the example of m. tibialis anterior (ТА); TES, depending on the gait cycle phase determined by shifts of the fifth foot toe (the curve under the electrodes); EMG is inverted and normalized to 100% (the dotted curve ТА, a), then the envelope curve was constructed (b); EMG is synchronized with kinematics data (c, stance phase; d, swing phase); subject D.G., walking speed 1.5 km/h, the right foot.
The possibility of activating the motor nuclei in flexors and extensors in a targeted manner, using epidural spinal cord stimulation, was shown in one study with the participation of patients with spinal injuries [4]. It has also been demonstrated in studies with the participation of healthy volunteers that TES applied to the points located 2 cm to the right or to the left of the central spinal axis at the level of vertebrae L1–L2 increased the force of extension at the knee and ankle joints on the side of stimulation [5]. To perform TES, 4 cathodes were positioned on the skin (ø 3 cm): (1) for activating extensors, ~2.5 cm to the right and to the left of the spinal axis along the course of spinal roots between vertebrae L1–L2, and (2) for activating flexors, ~2.5 cm to the right and to the left of the spinal column axis along the course of spinal roots between vertebrae T11–T12 (Fig. 1а). Two anodes (5 × 10 cm2) were symmetrically positioned on the skin over the crests of iliac bones. A BioStim or NeoStim-5 stimulator (Cosyma, Russia) was used for this stimulation. TES was performed using modulated monopolar current pulses (duration 1 ms, modulation 5 kHz) at frequencies 15 Hz (activation of extensors) and 30 Hz (activation of flexors). The currents used were within the 15–90 мА range. Both the materials and the TES method were described in detail in [3].
TES of the roots was performed at the T11–12 and L1–2 levels during the swing and stance phases of the ipsilateral leg, respectively. To detect the gait cycle phases, gyroscope sensors, including a tree-axial gyroscope and three-axial accelerator within the same microscheme LSM6DSL (ST Microelectronics, Switzerland), a microcontroller MSP430F5510 (Texas Instruments, USA), a radiomodule, and an accumulator, were fixed to the anterior thigh surfaces with elastic straps. The moment of putting the foot to the support surface and lifting it off was determined by the change of the sign in the hip angular velocity derivative recorded by the gyroscope.
To record the kinematic characteristics of lower limb movements, a Qualisys videosystem (Sweden) was used. The surface EMG of the knee joint flexor and extensor was recorded on both legs from the ankle muscles (mm. tibialis anterior (TA) and gastrocnemius lateralis (GL)), while the surface EMG of ankle joint flexor and extensor was recorded on both legs from the thigh muscles (mm. biceps femoris (BF) and vastus lateralis (VL)). A telemetric electroneuromyograph (ME 6000, MegaWin, Finland) was used in the investigations. EMG signals were synchronized with stimulation marks and video signals. To analyze EMGs, the gait cycle phases were determined by the shifts of the reflector marker fixed on shoes over the fifth toe. The materials and methods of EMG and the video images of walking, as well as their synchronization, were described in detail in [6].
The EMGs were noised by stimulation artefacts, due to TES of spinal roots. All EMG data, including also those recorded without TES, were filtered using the original software. The operational principle of the filter is based on searching a pattern characteristic of the stimulation artefact and replacing the EMG fragment containing a crosstalk for the dynamic average. At the same time, the variational EMG series remained almost intact. EMGs were further treated and analyzed in the RStudio environment. The EMG-activity signals were inverted into the positive region and normalized, and the envelope curve was constructed, EMGs were synchronized for each gait cycle phase, and the normalized EMG average was calculated (Fig. 1b) [7]. The envelope curve was constructed using the method of sliding average (window 60) and normalized using the method of scaling to 0 : 100. The normalized envelope curve of each cycle was approximated by 100 points using the linear interpolation method [8].
Since there is no golden standard for the calculations of CI [1], two well-known CI equations were used: Rudolph’s modified CI(1) equation [9, 10] and the CI(2) equation proposed by Latash et al. [1].
where \(EM{{G}_{H}}\) is the higher average activity in the pair of muscles, and \(EM{{G}_{L}}\) is the lower average activity. CI1 is averaged to the phase of gait (j = 1 : 200). The low CI(1) values indicate both the high activation level of one muscle synchronously with the low activation level in the other muscle of the pair and the low activation level in both muscles during the analyzed time period, while the high CI(1) values correspond to the high activity of both muscles in the analyzed time period [9].
where \(\int {EMG\,{\text{flexor}}} \) and \(\int {EMG\,{\text{extensor}}} \) are the normalized integrals for the muscle activities of the flexor and extensor. The minimal of the two values is taken per phase in the numerator and divided by the value of a combined activity per phase. The 0.5 value corresponds to the maximal CI and 0 corresponds to the absence of coactivation [1]. The CI(1) values were normalized to 1 to compare with the CI(2) values.
In contrast to CI(1), the CI(2) equation is nonsensitive to the average muscle activity level, since high CI(2) values reflect only a synchronous activity of muscles in the compared pair, while low values represent a low level in the coactivation of muscles in the pair. However, if the difference between the average activity of muscles in the compared pair is not too big, the percentage of randomness in the event the activity of what muscle will be taken in the numerator CI(2) increases, and therefore, the variability of values calculated using CI(2) equation will be high. The latter drawback is absent in the CI(1) equation.
In all phases, the ratio between the hip flexor and extensor (BF and VL), the ankle flexor and extensor (TA and GL) was calculated by the average EMG value per phase. The data obtained with TES of the right- and left-sided roots were analyzed together. To determine the root activation effect, the relative difference (Δ) was determined between the CIs in walking with TES and without TES for each investigation (pairwise difference, n = 15). The statistical analysis was performed, using Student’s t-test for paired samples. Differences were considered significant at p < 0.05.
RESULTS AND DISCUSSION
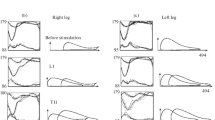
Muscle coactivation during walking without stimulation. The CI values for walking without TES calculated by both equations were given in Figs. 2a, 2b. Irrespective of the preferred method of calculation, we have found that the coactivation of ankle muscles during the swing phase was significantly lower than the coactivation of thigh muscles during this phase; and the coactivation of the ankle and thigh muscles did not differ during the stance phase. The coactivation of ankle muscles during the swing phase was significantly lower than during the stance phase; and the CI values for thigh muscles did not depend on the phase of gait.
The results of analogous investigations on the coactivation of ankle and thigh muscles, depending on the gait phase, during the treadmill walking of healthy subjects were published in [11]. It has also been shown that the CIs for the thigh muscles were higher than the CIs for the ankle muscles and that the CI values for the ankle muscles during the stance phase were higher than during the swing phase. However, the CI values for the thigh muscles in [11], as well as those of the ankle muscles, depended on the phase of gait and were higher during the stance phase than during the swing phase. The cause of differences was probably due to the fact that the subjects described in [11] walked at a speed of ~4.5 km/h, whereas the walking speed in our study was three times lower. Working on a treadmill at lower speed may possibly be associated with the reduced amplitude of movements at the knee joint, since the CI values for the thigh muscles (the knee stiffness indicator) during the swing phase do not decrease, compared with the CI values during the stance phase. Based on the analysis of CI changes depending on the gait cycle phases at different walking speeds, we may suggest that the voluntary regulation of walking speed takes place at the cost of controlling the range of movements at the knee joint.
Coactivation of muscles during walking with the activation of extensors during the stance phase. Whereas the CI values calculated for the thigh and ankle muscles, using the CI(1) equation, during the stance phase with TES at the L1 level were significantly higher than those for walking without TES during the stance phase (Fig. 3a), the positive increase in the corresponding values calculated using the CI(2) equation proved to be unreliable (Fig. 3b). The correlations calculated by the CI(1) and CI(2) equations between the CI values for walking with TES at the L1 level, considering the phase, remained the same as for walking without TES (Figs. 2a–2d). With the CI values calculated by the CI(1) equation during the stance phase (with TES), the averaged CI values for ankle muscles were significantly lower than the CI values averaged for thigh muscles (Fig. 2c), but this difference was unobserved in the absence of TES (Fig. 2a). The same correlation between the CI values during the stance phase was earlier observed in healthy subjects during their walk on a treadmill at a higher speed than under our investigations [11]. The proportion of the CI(1) values for ankle and thigh muscles, corresponding to the proportion obtained in [11], reflects an increased frequency of steps (a decrease in the stance phase length at a constant walking speed) with TES at the L1 level in the stance phase [3]. Irrespective of the preferred equation for calculations, the CI values for the swing phase with TES at the L1 level did not significantly differ during the stance phase from those during the swing phase for walking without TES (Fig. 3), which indirectly demonstrates the accuracy of detecting gait phases by the neuroprosthesis [3].
Coactivation of antagonists muscles during treadmill walking. Coactivation indices (CIs) calculated using the CI(1), CI(2), during the stance and the swing phases during walking (а, b) without TES, (c, d) with TES at the L1 level during the stance phase, (e, f) during walking with TES at the T11 level during the swing phase and (g, h) with alternating stimulation at the L1 level during the stance phase and at the T11 level during the swing phase, as in Fig. 1b. TA/GL and VL/B, are the CIs of the corresponding muscle pair. n = 15. Mean values and confidence intervals are shown. * p < 0.05.
Effect of spatiotemporal transcutaneous stimulation of the spinal cord on the muscle coactivation. (а, b) Difference (Δ) between the relative change in CI during walking with TES and without TES, which were calculated using the CI(1) and CI(2) equations, the pairwise comparison; L1, TES at the L1 level during the stance phase; T1, TES at the T11 level during the swing phase; L1+T1, TES at the L1 level during the stance phase and at the T11 level during the swing phase (Fig. 1b); TA/GL and VL/BF are the CIs of the corresponding muscle pair. n = 15. Mean values and confidence intervals are shown. * p < 0.05.
Coactivation of muscles during walking with the activation of flexors during the swing phase. With TES at the Т11 level during the swing phase, the CIs for the thigh and ankle muscles were significantly higher than those during the swing phase without TES, according to the CI(1)-based calculations (Fig. 3a), but according to the CI(2) calculations, the increase in the values was significant for ankle muscles and insignificant for the thigh muscles (Fig. 3b). The significant increase in the CI values calculated, using both equations, demonstrates that the ankle joint stiffness increased when motor nuclei of flexor muscles were activated during the swing phase. The correlations between the CI values calculated using both CI equations for walking with TES at the Т11 level differed from those observed during walking without TES; i.e., the difference is absent between the CI values of ankle muscles during the stance and swing phases; and the CI values for the ankle muscles with TES during the swing phase are the same as during the stance phase without TES (Figs. 2e, 2f). Therefore, with TES of the flexor roots during the swing phase, the ankle joint stiffness during this phase is the same, as during the stance phase without stimulation, i.e., with this method of TES, the ankle joint was recorded during the leg swing in a not fully extended position, i.e., in the same position as it was observed during the preceding stance phase without stimulation. The activation of the motor nuclei of flexor muscles during the swing phase in post-stroke patients may probably help to deal with the characteristic foot drop problem in the gait of these patients. The CIs during the stance phase with this method of TES did not significantly differ from those during the stance phase of walking without TES (Fig. 3), which once again proved the accuracy of detecting gait phases by the neuroprosthesis. This is an important result, since a variety of technical devices and methods were used in both CI calculations and the neuroprosthesis for the detection of gait phases.
Muscle coactivation during walking with the activation of flexors during the swing phase and extensors during the stance phase. When walking was accompanied by TES at the L1 level during the stance phase and by TES at the Т11 level during the swing phase (as in Fig. 1b), the CI values calculated using CI(1) for the ankle and thigh muscles during the stance phase were significantly higher than the corresponding values during walking without stimulation, and the CI(1) values for the ankle muscles during the swing phase also significantly differed from the corresponding values without TES (Fig. 3a). No significant differences have been observed in the calculations performed using the CI(2) equation (Fig. 3b). According to the CI(1) calculations, the CI values for the ankle muscles were significantly lower than the CI values for the thigh muscles during both the swing and the stance phases, and no significant differences have been found between the CI values of the ankle muscles obtained during the stance and swing phases for walking without TES (Figs. 2a, 2g). The correlations calculated by the CI(2) equation between the CI values for the thigh and ankle muscles during phases of walking with TES were the same as those for walking without TES (Figs. 2b, 2h).
No significant differences have been found between the CI values by the paired comparison of TES during either the stance or the swing phase: L1/T11, L1/(L1 + T11), T11/(L1 + T11). The absence of differences was associated with the phenomenon that stimulation used during one of the gait cycle phases, changing the kinematics of movement in this phase, caused also compensational changes in the kinematics of the other phase, since the walking speed was constant and specified by the treadmill velocity.
The CI(1) values for the stance phase with TES of the roots of extensor muscles during the stance phase and during the swing phase with TES of the roots of flexor muscles during the swing phase were significantly higher than the CI(1) values during the corresponding phases of walking without TES for both the ankle and the thigh muscles (Fig. 3a). The increase in the CI(1) value reached 5–7% for ankle muscles, and 11–12% for thigh muscles. As has been noted in [9], the high CI(1) values correspond to the high averaged activity of both muscles in the analyzed pair. TES of the roots of extensor muscles has been confirmed to increase the amplitude of the reflector response of extensor muscles on the side of stimulation [5], as has been shown in patients with spinal impairments, the epidural electrical stimulation of the spinal cord segments, where the nuclei of flexor muscles are located, increases the activity of flexor muscles, as well as the stimulation of the spinal cord segments where the nuclei of extensor muscles are located increases the activity of flexor muscles for extensors [4]. The CI(2) values with TES in the same conditions increased insignificantly (Fig. 3b); this is associated with high variability of values calculated using this equation, which is manifested in a small difference between the EMG amplitudes in the compared muscles (see the Methods). Therefore, changes in the CI(2) values with TES indirectly indicate that during stimulation of the roots the activity of muscles in the analyzed pairs differed insignificantly. The increased average activity of antagonist muscles may possibly be associated with the crosstalk phenomenon, i.e., a possible transfer of excitation between neighboring muscles by surface electrodes during EMG recording [12]. On the one side, the majority of ankle muscles during the subjects’ walking at different speeds has been shown that the difference between the results of recording by the methods of surface and intramuscular EMG is minimal or insignificant; i.e., the crosstalk phenomenon is absent [13, 14]. On the other side, surface EMG in the same conditions recorded the presence of activity for m. soleus and m. tibialis anterior, whereas the intramuscular EMG demonstrated the absence of activity [14], and the authors of the study made the conclusion that the results of surface EMG of these muscles had to be carefully interpreted. Therefore, it is not excluded that the crosstalk phenomenon between the neighboring muscles made its contribution to the recorded increase in the coactivation values of antagonist muscles, which we recorded using TES.
CONCLUSIONS
The results of investigations on the coactivation of antagonist muscles in a given phase during the targeted activation of the investigated muscles using TES of the motor pools of extensor muscles during the stance phase have demonstrated that the coactivation of ankle muscles was significantly lower than the coactivation of thigh muscles, which could not be observed without stimulation. Based on the earlier published data, we can suggest that this was associated with the recorded increase in the stepping frequency (a reduction in the step length at constant walking speed [3]).
TES at the level of the motor pools of flexor muscles during the swing phase has shown that the coactivation of ankle muscles during the swing phase was the same as during the stance phase of walking without stimulation. Therefore, due to this targeted TES, the ankle joint was recorded during the swing phase in a not fully extended position, in the same in which it was during the stance phase without stimulation.
The ankle joint stiffness remained the same during both the swing and the stance phases of walking accompanied by alternating TES of the motor pools of flexor muscles during the swing phase and the motor pools of extensor muscles during the stance phase.
Irrespective of the coactivation calculation technique, the CI values during the swing phase with TES during the stance phase did not significantly differ from the CI values during the swing phase of walking without TES and, on the contrary, with TES during the swing phase did not significantly differ from CI values during the stance phase of walking without TES. This demonstrates the accuracy of technology for detecting the gait cycle phases by the neuroprosthesis.
Coactivation of antagonist muscles has been determined for the first time during movements accompanied by noninvasive electrical stimulation of the spinal cord and an increase, compared with movements without stimulation, has been for the first time recorded in the coactivation of all investigated muscle pairs. Therefore, a further research should be conducted to find if this is associated with excitation crosstalk between muscles or with an increase in the stiffness of joints, since the increased stiffness of joints is associated with the risks of falling and gait disorders [11].
REFERENCES
Latash, M.L., Muscle coactivation: definitions, mechanisms, and functions, J. Neurophysiol., 2018, vol. 120, no. 1, p. 88.
Solopova, I.A., Sukhotina, I.A., Zhvansky, D.S., et al., Effects of spinal cord stimulation on motor functions in children with cerebral palsy, Neurosci. Lett., 2017, vol. 639, p. 192.
Gorodnichev, R.M., Pukhov, A.M., Moiseev, S.A., et al., Regulation of gait cycle phases during noninvasive electrical stimulation of the spinal cord, Hum. Physiol., 2021, vol. 47, no. 1, p. 60.
Wagner, F.B., Mignardot, J.B., Le Goff-Mignardot, C.G., et al., Targeted neurotechnology restores walking in humans with spinal cord injury, Nature, 2018, vol. 563, no. 7729, p. 65.
Calvert, J.S., Manson, G.A., Grahn, P.J., and Sayenko, D.G., Preferential activation of spinal sensorimotor networks via lateralized transcutaneous spinal stimulation in neurologically intact humans, J. Neurophysiol., 2019, vol. 122, no. 5, p. 2111.
Gerasimenko, Y., Gad, P., Sayenko, D., et al., Integration of sensory, spinal, and volitional descending inputs in regulation of human locomotion, J. Neurophysiol., 2016, vol. 116, no. 1, p. 98.
Guerrero, J.E. and Macias-Diaz, J.A., Biosignal EMG: tools for electromyogram signals (EMG) analysis, R Package, 2015. https://CRAN.R-project.org/package=biosignalEMG.
Becker, R., The New S Language, Boca Raton, FL: CRC Press, 2018. ISBN 9781315895888
Rudolph, K.S., Axe, M.J., and Snyder-Mackler, L., Dynamic stability after ACL injury: who can hop? Knee Surg., Sports Traumatol., Arthroscopy, 2000, vol. 8, no. 5, p. 262.
Martino, G., Ivanenko, Y.P., Serrao, M., et al., Locomotor patterns in cerebellar ataxia, J. Neurophysiol., 2014, vol. 112, no. 11, p. 2810.
Acuña, S.A., Francis, C.A., Franz, J.R., and Thelen, D.G., The effects of cognitive load and optical flow on antagonist leg muscle coactivation during walking for young and older adults, J. Electromyogr. Kinesiol., 2019, vol. 44, p. 8.
De Luca, C.J. and Merletti, R., Surface myoelectric signal cross-talk among muscles of the leg, Electroencephalogr. Clin. Neurophysiol., 1988, vol. 69, no. 6, p. 568.
Vieira, T.M., Botter, A., Muceli, S., and Farina, D., Specificity of surface EMG recordings for gastrocnemius during upright standing, Sci. Rep., 2017, vol. 7, no. 1, p. 1.
Péter, A., Andersson, E., Hegyi, A., et al., Comparing surface and fine-wire electromyography activity of low-er leg muscles at different walking speeds, Front. Physiol., 2019, vol. 10, art. ID 1283. https://doi.org/10.3389/fphys.2019.01283
ACKNOWLEDGMENTS
The authors thank R.М. Gorodnichev, А.М. Pukhov, Е.А. Pivovarova, S.М. Ivanov, and V.V. Markevich for their aid in the experimental work.
Funding
The research study no. 2/2019 of 01.12.2019 in collaboration with Cosyma company was supported in the framework of the project Development of a Neurostimulating Device for Regulating the Locomotor Functions in Patients with Complications of Acute Cerebral Circulatory Dysfunction and by Basic Research Program no. 1.42 of the Presidium of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All studies were conducted in correspondence with the principles of biomedical ethics stipulated under the 1964 Helsinki Declaration and its subsequent revisions and approved by the Bioethics Committee of VLSAPhC (Velikie Luki, Russia).
INFORMED CONSENT
Each participant of the study gave his voluntary informed consent signed by him after informing about potential risks and gains, as well as about the nature of the forthcoming investigations.
CONFLICT OF INTERESTS
The authors declare the absence of obvious and potential conflicts of interests associated with the publication of this article.
Additional information
Translated by N. Tarasyuk
Rights and permissions
About this article
Cite this article
Moshonkina, T.R., Shandybina, N.D., Moiseev, S.A. et al. Muscle Coactivation Phenomenon in the Modulation of Walking by Electrical Stimulation of the Spinal Cord. Hum Physiol 47, 175–182 (2021). https://doi.org/10.1134/S0362119721020092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119721020092