The article proposes an adsorption technology for the treatment of salt wastewater and concentrate of reverse osmosis plants to remove sulfates and chlorides by multi-tonnage waste from the energy industry — chemical water treatment sludge formed at the wastewater pre-treatment stage. Curves of adsorption of sulfate and chloride ions by granular sorption material in dynamic conditions were obtained. According to the Shilov equation, the time and coefficient of protective action of the loading layer during adsorption purification from sulfate and chloride ions under dynamic conditions were calculated. The basic technological scheme of the pilot wastewater treatment plant and reverse osmosis concentrates of the plant at PJSC Nizhnekamskneftekhim is proposed. The economic benefit and prevented environmental damages implemented using this technology are calculated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
A number of industrial enterprises face the problem of the treatment of salt wastewater after chemical desalting and reverse osmosis. All chemically contaminated effluents (not only from electroplating and pickling sites, but also effluents from thermal stations, boiler houses, machining shops and other industries) are sent to neutralization stations to create pH = 6.5–8.5 and precipitate heavy metals from salt wastewater. As a result, a large amount of highly salinized sewage of increased hardness (up to 15 g/L) and salinity is formed, which makes it impossible not only to return to the circulating cycle, but also to discharge it to the city collector. The characteristic composition of the average chemical desalination flow is presented in Table 1.
The currently used technologies for the treatment of salt wastewater are characterized by complexity, as well as high capital, energy and operating costs. Reagent treatment (the most common method of wastewater treatment) does not yield wastewater salinity values necessary for returning water to production.
This paper proposes an adsorption technology for the purification of saline wastewater and concentrate from reverse osmosis plants for removing sulfate and chloride ions by a multi-tonnage waste of the energy industry —chemical water treatment sludge formed at the wastewater pre-treatment stage.
Chemical water treatment sludge (CWTS) is waste from the processes of liming and coagulation at water treatment plants of thermal power plants. In experimental studies, carbonate sludge (moisture 3%) from Kazan CHP-1 was used. The dried sludge is a fine light yellow to brown powder. The chemical composition of the sludge was determined by X-ray diffraction analysis of the sludge on a D8 ADVANCE diffractometer (Bruker): calcite (CaCO3) — 73%; Brucite (Mg(OH)2) — 8%; Portlandite (Ca(OH)2) — < 1%; quartz (SiO2) — 0.4%; remainder (other substances) — 17.6%.
In [2], carbonate sludge is considered as a sorption material for industrial wastewater purification from heavy metal ions Fe3+, Cr3+, Zn2+, Cu2+, Ni2+.
This article discusses the fundamental possibility of purification of saline wastewater and reverse osmosis concentrate to remove sulfate and chloride ions by carbonate sludge (CWTS). Previously [3], the adsorption capacity of the sludge was determined experimentally with respect to these ions, adsorption isotherms of sulfate ions and chloride ions were constructed, and the effect of pH on the adsorption of sulfate ions and chloride ions was also shown [3].
The adsorption isotherms for sulfate ions and chloride ions are H-type Smith-class isotherms and describe the chemisorption process. The high values of the enthalpy of adsorption can be considered due to the formation of a strong bond between sulfate ions, chloride ions and functional groups of the sludge as a result of the following chemical reaction:
In practice, ion-exchange filters with granular loading are used for wastewater desalination at industrial plants. Adsorption in dynamic conditions has significant technological, operational and economic advantages (compared with adsorption in static conditions) and provides the possibility of utilizing the adsorption capacity of the sorbent more fully. Therefore, granular sorption material (GSM) was developed on the basis of fine sludge.
To obtain granules, fine slurry with a particle size of 0.01–0.09 mm is mixed with liquid sodium glass at a mass and volume ratio of 2 : 1, respectively (this ratio was chosen experimentally: with a smaller ratio, the sludge does not get fully saturated with the liquid sodium glass, and the granules fall apart during the subsequent firing; with a larger ratio, the use of excess binder is wasteful). The mixture is brought to homogeneity, then hand-rolled to obtain granules, the granules are kept in an oven for 3 hrs at 400 °C [4]. Next the granules are cooled to room temperature in a desiccator. Characteristics of granules are the following: size 0.5–2.5 mm; abrasion resistance 78%; hydrophilicity is medium.
Technological characteristics of the obtained GSM are the following: adsorption capacity for sulfate ions 130 mg/g; adsorption capacity for chloride ions 116 mg/g; total pore volume 0.592 cm3/g; specific surface area 46.2 m2/g.
For production processes, adsorption of impurities in dynamic conditions is most important. The process of dynamic adsorption of sulfate ions and chloride ions by a hydrophobic sorption material with a fraction of 0.5–2.5 mm was studied in a laboratory setup (filter column 2.5 cm in diameter): the concentration of model solutions of Na2SO4 and NaCl 300 mg/dm3; the height of the GSM loading layer 20 cm; weight 54.38 g; filtration rate 3.5 m/hr.
Equal volumes of model solutions were passed through the GSM loading in portions of 1 dm3. Breakthrough of sulfate ions was observed around 20 mg/dm3 and appeared in the filtrate at passing 120.6 dm3 of the model solution of Na2SO4. Full saturation of the GSM occurred after passing 162.3 dm3 of solution. The process of chemisorption was stopped when the concentration of sulfate ions in the filtrate became equal to the initial concentration of sulfate ions at the entrance to the filter. The values of dynamic (DEC) and total exchange capacity (TEC) of GSM with respect to sulfate ions were: DEC = 665.3 mg/g; TEC = 895.4 mg/g.
Fig. 1 shows the output curve of the adsorption of sulfate ions by a granular sorption material under dynamic conditions.
A similar experiment was conducted with a model solution of NaCl. The breakthrough of chloride ion was observed at 10 mg/dm3 and appeared in the filtrate after passing 240.4 dm3 of a model NaCl solution. Full saturation of GSM occurred after passing 292.3 dm3 of solution. The process of chemisorption was stopped when the concentration of chloride ions in the filtrate became equal to the initial concentration of chloride ions at the entrance to the filter. The values of the dynamic and total exchange capacity of GSM with respect to chloride ions were:
Fig. 2 shows the output curve of adsorption of chloride ions by a granular sorption material under dynamic conditions.
The time τ and the coefficient K of protective action of the GSM loading layer during adsorption purification to remove sulfate ions and chloride ions under dynamic conditions were calculated according to the Shilov equation [5]: for sulfate ions: τ = 69.2 hr, K = 354.8 hr/m; for chloride ions: τ = 130.4 hr, K = 707.3 hr/m;
where τ is the time of the protective action of the GSM loading layer, hr; K is the coefficient of protective action of GSM loading layer, hr/m; L is the height of the GSM loading layer, m; τ0 is the loss of the external protective effect of the GSM loading layer, hr.
The quality indicators of the filtrate are determined by passing different volumes of model solutions through the GSM loading under dynamic conditions. The indicators satisfy MPC standards for substances in drinking and household water reservoirs (SanPin 2.1.4.10749-01), which indicates that the sorption material does not introduce secondary pollution to water bodies (Table 2).
Water passed through the GSM was subjected to biotesting for acute toxicity for fish Poecillia reticulate Pet. and crustaceans Daphnia magna Str. The results obtained confirmed that the GSM is class V in terms of environmental hazard (practically non-hazardous).
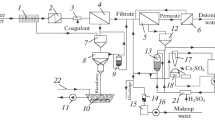
The use of granulated sorption material for purification of reverse osmosis concentrate produced by a plant included in the pilot scheme of purification of conditionally clean wastewater of PJSC Nizhnekamskneftekhim (Fig. 3) is proposed.
Principal flow chart of a pilot plant for the treatment of wastewater from PJSC Nizhnekamskneftekhim including the production line for granulated sorption material: 1 — mechanical purification unit; 2 — coagulation and disinfection unit; 3 — ultrafiltration unit; 4 — reverse osmosis unit; 5 — adsorption filter; 6 — sorbent supply bunker; 7 — storage bin of the finished sorbent; 8 — drying chamber; 9 — muffle furnace; 10 — granulator-mixer.
At the pilot plant, five process steps are carried out sequentially: mechanical purification; coagulation; disinfection; ultrafiltration; reverse osmosis.
The plant capacity is 24.7 m3/hr.
After mechanical purification, the waste water is sent to a tank for coagulation and disinfection.
Coagulants (aluminum oxychloride and sodium hypochlorite) are fed into the pipeline by a dosing pump, the dose of coagulants is determined depending on the quality of incoming water. Then the wastewater enters the ultrafiltration node UFS-M and fine water purification from mechanical impurities is performed. To prevent biofouling, membranes are washed with chemical reagents.
The scheme for the production of granulated sorption material (see Fig. 3, positions 6–10) includes the following operations:
– granulation — mixing sludge with a binder (liquid sodium glass) in a granulator-mixer TL-080. The size of the obtained granules is 0.5–2.5 mm;
– calcination of granules in a chamber furnace PKO-1.2–200 at a temperature of 400 °C for 3 hrs;
– drying of the impregnated granules in a PE-0041 drying cabinet at a temperature of 150 °C to a constant mass. After drying, the granules enter the storage bin of the finished sorption material.
The economic efficiency of the proposed technology was calculated by comparing the costs and expenses of PJSC Nizhnekamskneftekhim. Environmental damage, preventable by using the treatment of salt wastewater and reverse osmosis concentrate by carbonate sludge, was calculated.
Also estimated are energy costs, capital costs (including equipment costs, prices as of December 2018) and environmental damage prevented by adsorption purification of reverse osmosis concentrate to remove sulfate ions and chloride ions by carbonate sludge:
Prevented environmental damage for sulfate ions, thousand rubles/year ................... | 228 |
Prevented environmental damage for chloride ions, thousand rubles/year ................. | 236 |
Capital costs, thousand rubles .................................................................................... | 470 |
Material costs, rub ....................................................................................................... | 2386 |
Energy costs, rubles/day. (for the production of 420 kg of material) ......................... | 516 |
Conclusions
An adsorption technology was proposed for the purification of salt wastewater and reverse osmosis concentrate to remove sulfate ions and chloride ions using multi-tonnage waste from the energy industry — sludge from chemical treatment of water generated at the stage of wastewater pre-treatment.
Curves of adsorption of sulfate ions and chloride ions by a granular sorption material under dynamic conditions were obtained.
The time and coefficient of protective action of the granular sorption material loading layer are calculated according to the Shilov equation.
A schematic flow chart of the pilot plant for the treatment of wastewater and reverse osmosis concentrates at PJSC Nizhnekamskneftekhim is presented.
The economic effect and environmental damage prevented by the introduction of this technology are calculated.
References
O. V. Peshcherova and N. S. Popov, “A method for improving the energy efficiency of a reverse osmosis plant used in water desalination processes” [in Russian], Usp. Khim. Khim. Tekhnol., 28, No. 4 (153), 86–90 (2014).
T. G. Lupeiko, E. M. Bayan, and M. O. Gorbunova, “Study of technogenic carbonate-reducing waste for the purification of aqueous solutions from nickel (II) ions” [in Russian], Zh. Prikl. Khim., 77, No. 1, 87–91 (2004).
L. A. Nikolaeva, E. N. Borodai, and M. A. Golubchikov, “Sorption properties of sludge clarifiers in the treatment of wastewater from power plants to remove petroleum products” [in Russian], Izv. VUZov, Problemy Energetiki, No. 1–2, 132–137 (2011).
L. A. Nikolaeva and M. A. Golubchikov, “Investigation of oil product sorption of power plants by modified sludge from TPP clarifiers” [in Russian], Teploenergetika, No. 5, 59–62 (2012).
A. G. Kasatkin, Basic Processes and Devices of Chemical Technology [in Russian], TID “Alliance”, Moscow (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimicheskoe i Neftegazovoe Mashinostroenie, Vol. 55, No. 5, pp. 43–46, May, 2019.
Rights and permissions
About this article
Cite this article
Nikolaeva, L.A., Khamitova, É.G. The Use of Energy Industry Waste as Sorption Material in the Purification of Reverse Osmosis Concentrate. Chem Petrol Eng 55, 427–432 (2019). https://doi.org/10.1007/s10556-019-00640-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10556-019-00640-7