Abstract
The results of an analysis of mathematical models for a continuous fermentation process for lactic acid production have been presented. Mathematical models are represented by equations with the kinetics of the formation of lactic acid for strains of the following types: strains that consume only the main substrate (most frequently, glucose) and do not form by-products; strains that form by-products; strains that consume not only the main substrate, but also the substrate that forms during synthesis from the corresponding component of raw materials; and strains that use both the substrate from the component that forms during synthesis and simultaneously form a by-product in sufficiently large amounts. Kinetic relationships take into account inhibition by biomass (X), a substrate (S), and a product (P). The resulting relationships for the concentrations of the products (in the general case, X, S, P, and B) have been presented, and, most importantly, inlet characteristics (in the general case, D, S0, and M0) that ensure the real implementation of the technology have been estimated. Computational relationships and algorithms for the calculation of characteristics in different variants of the formulation of problems have been given. It has been pointed out that, in most solutions, there is the possibility of obtaining characteristics that ensure a set of implementing technologies. Data on the sources of raw materials used in published scientific studies have been presented. A table with algorithms for calculating the characteristics of steady states and numerical examples of calculating the characteristics of steady states have been given.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
A study of the kinetics of a biotechnological process for lactic acid production and an analysis of a batch fermentation method [1, 2] have shown that researchers have great interest in the synthesis process with the use of strains that produce lactic acid.
In [3], it is pointed out that lactic acid is considered one of the most useful products used in the food, textile, pharmaceutical, and chemical industries as a raw material for the production of propylene glycol, acrylic acid, acetaldehyde, etc. Interest in lactic acid has increased due to the possibility of using it as a monomer for the production of biodegradable polymers (PLA).
The potential of using lactic acid for the manufacture of various products was shown in [4].
An extensive study of achievements in the field of lactic acid production by microbiological fermentation was presented in [5]. The article contains 326 scientific references. The main sections of the article are as follows: (1) Introduction, (2) Microbial Lactic Acid Producers, (3) Alternative Fermentation Substrates for Lactic Acid Production, (4) Advances in Fermentation Processes for Enhanced Lactic Acid Production, and (5) Improved Lactic Acid Fermentation with High Cell Density.
Naturally, the selection of microorganisms is the key element determining the biotechnology of the process. A list of microorganisms that produce lactic acid was presented in [6]. The list is quite impressive (61 items). The types of substrates consumed by microorganisms were also given in this article. However, as will be shown below, naturally, not all of the listed microorganisms have attracted attention for the purpose of developing a biotechnological process.
Ensuring the possibility of developing a biotechnological process for lactic acid production is associated with selecting the sources of raw materials with a comparatively low cost. Raw materials with a comparatively low cost for the production of lactic acid are listed in Table 1 [3]. Data on productivity attained in cultivation using different types of raw materials are also given in this table. Naturally, Table 1 does not exhaust the complete list of possible raw materials for the production of lactic acid.
One important component for developing the technology of the process is the construction of equations for the kinetics of the process that determines the rate of the growth of a population of microorganisms and, as a consequence, the intensity of the formation of lactic acid. Kinetic relationships were presented in the review [1], and they are not considered in this study. It should only be noted that, most frequently, the form of kinetic relationships also determines the mathematical expressions of a particular mathematical model for a continuous process, since the conditions of inhibition for a particular strain of microorganisms are taken into account in some kinetic relationships and they are not taken into account in others.
The mathematical models of continuous fermentation processes can be represented by the three groups determined by the types of strains: a group of strains of microorganisms that consume only the main substrate (most frequently, glucose) and do not form by-products (or an insignificant amount of them); a group of strains of microorganisms that form a by-product which is sometimes quite valuable in a sufficiently large amount; and a group of strains of microorganisms that use, in addition to the main substrate, the components of raw materials that produce the main substrate during synthesis (with and without the formation of by-products).
Some other features of the mathematical modeling of continuous fermentation should also be pointed out. First and foremost, this is the necessity of obtaining the estimates of inlet characteristics that ensure the possibility of the real implementation of the process. Another feature is that the results of modeling make it possible to obtain a set of the estimates of inlet characteristics for the same value of productivity with respect to lactic acid QP (g/(L h)), where QP = PD; P is the concentration of lactic acid, g/L; and D is the dilution rate, h–1. There is the possibility of obtaining estimates for optimal conditions: maxQP and others.
MATHEMATICAL MODELING OF CONTINUOUS FERMENTATION
A system of equations of a mathematical model for the process that pertains to the first group for strains of microorganisms that consume only the main substrate and do not form by-products has the following form:
System of equations (1)–(3) was presented in several studies cited below.
The calculation of the characteristics of the process based on the solution to (1)–(3) depends on the form of the relationship for the specific growth rate μ. A sufficiently complete review of the forms of kinetic relationships for μ was presented in [1, 2], and it is not given here, except for the variants that are used in the present review. One of the first relationships for μ was derived in [25] and has the following form:
A more complex relationship was used in [26]:
However, relationship (5), as well as (4), takes into account inhibition only by the concentration of substrate S, and it is very seldom used for the modeling of the continuous process.
The equation for the specific growth rate in the following form is most frequently used in modeling:
in which inhibition by the concentrations of the substrate S and the product P (lactic acid) is taken into account.
A list of constants with numerical values [27, 28], which were used in most numerical calculations in modeling, is given in Table 2.
STEADY STATES
An analytical solution to system of equations (1)−(3), (6) was presented in [29–31]. Block diagrams of algorithms for calculating the characteristics of steady states in different variants of the formulation of problems were also given in these publications. In the generalized form, these algorithms are given in Table 3.
In addition to initial data, constants from Table 2 were used in algorithms presented in Table 3. Numerical examples of the implementation of algorithms were given in [29–31].
The graphical interpretation of steady states was presented in [29, 31–35].
The following features were pointed out:
The extremal character of the dependences of the productivity QP (g/(L h)) on Sf at a given value of D and on D at a given value of Sf, as well as concentration P (g/L) on Sf at a given value of D;
The presence of the multiplicity of steady states for the same value of QP;
The limiting value of the dilution rate D = Dlim (h–1) at which the substrate is washed out from the fermenter without having time to enter into the synthesis process.
This emphasizes that there is the boundedness of the range of values for the inlet characteristics Sf and D that ensure the real conditions of the existence of the technology.
The following two characteristics relate to constructing the region of real implementation of the technology and are very important for estimating the capabilities of the technological process: Dlim and QP.
The calculation of Dlim was presented in [32, 33] using the following equation:
i.e., the value of D at which the substrate is washed out from the fermenter without having time to enter into the synthesis process. In this case, we have X = 0, P = 0, and S = Sf.
The maximum possible value of Dlim was also given in these publications:
which is determined only by the values of kinetic constants. For max Dlim, the value of Sf = (KmKi)1/2 was obtained.
It is evident that the real value of D in the technology should satisfy the following condition:
The second characteristic max QP was presented in [32] and calculated using the formula
Formulas for calculating the following characteristics of the process were also given in [32]: Xopt, Sopt, Popt, \(S_{f}^{{{\text{opt}}}}\), and Dopt for max QP. The general formulation of the optimization problem was presented in [36].
The region for estimating the initial characteristics Sf and D is constructed in the form of dependence for a given value of QP < max QP:
Relationships (11)–(12) were derived in [37]. The coordinates of the so-called singular points that bound the range of values for D and the range of values for Sf were also given in this publication. The quantity D1 is the minimum value of D for both of Eqs. (11) and (12), and the quantity D2 is the maximum value of D for both of Eqs. (11) and (12):
The calculation of the corresponding values of Sf, i.e., Sf(D1) and Sf(D2), was presented.
Two more singular points for the range of allowable values, namely, \(S_{f}^{{{\text{max}}}}\) and \(S_{f}^{{{\text{min}}}}\), were calculated using the necessary condition with respect to D:
The values of D for \(S_{1}^{{{\text{max}}}}\) and D for \(S_{2}^{{{\text{min}}}}\) were calculated. Thus, a portrait of the allowable values of Sf and D for any QP < maxQP was determined.
Relationships that substantiate the existence of the multiplicity of steady states for the process under consideration were presented in [37–39]. This issue was considered in more detail elsewhere [33, 39]. Computational relationships based on which two values of Sf that ensure the same value of QP at a given value of D in a real technology are determined (i.e., there are two technological processes) were presented in [33]. Algorithms for calculating the characteristics of the process under the conditions of multiplicity were given. An example of the results of numerical calculation for two steady states was presented.
A more intricate problem was solved in [37], where Sf was taken as the initial value and the values of D that ensure the same value of QP were calculated. In this variant, the range of allowable values [37] is divided into three sections, for each of which based on a given value of Sf its own computational relationship is used. However, as was the case in [33], two values of D are obtained for each value of Sf; i.e., there are two steady states for the same value of QP. Examples of numerical calculations for each of the sections were given.
It was shown in [40] that taking into account inhibition by the concentration of biomass and the concentration of the product in a relationship for the specific growth rate can be written in the more general form as follows:
Estimates for f and h in the processing of six experiments with different concentrations of the lactose of a strain of Lactobacillus casei were also obtained in the same study. The values of f and h for all of the experiments were 0.5. Only one experiment showed f = 0.7 at h = 0.5.
These data were subsequently used in the modeling of processes with other strains of microorganisms.
In the modeling of steady states, the authors of [41, 42] considered the possibility of using raw materials that produce the main substrate during synthesis [41] and strains of microorganisms that form a by-product in sufficiently large amounts [42].
A process for producing lactic acid from wheat flour was considered and modeled in [41]. Maltose, during the hydrolysis of which the main substrate (glucose) was formed, was used as a component that produces the main substrate. Computational relationships for the characteristics of the steady-state process X, P, S, and M0 were presented based on the solution to the following equations of the model:
where M0 is the concentration of maltose in the inlet stream, g/L, and S0 is the concentration of glucose in the inlet stream, g/L.
The specific rate of microorganism growth μ was used in the following form:
i.e., inhibition by the substrate and product was taken into account.
Experimental studies were conducted in two variants: (1) S0 = 125 g/L and M0 = 60 g/L and (2) S0 = 115 g/L and M0 = 70 g/L.
The estimates of constants for (16)–(20), the values of which are given in Table 4, were obtained [41].
The numerical values of the characteristics of steady-state processes, including the optimal one (maxQP), were given.
Relationships for calculating the characteristics of a process for lactic acid production using a strain of Lactococcus lactis ssp. lactis ATCC 19435 were presented in [42]. The specific feature of the process is that, in contrast to standard conditions, a by-product is formed at a temperature of above 30°C. Thus, the specific growth rate was used in the following form:
The rate of the formation of the product rP was as follows:
The rate of the formation of the by-product \({{r}_{{{{P}_{2}}}}}\) was written in the following form:
Under standard conditions (pH 6.0 and 30°C), a by-product does not form. For these conditions, we have n = 2.06, α = 13.2, and β = 6.45 × 10–2.
The dependence of process parameters on temperature was written in the following form:
The values for αa were as follows: A1 = 2.88 and E1 = 53.9. The values for βa were as follows: A1 = 2.97 × 10–2 and E1 = 543; A2 and E2 were not specified.
The temperature dependence was studied in the temperature range from 30 to 37°C.
A more complex mathematical model for the description of a steady state in a fermenter using a strain of Lactococcus lactis NZ133 was developed in [43].
The complexity of the mathematical model is that it includes a large number of constants. The equations of the model were as follows:
The values of constants were determined using experimental data under the conditions of batch cultivation.
An algorithm for calculating the characteristics of the process X, S, P, and QP for different values of the inlet characteristics Sf and D was developed and numerically implemented in [44] using the mathematical model presented in [43].
The numerical implementation of the algorithm was performed using constants from [43]. One important characteristic is the presence of the extremal dependence of productivity QP on D at values of Sf = 40 g/L and Sf = 60 g/L. The extremal dependence emphasizes that there is the multiplicity of steady states for the technology with this strain.
Relationships for optimal conditions and computational relationships for estimating the multiplicity of characteristics for steady-state processes based on a given value of the dilution rate D and the value of the substrate concentration Sf were presented using (25)–(27) in [45–47].
It was shown [45] that the value of productivity is determined by two characteristics, namely, the concentration of the substrate S and the dilution rate D; i.e., QP = QP(S, D).
The extremum condition was written using two relationships
with constraints on the inlet characteristics Sf and D.
The following relationship for the maximum productivity was derived:
Three algorithms were described and numerically implemented. The main algorithm solves the problem of determining the values of Sf and D that ensure maхQP.
The sequence of estimating the characteristic Sf based on a given value of D under the condition that QP < maxQP was presented in [46]. The range of possible values of D that does not contradict material balance equations (25)–(27) is determined beforehand. The value of D is specified, and two values of Sf are calculated. Thus, two steady states at one value of D are determined. An algorithm of computations and numerical calculation using constants from [43] were presented.
A more complex variant of estimating a set of steady states based on a given value of Sf at QP < maxQP was described in [47]. The value of Sf can be specified using material balance equations in three different variants:
variant 1: Sf1 ≤ Sf < maxSf;
variant 2: Sf2 < Sf < Sf1;
variant 3: minSf < Sf ≤ Sf2.
Relationships for calculating the value of D for each of the variants are different. Since the values of D are limited for the conditions of practical implementation of the technology D1 < D < D2, we have \({{S}_{{f1}}} = {{S}_{f}}\) at D1 and \({{S}_{{f2}}}\) at D2; maxSf and minSf are the limiting values of Sf for a given strain of microorganisms.
The sequence of calculating the value of D when Sf is specified for each of the variants was presented. For the same variants, two values of D for each value of Sf were numerically calculated; i.e., two steady states were determined. Numerical calculations were performed using constants from [43].
A more general mathematical model that takes into account the formation of the by-product B (a set of all by-products) and the use of the components of raw materials that produce the main substrate during synthesis M0 was constructed in [48].
The mathematical model has the following form:
The mathematical model contains elements that take into account the possibility of inhibition, which are included into a relationship for kinetics: inhibition by biomass (Xmax, n1), a product (Pmax, n2), and a substrate (Ki).
The results of the transformation of system of equations (30)–(35) are given in the appendix (formulas (A.1)–(A.11)), which are necessary for solving the formulated problem.
The sequence of solving the optimization problem at maxQP was presented in [48]. The specific feature of solving it is that it is possible to obtain a set of values for the characteristics of the process \(S_{0}^{{{\text{opt}}}}\) and \(M_{0}^{{{\text{opt}}}}\) for the same value of maxQP. The results of numerical calculations using constants from literature sources were given.
A detailed analysis of the generalized mathematical model from the standpoint of the assessment of steady states was presented in [49].
Computational relationships were given for three variants of the formulation of the problem. The most interesting variant is the third, which is the most general.
For all of the variants, formulas for calculating the maximum value of Dlim (when there is no feed, i.e., M0 = 0) were presented. The range of values for D is formed using the following equation:
In variant 3, the value of S was given using the mathematical model
An algorithm for solving the problem of estimating X, S, P, B, M, and QP with the numerical values of constants in equations was presented in [49].
In [50], computational relationships were given for estimating the characteristics of a steady state under the conditions of specifying the value of the dilution rate D, which are determined as the extreme values D1 and D2; i.e., D can be specified according to the following condition:
Computational relationships for D1 and D2 were presented.
Multiplicity is formed under the conditions of specifying D according to (37) and calculated using formulas for \(S_{1}^{'}\left( D \right)\) and \(S_{2}^{'}\left( D \right)\) (formulas (A.2), (A.6), and (A.7) in the appendix).
Relationships for constructing a set of steady states that, in fact, ensure lactic acid production were derived using the equations of the mathematical model (30)–(35) in [51, 52].
In [51], the characteristics of multiplicity were estimated based on the condition of specifying S0 within the permissible limits, and M0 and D were determined at a given value of productivity subject to the condition that QP < maxQP.
The region of the possible assignment of S0 was marked. The determination of the region was performed by simultaneously solving Eqs. (A.6) and (A.7) using (A.2). In (A.6) and (A.7), we have \(S_{1}^{'} = {{S}_{0}}\) and \(S_{2}^{'} = {{S}_{0}}\) at M0 = 0. The region is bounded by the coordinates of singular points. Singular points 1 and 2 were obtained based on the solution to (A.8) with the subsequent estimation of S0 for D1 and D2. Two more singular points 3 and 4 were determined as the maximum value of S0 using (A.6) (singular point 3) and the minimum value of S0 using (A.7) (singular point 4), and point 5 is the extremum point.
In the assignment of S0 for each of the singular points, a steady state is always unique; i.e., there is no set.
The entire region of the possible assignment of S0 is divided into three sections. This is due to the fact that it is necessary to calculate the characteristics of multiplicity using different relationships for each of the sections:
where \({{S}_{3}} = S_{1}^{'}\left( {{{D}_{3}}} \right)\), \({{S}_{2}} = S_{1}^{'}\left( {{{D}_{2}}} \right)\), \({{S}_{1}} = S_{1}^{'}\left( {{{D}_{1}}} \right)\), and \({{S}_{4}} = S_{2}^{'}\left( {{{D}_{4}}} \right)\).
It was shown that \(S_{1}^{'} > S_{2}^{'}\). The construction of sets of steady states for the assumed value of QP was considered separately for each of the sections.
A table with formulas for calculating M0 and D for a given value of S0 for each of the sections was obtained and presented. The results of numerical calculations for each of the sections were given; i.e., the coordinates of singular points using constants given in the publication for the assumed value of QP were determined and the characteristics of the sets Set1*, Set2*, and Set3* for each of the sections, respectively, were formed.
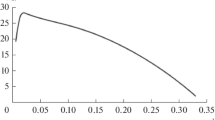
Figure 1 presents a portrait of the dependence of S0 on D for QP = 6.0 g/(L h) with the boundaries of the sections of calculation.
The characteristics of the estimation of multiplicity for a given concentration of the component that produces the main substrate during synthesis M0 were presented in [52]. As was the case in [51], the region of the assignment of M0 within the permissible limits and the corresponding values of S0 and D at a given value of QP < maxQP was determined.
The region of the possible assignment of M0 was determined by simultaneously solving Eqs. (A.6) and (A.7) using (A.2). Here, in (A.6) and (A.7), the value of \(S{\kern 1pt} '\) is according to (A.4) at S0 = 0.
Thus, we obtain the following two equations:
using (A.6):
using (A.7):
The region is bounded by the coordinates of singular points. Singular points 1 and 2 are calculated based on the solution to (A.8), from which we obtain D1 and D2. For D1 and D2, the values of M0 are calculated:
Two more singular points 3 and 4 are calculated as the maximum value of M0 using (42) (singular point 3) and the minimum value of M0 using (43) (singular point 4). The value of М0 for singular point 3 is calculated using (42) at D = D3; the value of М0 for singular point 4 is calculated using (43) at D = D4.
As a result, the region of values for M0 was divided into the following three sections:
where \({{M}_{0}}\left( {{{D}_{2}}} \right) > {{M}_{0}}\left( D \right)\).
The construction of sets of steady states for the assumed value of QP was considered separately for each of the sections.
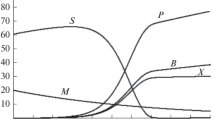
A table with formulas for calculating S0 and D for a given value of M0 for each of the sections was obtained and presented. The results of numerical calculations for each of the sections were given, and the characteristics of the sets Set1**, Set2**, and Set3** were formed. Figure 2 presents a portrait of the dependence of M0 on D with the indication of the positions of singular points and the boundaries of sections I, II, and III. The portrait was constructed for QP = 6.0 g/(L h) using table constants [52]; point 5 is the extremum point.
Concluding this part of the review, we cite one more study [53], which falls out of the general scheme of the review, since it describes a special apparatus for lactic acid production. The apparatus is a tube with a diameter of 10 mm and a length of 400 mm with a biofilm. The modeling (experimental) of a continuous process was described and the results of experiments were presented. There are no quantitative estimates (the equations of the material balances) in the cited study; however, based on conclusions, the apparatus is promising for use.
CONCLUSIONS
In conclusion, we cite two studies [54, 55] in which there are no calculations of the characteristics of steady states for lactic acid production; moreover, these publications themselves are review articles. The study [54] contains 63 references, and the study [55] has 35 references.
The publication [54] is a review of current developments in the continuous fermentation of lactic acid and a detailed study of the recycling of inexpensive raw materials. A list of microorganisms with the main characteristics of their cultivation in continuous fermentation, including that with cell recycle, was presented. The possibilities of using alternative substrates were described, and the general economic estimates for continuous fermentation processes were given. It should be noted that most references in the cited review pertain to 2016 or earlier.
Interest in the study [55] is due to the fact that, to a certain extent, this review broadens the review presented in [3] in the field of the food industry. In particular, the role of fermentation in food supply was pointed out; i.e., it was emphasized that the following properties of food are improved by lactic acid bacteria (LAB): aroma, the preservation of properties, poison prevention, and antibiotic properties.
A list of LAB for the food industry was presented: the preparation of cottage cheese, yoghurts, bread products, etc. It should be noted that there are no quantitative characteristics in the cited publication; thus, we have a purely literature review with the substantiation of conclusions from references to publications.
NOTATION
B | concentration of the total number of by-products, g/L |
D | dilution rate, h–1 |
K i | inhibition constant, g/L |
K m | substrate saturation constant, g/L |
k M | constant that determines the amount of produced substrate, h–1 |
M | concentration of raw materials that additionally produce the substrate, g/L |
P | concentration of the product, g/L |
Q P | productivity, g/(L h) |
S | concentration of the substrate, g/L |
X | concentration of biomass, g/L |
Y X/S | stoichiometric coefficient, g/g |
α, αB, β, βB | constants |
µ | specific rate of microorganism growth, h–1 |
SUBSCRIPTS AND SUPERSCRIPTS
0 | initial value |
max | maximum value |
opt | optimum value |
REFERENCES
Gordeev, L.S., Koznov, A.V., Skichko, A.S., and Gordeeva, Yu.L., Unstructured mathematical models of lactic acid biosynthesis kinetics: A review, Theor. Found. Chem. Eng., 2017, vol. 51, no. 2, pp. 175–190. https://doi.org/10.1134/S0040579517020026
Gordeeva, Yu.L., Rudakovskaya, E.G., Gordeeva, E.L., and Borodkin, A.G., Mathematical modeling of biotechnological process of lactic acid production by batch fermentation: A review, Theor. Found. Chem. Eng., 2017, vol. 51, no. 3, pp. 282–298. https://doi.org/10.1134/S0040579517030058
Wee, Y-J., Kim, J.-N., and Ryu, H.-W., Biotechnological production of lactic acid and its recent applications, Food Technol. Biotechnol., 2006, vol. 44, no. 2, pp. 163–172.
Datta, R. and Henry, M., Lactic acid: Recent advances in products, processes and technologies—A review, J. Chem. Technol. Biotechnol., 2006, vol. 81, no. 7, pp. 1119–1129. https://doi.org/10.1002/jctb.1486
Abdel-Rahman, M.A., Tashiro, Y., and Sonomoto, K., Recent advances in lactic acid production by microbial fermentation processes, Biotechnol. Adv., 2013, vol. 31, no. 6, pp. 877–902. https://doi.org/10.1016/j.biotechadv.2013.04.002
Hofvendahl, K. and Hahn-Hägerdala, B., Factors affecting the fermentative lactic acid production from renewable resources, Enzyme Microb. Technol., 2000, vol. 26, pp. 87–107. https://doi.org/10.1016/S0141-0229(99)00155-6
Kotzamanidis, Ch., Roukas, T., and Skaracis, G., Optimization of lactic acid production from beet molasses by Lactobacillus delbrueckii NCIMB 8130, World J. Microbiol. Biotechnol., 2002, vol. 1, no. 5, p. 441.
Wee, Y.J., Kim, J.N., Yun, J.S., and Ryu, H.W., Utilization of sugar molasses for economical L(+)-lactic acid production by batch fermentation of Enterococcus faecalis, Enzyme Microb. Technol., 2004, vol. 35, nos. 6–7, p. 568.
Richter, K. and Berthold, C., Biotechnological conversion of sugar and starchy crops into lactic acid, J. Agric. Eng. Res., 1998, vol. 71, no. 2, p. 181.
Richter, K. and Trӓger, A., L(+)-lactic acid from sweet sorghum by submerged and solid-state fermentations, Acta Biotechnol., 1994, vol. 14, p. 367.
Hofvendahl, K. and Hahn-Hӓgerdal, B., L-lactic acid production from whole wheat flour hydrolysate using strains of Lactobacilli and Lactococci, Enzyme Microb. Technol., 1997, vol. 20, no. 4, p. 301.
Oh, H., Wee, Y.J., Yun, J.S., Han, S.H., Jung, S., and Ryu, H.W., Lactic acid production from agricultural resources as cheap raw materials, Bioresour. Technol., 2005, vol. 96, no. 13, p. 1492.
Xiaodong, W., Xuan, G., and Rakshit, S.K., Direct fermentative production of lactic acid on cassava and other starch substrates, Biotechnol. Lett., 1997, vol. 19, no. 9, p. 841.
Yun, J.S., Wee, Y.J., Kim, J.N., and Ryu, H.W., Fermentative production of DL-lactic acid from amylase-treated rice and wheat brans hydrolyzate by a novel lactic acid bacterium, Lactobacillus sp., Biotechnol. Lett., 2004, vol. 26, no. 20, p. 1613.
Linko, Y.Y. and Javanainen, P., Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch, Enzyme Microb. Technol., 1996, vol. 19, no. 2, p. 118.
Vishnu, C., Seenayya, G., and Reddy, G., Direct fermentation of various pure and crude starchy substrates to L(+)-lactic acid using Lactobacillus amylophilus GV6, World J. Microbiol. Biotechnol., 2002, vol. 18, no. 5, p. 429.
Yáñez, R., Moldes, A.B., Alonso, J.L., and Parajó, J.C., Production of D(–)-lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp. Torquens, Biotechnol. Lett., 2003, vol. 25, no. 14, p. 1161.
Miura, S., Arimura, T., Itoda, N., Dwiarti, L., Feng, J.B., Bin, C.H., and Okabe, M., Production of L-lactic acid from corncob, J. Biosci. Bioeng., 2004, vol. 97, no. 3, p. 153.
Yáñez, R., Alonso, J.L., and Parajó, J.C., D-lactic acid production from waste cardboard, J. Chem. Technol. Biotechnol., 2005, vol. 80, no. 1, p. 76.
Park, E.Y., Anh, P.N., and Okuda, N., Bioconversion of waste office paper to L(+)-lactic acid by the filamentous fungus Rhizopus oryzae, Bioresour. Technol., 2004, vol. 93, no. 1, p. 77.
Moldes, A.B., Alonso, J.L., and Parajó, J.C., Strategies to improve the bioconversion of processed wood into lactic acid by simultaneous saccharification and fermentation, J. Chem. Technol. Biotechnol., 2001, vol. 76, no. 3, p. 279.
Wee, Y.J., Yun, J.S., Park, D.H., and Ryu, H.W., Biotechnological production of L(+)-lactic acid from wood hydrolyzate by batch fermentation of Enterococcus faecalis, Biotechnol. Lett., 2004, vol. 26, no. 1, p. 71.
Schepers, A.W., Thibault, J., and Lacroix, C., Lactobacillus helveticus growth and lactic acid production during pH-controlled batch cultures in whey permeate/yeast extract medium. Part II: Kinetic modeling and model validation, Enzyme Microb. Technol., 2002, vol. 30, no. 2, p. 187.
Büyükkileci, A.O. and Harsa, S., Batch production of L(+)-lactic acid from whey by Lactobacillus casei (NRRL B-441), J. Chem. Technol. Biotechnol., 2004, vol. 79, no. 9, p. 1036.
Monod, J., Recherches sur la croissance des cultures bactériennes, Paris: Hermann & Cie, 1942.
Agrawal, P., Koshy, G., and Ramseier, M., An algorithm for operating a fed-batch fermentor at optimum specific-growth rate, Biotechnol. Bioeng., 1989, vol. 33, no. 1, p. 115.
Henson, M.A. and Seborg, D.E., Nonlinear control strategies for continuous fermenters, Chem. Eng. Sci., 1992, vol. 47, no. 4, p. 821.
Kumar, G.P., Sastry, I.V.K.S., and Chidambaram, M., Periodic operation of a bioreactor with input multiplicities, Can. J. Chem. Eng., 1993, vol. 71, no. 5, pp. 766 –770. https://doi.org/10.1002/cjce.5450710515
Gordeeva, Yu.L., Ivashkin, Yu.A., Gordeev, L.S., and Glebov, M.B., Modeling of Microbiological Synthesis Processes with the Nonlinear Kinetics of Microorganism Growth, Moscow: Ross. Khim.-Tekhnol. Univ. im. D.I. Mendeleeva, 2011.
Gordeeva, Yu.L., Komissarov, Yu.A., and Borodkin, A.G., Algorithms for calculating the characteristics of microbiological synthesis processes with the nonlinear kinetics of microorganism growth, Vestn. Astrakh. Gos. Tekh. Univ., Ser.: Upr., Vychisl. Tekh. Inf., 2014, no. 2, p. 128.
Gordeeva, Yu.L., Algorithms for estimating the characteristics of biosynthesis processes, Program. Prod. Sist., 2012, no. 2, p. 94.
Gordeeva, Yu.L. and Gordeev, L.S., Optimization of continuous microbiological synthesis processes with nonlinear microbial growth kinetics, Theor. Found. Chem. Eng., 2015, vol. 49, no. 6, pp. 829–835. https://doi.org/10.1134/S0040579515060044
Gordeeva, Yu.L., Menshutina, N.V., Gordeeva, E.L., and Komissarov, Yu.A., An algorithm for ensuring the real conditions of multiplicity in microbiological synthesis processes at a given dilution rate, Vestn. Astrakh. Gos. Tekh. Univ., Ser.: Upr., Vychisl. Tekh. Inf., 2016, no. 2, p. 60.
Gordeeva, Yu.L., Shcherbinin, M.Yu., and Gordeev, L.S., Steady states of biotechnological processes with the nonlinear kinetics of microorganism growth: Multiplicity at a given dilution rate, Entsikl. Inzh.-Khim., 2012, no. 8, p. 23.
Gordeeva, E.L., Glebov, M.B., Borodkin, A.G., and Gordeeva, Yu.L., Evaluating the real conditions that provide multiplicity in processes of microbial synthesis at a given substrate concentration, Theor. Found. Chem. Eng., 2016, vol. 50, no. 4, pp. 476–478. https://doi.org/10.1134/S0040579516040333
Saha, P., Patwardhan, S.C., and Ramachandra Rao, V.S., Maximizing productivity of a continuous fermenter using nonlinear adaptive optimizing control, Bioprocess Eng., 1999, vol. 20, p. 15.
Gordeeva, Yu.L., Shcherbinin, M.Yu., Gordeev, L.S., and Komissarov, Yu.A., Steady states of biotechnological processes with the nonlinear kinetics of microorganism growth: Multiplicity at a given substrate concentration in the inlet stream, Vestn. Astrakh. Gos. Tekh. Univ., Ser.: Upr., Vychisl. Tekh. Inf., 2013, no. 1, p. 21.
Gordeeva, Yu.L., Ponkratova, S.A., and Gordeev, L.S., Information systems in biotechnology: Multiplicity of steady states, Vestn. Kazan. Tekhnol. Univ., 2011, vol. 14, no. 18, p. 137.
Gordeeva, Yu.L. and Gordeev, L.S., Algorithms for estimating the multiplicity of the steady states of a biotechnological process for producing lactic acid, Vestn. Astrakh. Gos. Tekh. Univ., Ser.: Upr., Vychisl. Tekh. Inf., 2014, no. 1, p. 7.
Altiok, D., Tocatli, F., and Harsa, S., Kinetic modelling of lactic acid production from whey by L. casei (NRRL B-441), J. Chem. Technol. Biotechnol., 2006, vol. 81, p. 1190.
Gonzalez, K., Tebbani, S., Lopes, F., Thorigné, A., Givry, S., Dumur, D., and Pareau, D., Modeling the continuous lactic acid production process from wheat flour, Appl. Microbiol. Biotechnol., 2016, vol. 100, no. 1, pp. 147–159. https://doi.org/10.1007/s00253-015-6949-7
Åkerberg, C., Hofvendahl, K., Zacchi, G., and Hahn-Hägerdal, B., Modelling the influence of pH, temperature, glucose and lactic acid concentrations on the kinetics of lactic acid production by Lactococcus lactis ssp. lactis ATCC 19435 in whole-wheat flour, Appl. Microbiol. Biotechnol., 1998, vol. 49, no. 6, pp. 682–690. https://doi.org/10.1007/s002530051232
Boonmee, M., Leksawasdi, N., Bridge, W., and Rogers, P.L., Batch and continuous culture of Lactococcus lactis NZ133: Experimental data and model development, Biochem. Eng. J., 2003, vol. 14, p. 127.
Gordeeva, Yu.L., Ivashkin, Yu.A., and Gordeev, L.S., Modeling the continuous biotechnological process of lactic acid production, Theor. Found. Chem. Eng., 2012, vol. 46, no. 3, pp. 279–283. https://doi.org/10.1134/S0040579512030049
Gordeeva, Yu.L., Ivashkin, Yu.A., and Gordeev, L.S., Algorithms for optimizing a continuous process for lactic acid biosynthesis, Program. Prod. Sist., 2012, no. 3 (99), p. 244.
Gordeeva, Yu.L., Ivashkin, Yu.A., and Gordeev, L.S., Steady states of a biotechnological process for producing lactic acid at a given dilution rate, Theor. Found. Chem. Eng., 2013, vol. 47, no. 2, pp. 149–152. https://doi.org/10.1134/S0040579513020036
Gordeeva, Yu.L. and Gordeev, L.S., Steady states of a biotechnological process for producing lactic acid at a given substrate concentration in the inlet stream, Theor. Found. Chem. Eng., 2014, vol. 48, no. 3, pp. 262–266. https://doi.org/10.1134/S0040579514030063
Gordeeva, Yu.L., Borodkin, A.G., and Gordeev, L.S., Optimal process parameters of the synthesis of lactic acid by continuous fermentation, Theor. Found. Chem. Eng., 2018, vol. 52, no. 3, pp. 386–392. https://doi.org/10.1134/S0040579518030090
Gordeeva, Yu.L., Borodkin, A.G., Gordeeva, E.L., and Rudakovskaya, E.G., Mathematical modeling of continuous fermentation in lactic acid production, Theor. Found. Chem. Eng., 2019, vol. 53, no. 4, pp. 501–508. https://doi.org/10.1134/S0040579519040183
Gordeeva, Yu.L., Borodkin, A.G., and Gordeeva, E.L., Steady states of a continuous fermentation process for lactic acid production: The multiplicity for a given dilution rate, Theor. Found. Chem. Eng., 2020, vol. 54, no. 3, pp. 482–488. https://doi.org/10.1134/S0040579520020062
Gordeeva, E.L., Ravichev, L.V., and Gordeeva, Yu.L., Steady states of a fermentation process for lactic acid production at a given concentration of the main substrate, Theor. Found. Chem. Eng., 2020, vol. 54, no. 4, pp. 569–580. https://doi.org/10.1134/S0040579520040181
Gordeeva, Yu.L., Ravichev, L.V., and Gordeeva, E.L., Estimating the multiplicity of the steady states of a fermentation process for lactic acid production at a given concentration of the component producing the main substrate, Theor. Found. Chem. Eng., 2020, vol. 54, no. 6, pp. 1256–1266. https://doi.org/10.1134/S0040579520060160
Cuny, L., Pfaff, D., Luther, J., Ranzinger, F., Ödman, P., Gescher, J., Guthausen, G., Horn, H., and Hille-Reichel, A., Evaluation of productive biofilms for continuous lactic acid production, Biotechnol. Bioeng., 2019, vol. 116, no. 14, p. 2687.
López-Gómez, J.P., Alexandri, M., Schneider, R., and Venus, J., A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials, Process Biochem., 2019, vol. 79, p. 1.
Admassie, M., A review on food fermentation and the biotechnology of lactic acid bacteria, World J. Food Sci. Technol., 2018, vol. 2, no. 1, pp. 19–24. https://doi.org/10.11648/j.wjfst.20180201.13
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Uteshinsky
APPENDIX
APPENDIX
The equations for calculating maxQP (the maximum value of QP) and the corresponding value of the dilution rate Dopt have the following form:
Rights and permissions
About this article
Cite this article
Gordeeva, E.L., Ravichev, L.V., Borodkin, A.G. et al. Mathematical Modeling of a Biotechnological Continuous Fermentation Process for Lactic Acid Production: A Review. Theor Found Chem Eng 55, 1192–1203 (2021). https://doi.org/10.1134/S0040579521060038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579521060038