Abstract
Results of an experimental study on a semibatch multistage chromatographic extraction process for the separation of a binary mixture of components in a multistage setup with eluent recycling under different mixture-loading conditions are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
As a rule, liquid–liquid chromatography is widely used for analytic and preparative purposes, but interest in it has also grown in recent years in the production of medicines on an industrial scale [1–8]. New liquid–liquid chromatography methods (in which less expensive and easily maintainable extraction equipment, i.e., a cascade of multistage columns or mixing-settling extractors modified in a proper way, is proposed for use instead of centrifugal chromatographs) are being developed and studied at the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences [9–12]. One advantage of this approach is also the absence of serious obstacles for increasing the scales to industrial application. Liquid chromatography with a free stationary phase or liquid chromatography without a solid carrier, which is also called centrifugal chromatography, is a comparatively new method of chromatography [13–16]. One specific feature of such processes is the absence of a solid carrier for the stationary phase retained in an apparatus in a free state by centrifugal forces and, therefore, it is more appropriate to call it the nonflowing phase, in contrast to the flowing (mobile) phase.
Unlike chromatography, the injection of a mixture into the system in the chromatographic extraction processes of separation considered in this paper is performed not in the form of a short-term pulse, but periodically for a certain time interval. This paper is devoted to studying the effect of regimes used for the injection of a flowing phase and a separated mixture of components into the chromatographic extraction setup designed in the form of a cascade of consecutively connected multistage columns on the efficiency of separation, when the process is performed in a closed loop. As was shown in [11], the application of mobile phase recycling simulates an increase in the number of stages in a chromatographic device, thus improving the efficiency of separation, whereas an increase in the separated mixture injection time elevates the productivity of this process [10, 12]. However, an increase in productivity has limits due to a decrease in the degree of separation, i.e., in the process selectivity.
EXPERIMENTAL SETUP AND METHOD OF EXPERIMENTS
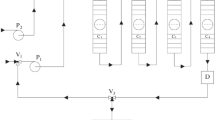
Experimental studies were performed on a laboratory setup (Fig. 1) which had a total volume of 119 mL and was composed of four consecutively connected multistage columns sectioned by perforated trays installed at every 35 mm with a diameter of 6.4 mm and 26 stages in each. The trays are PTFE plates 3 mm in thickness and have 13 orifices 0.25 mm in diameter. The experiments were performed with the use of a two-phase hexane–isopropanol–water system at a volumetric ratio of 1 : 1 : 1, and caffeine and coumarin were used as separated substances. The binary mixture solution in the flowing phase was periodically fed to the initial stage of the first column for a certain time period in an alternating sequence with the pure flowing phase. The separated mixture and mobile phase were injected into the setup with the use of dosing pumps with adjustable frequency and velocity of the piston motion. The specified operational parameters of the dosing pumps provided the displacement of discrete mobile phase volumes through the columns (pulsed feeding in the form of individual injections) with intense phase contact necessary for interphase mass transfer. The flowing phase was withdrawn from the last stage of the fourth column after passing through all the stages in the cascade of columns. The outlet of the last (fourth) column was connected in the regime of recycling to the inlet of the first column with an FEP tube, which had an inner diameter of 1.6 mm and a length of 26.5 m and provided the recycling system volume equal to 0.48 from the device volume. Outlet concentration curves were continuously recorded at the outlet from the setup with the use of UVV 101.4 M spectrophotometric detector connected to a computer. The productivity of the considered semibatch chromatographic extraction process is governed by the time of loading periods (the number of injections for the solution of components) and the intervals between neighboring loads. For this reason, the time taken to inject the solution of components into the system (the number of injections, i.e., the volume of the loaded separated mixture) and the intervals in which new solution portions were injected were varied in the experiments. The experiments were performed for two sequential loadings of the solution of components into the system.
Process flowsheet of the laboratory chromatographic extraction multistage setup with mobile phase recycling: V1, V2, and V3 are three-way valves; P1 and P2 are dosing pumps; and D is a detector. (a) Separated mixture injection in the open loop, (b) separation of components in the closed loop, (c) supply of the fresh flowing phase and withdrawal of components from the setup in the open system.
For the individual components of the selected pharmaceutical mixture (caffeine and coumarin), the distribution coefficients and the numbers of theoretical stages were preliminarily determined from the experimental chromatograms.
The distribution coefficients were calculated by the formula
where K is the distribution coefficient, if is the number of a flowing phase portion leaving the setup and corresponds to the yield of the entire volume of this phase (solvent front yield), imax is the number of a portion with a maximum concentration, and S is the nonflowing phase fraction.
Taking into account the cyclic flowing phase motion regime, the parameter if is equal to the total number of stages in the cascade of columns, i.e., if = ns = 104.
The number of theoretical stages (trays) was determined from the peak base width W as
RESULTS AND DISCUSSION
The distribution coefficients K calculated by Eq. (1) were K1 = 0.10 for caffeine and K2 = 0.46 for coumarin. The numbers of theoretical stages according to Eq. (2) was N1 for caffeine and N2 = 26 for coumarin.
The experimental concentration curves at the outlet from the setup are plotted in Fig. 2 in the absorbance (mAu)–time (s) coordinates for different conditions of mixture loading into the apparatus in the regime with eluent recycling. As can be seen from the experimental curves, the separation of components is attained after two cycles (after two passages of the separated mixture through the setup). As can be seen from the experimental curves, the separation of components is attained after two cycles (after two passages of the separated mixture through the setup). When the load is increased from 1 to 40 injections, the separation efficiency of the components becomes slightly worse, but productivity grows many times. Moreover, as follows from the comparison of Figs. 2a–2e, the concentration of components in the separated fractions grows by more than an order of magnitude, when the separated mixture load is increased from 1 to 40 injections.
Experimental concentration curves of components at the outlet from the setup for two sequential loadings of the binary caffeine–coumarin mixture for the separation process performed under mobile phase recycling conditions at different separated mixture loads into the setup by (a) 1, (b) 10, (c) 20, (d) 30, and (e) 40 injections.
CONCLUSIONS
The results demonstrate the possibility and prospects of organizing the continuous chromatographic extraction process for the separation of components in a cascade of consecutively connected extraction columns in the regime of flowing (mobile) phase recycling. For the intensification of mass transfer, especially in cases when the extraction process is accompanied by a chemical reaction at the phase interface, it is possible to use multistage (sectioned) columns with stirrers [17].
REFERENCES
Müller, M., Wasmer, K., and Vetter, W., Multiple injection mode with or without repeated sample injections: Strategies to enhance productivity in countercurrent chromatography, J. Chromatogr. A, 2018, vol. 1556, pp. 88–96. https://doi.org/10.1016/j.chroma.2018.04.069
Chami, M.C., Bouju, E., Lequemener, C., de Vaumas, R., Hadji-Minaglou, F., Fernandez, X., and Michel, T., Purification of two valepotriates from Centranthus ruber by centrifugal partition chromatography: From analytical to preparative scale, J. Chromatogr. A, 2018, vol. 1580, pp. 126–133. https://doi.org/10.1016/j.chroma.2018.10.044
Huang, X.-Y., Pei, D., Liu, J.-F., and Di, D.-L., A review on chiral separation by counter-current chromatography: Development, applications and future outlook, J. Chromatogr. A, 2018, vol. 1531, pp. 1–12. https://doi.org/10.1016/j.chroma.2017.10.073
Fang, Y., Li, Q., Shao, Q., Wang, B., and Wei, Y., A general ionic liquid pH-zone-refining countercurrent chromatography method for separation of alkaloids from Nelumbo nucifera Gaertn, J. Chromatogr. A, 2017, vol. 1507, pp. 63–71. https://doi.org/10.1016/j.chroma.2017.05.048
Sutherland, I.A., Hewitson, P., Siebers, R., van den Heuvel, R., Arbenz, L., Kinkel, J., and Fisher, D., Scale-up of protein purifications using aqueous two-phase systems: Comparing multilayer toroidal coil chromatography with centrifugal partition chromatography, J. Chromatogr. A, 2011, vol. 1218, pp. 5527–5530. https://doi.org/10.1016/j.chroma.2011.04.013
Wang, Y., Zhang, L., Zhou, H., Guo, X., and Wu, S., K-targeted strategy for isolation of phenolic alkaloids of Nelumbo nucifera Gaertn by counter-current chromatography using lysine as a pH regulator, J. Chromatogr. A, 2017, vol. 1490, pp. 115–125. https://doi.org/10.1016/j.chroma.2017.02.022
Boonloed, A., Weber, G.L., Ramzy, K.M., Dias, V.R., and Remcho, V.T., Centrifugal partition chromatography: A preparative tool for isolation and purification of xylindein from Chlorociboria aeruginosa, J. Chromatogr. A, 2016, vol. 1478, pp. 19–25. https://doi.org/10.1016/j.chroma.2016.11.026
Berthod, A., Friesen, J.B., Inui, T., and Pauli, G.F., Elution–extrusion countercurrent chromatography: Theory and concepts in metabolic analysis, Anal. Chem. (Washington, DC, U. S.), 2007, vol. 79, no. 9, pp. 3371–3382. https://doi.org/10.1021/ac062397g
Kostanyan, A.E. and Voshkin, A.A., Support-free pulsed liquid–liquid chromatography, J. Chromatogr. A, 2009, vol. 1216, no. 45, pp. 7761–7766. https://doi.org/10.1016/j.chroma.2009.09.007
Kostanyan, A.E. and Erastov, A.A., Steady state preparative multiple dual mode counter-current chromatography: Productivity and selectivity. Theory and experimental verification, J. Chromatogr. A, 2015, vol. 1406, pp. 118–128. https://doi.org/10.1016/j.chroma.2015.05.074
Kostanyan, A., Martynova, M., Erastov, A., and Belova, V., Simultaneous concentration and separation of target compounds from multicomponent mixtures by closed-loop recycling countercurrent chromatography, J. Chromatogr. A, 2018, vol. 1560, pp. 26–34. https://doi.org/10.1016/j.chroma.2018.05.032
Martynova, M.M., Kostanyan, A.E., and Tsareva, Yu.V., Investigation into the extraction–chromatographic separation of a binary mixture in a series of multistage columns, Theor. Found. Chem. Eng., 2019, vol. 53, pp. 950–953. https://doi.org/10.1134/S0040579519050142
Conway, W.D., Countercurrent Chromatography: Apparatus, Theory and Applications, New York: VCH, 1990.
Ito, Y., Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography, J. Chromatogr. A, 2005, vol. 1065, pp. 145–168. https://doi.org/10.1016/j.chroma.2004.12.044
Friesen, J.B., McAlpine, J.B., Chen, S.-N., and Pauli, G.F., Countercurrent separation of natural products: An update, J. Nat. Prod., 2015, vol. 78, no. 7, pp. 1765–1796. https://doi.org/10.1021/np501065h
Ignatova, S. and Sutherland, I., The 8th International Conference on Counter-Current Chromatography held at Brunel University, London, UK, July 23–25, 2014, J. Chromatogr. A, 2015, vol. 1425, pp. 1–7. https://doi.org/10.1016/j.chroma.2015.10.096
Rozen, A.M. and Kostanyan, A.E., Enhanced extractors: An element approach, modeling, calculation, and prospects for the development of new high-efficiency designs, Zh. Prikl. Khim., 1986, vol. 59, no. 9, pp. 2083–2090.
Funding
This work was performed as part of a state task of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental sciences. Financial support was provided in part by the Russian Foundation for Basic Research (project no. 18-33-00081 mol_a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Martynova, M.M., Apostolov, A.G. & Kostanyan, A.E. Experimental Study of the Chromatographic Extraction Process of Separation in a Closed Multistage Loop. Theor Found Chem Eng 55, 1107–1110 (2021). https://doi.org/10.1134/S0040579520050176
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520050176