Abstract
A periodic method of extraction-chromatographic separation of a mixture of components in a closed cascade of mixer–settler extractors was proposed. The processes of extraction separation in a closed cascade operating in the chromatography mode were analyzed. The theoretical foundations of the method were developed, which are necessary for modeling of extraction-chromatographic separation processes; examples of modeling different variants of such processes were given. The proposed separation method can be especially useful in the development of technologies for obtaining pure and special-purity substances, in particular, in the production of rare earth metals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The efficiency of chromatographs, as well as extractors, is determined by the interphase mass-transfer rate and the longitudinal dispersion rate in both phases. Unlike continuous stationary processes of countercurrent extraction, the transfer processes in liquid–liquid chromatography are nonstationary, and one of the phases—the stationary phase—is not removed from the chromatographic column [1–15]. The methods and equipment of liquid–liquid chromatography have low productivity and cannot be used in industry, in particular, in hydrometallurgy, where large volumes of process solutions should be processed. At the same time, chromatographic methods have a number of advantages over extraction methods: the former can separate a great number of components within a single process stage, give high-purity products, are environmentally more friendly because they use relatively low volumes of organic solvents, and are less intensive in materials and energy because the number of process stages and the amounts of the used reagents are reduced. Previously, we have developed high-productivity extraction-chromatographic separation methods using industrial separation equipment: a cascade of pulsed tray columns [3, 4, 14] and a cascade of centrifugal extractors [15]. Various designs and operating conditions of the considered separation processes are possible. For practical implementation of these processes, their theoretical description is necessary. The purpose of this work was the theoretical analysis of one of the most promising variants of such processes, namely, extraction-chromatographic separation of a mixture of components in a closed cascade of mixer–settler extractors.
MATHEMATICAL MODEL
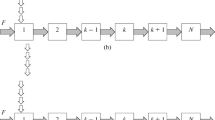
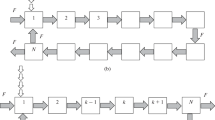
As in the cascade of centrifugal extractors, in each stage of the modeled cascade, there is full recirculation of the organic phase between the settler and the mixer, and the stages of the cascade are coupled only by the flow of the aqueous phase. Figure 1 presents two variants of the design of the cascade.
(a) Uniform loop and (b) loop with recirculation line: A is the point of supply of the aqueous phase or a mixture of components, B is the point of the recording of the concentrations and the removal of the fractions of components, N is the number of stages in the cascade (1, 2, 3, …, k is no. of a stage), and F is the volume flow rate of supply of the aqueous phase to the cascade.
The calculation dependences were derived using two approaches that we proposed before [16]: (1) cycles of circulation of components of the mixture in the system are represented as their transport through a number of series-connected identical cascades, and (2) it is assumed that the outlet concentration profiles of components after each cycle can be described by the Gaussian distribution. To simplify mathematical manipulations, we consider extraction systems with a linear dependence of the equilibrium concentrations (the distribution coefficient KD is independent of concentration). Note that, in some cases, the equilibrium curve can be approximated by a straight line with an accuracy sufficient for practical calculations. To further simplify the mathematical model, it can be assumed that the flow circulating through the cascade in a long pipeline (Fig. 1b), connecting the last stage with the first stage (in the recycle line), moves in the ideal displacement mode.
Using the first approach, after a number of mathematical manipulations, the following equation was derived for the outlet concentration profile of a component with distribution coefficient KD after cycle n:
Using the Gaussian distribution, a simpler dependence can be obtained:
In Eqs. (1) and (2), the following notation is accepted: \(a = \frac{1}{{1 - S + S{{K}_{{\text{D}}}}}}\), b = Vr/Vс is the ratio of the volume Vr of the recirculation line to the volume Vс of the cascade of extraction stages, N is the number of stages in the cascade, S is the fraction of the volume that is filled in a stage by the organic (nonflowing) phase, \(X = x{\text{/}}\bar {x}\) is the dimensionless concentration, \(\bar {x} = {Q \mathord{\left/ {\vphantom {Q {{{V}_{{\text{c}}}}}}} \right. \kern-0em} {{{V}_{{\text{c}}}}}}\) = \({{{{x}_{{\text{s}}}}F{{\tau }_{{\text{s}}}}} \mathord{\left/ {\vphantom {{{{x}_{{\text{s}}}}F{{\tau }_{{\text{s}}}}} {{{V}_{c}}}}} \right. \kern-0em} {{{V}_{c}}}}\) is the average concentration in the cascade after the loading of the mixture, Q is the amount of the component with distribution coefficient KD in the loaded mixture, xs is the concentration of the component in the mixture, F is the volume flow rate of supply of the aqueous phase and the mixture of components and circulation in the loop, \(t = \frac{{\tau F}}{{{{V}_{{\text{c}}}}}}\) is the dimensionless time, \({{t}_{{\text{s}}}} = {{{{\tau }_{{\text{s}}}}F} \mathord{\left/ {\vphantom {{{{\tau }_{{\text{s}}}}F} {{{V}_{{\text{c}}}}}}} \right. \kern-0em} {{{V}_{{\text{c}}}}}}\) is the dimensionless time of loading of the mixture of components, τs is the duration of the period of loading of the mixture of components, and i is no. of a cycle.
The calculations showed that, for modeling of separation processes, instead of Eq. (1), simpler dependence (2) can be used.
To take into account the mutual effect of the neighboring cycles, the concentration profiles of all the cycles should be summed:
ANALYSIS OF MATHEMATICAL MODEL
Let us consider several examples of separation of mixtures of components in the uniform loop (b = 0) and in the loop with the recirculation line. Figure 2 presents the results of numerical modeling of the separation of a ternary mixture (KD1 = 0.4, KD2 = 0.7, KD3 = 1.2) in two cycles in a cascade of hundred stages (N = 100) at various values of the parameter b: the concentration profiles after the second cycle, calculated from Eq. (2); and the concentration profiles throughout the recirculation process, calculated from Eq. (3). As is seen, the uniform loop (b = 0) after the second cycle could separate components if the concentration profiles after the first and second cycles did not overlap. The nonuniform loop owing to an increase in the parameter b can separate the mixture in two cycles.
After each passage of the mixture through the cascade of extractors, the degree of separation of components increases. However, the approach and overlap of the concentration profiles of the neighboring cycles, which are caused by their dispersion during their passage through the cascade, limits the efficiency of separation in the uniform loop. The long recirculation pipe in the nonuniform loop favors the separation of the concentration profiles of the neighboring cycles, thus making possible to increase the number of cycles and increase the quality of separation.
Figure 3 by the example of the separation of two binary systems (KD1 = 0.8, KD2 = 1.4 and KD1 = 1.4, KD2 = 2.4) illustrates the effect of the parameter ts on the quality of separation of components. In the first case, the separation of components is reached in three cycles (n = 3). An increase in ts from 0.01 to 0.3, which corresponds to an increase in the selectivity by a factor of 30, did not significantly affect the selectivity of the separation. At ts = 0.5, because of the partial overlap of the concentration profiles of the neighboring cycles, the quality of separation impairs. In the second case, the separation of components is reached in two cycles, and the high selectivity of the process also remains at ts = 0.5.
Figure 4 presents the results of modeling the two-stage separation of a ternary mixture (KD1 = 0.2, KD2 = 0.5, KD3 = 1). After the second cycle, the loop is opened; to the first stage of the cascade, the aqueous phase is fed; and from the last stage, the fraction of component 3 is removed for the time t = 2.6–3.2. Then, the loop is closed; the separation continues; and after the fourth cycle, the fractions of components 1 and 2 are removed from the cascade. The concentration profiles are calculated from Eq. (3) by substituting n = 2 for the first stage and n = 4 for the second stage. The multistage separation method can be used for the separation of complex multicomponent mixtures.
CONCLUSIONS
The advantages of the proposed method of extraction separation in a closed cascade of mixer–settler extractors are an increase in the quality of separation (purity of the separated components) owing to the multiple passage of a mixture through the cascade and a decrease in the consumption of reagents (the flow of the mixture (mobile phase) can circulate in the cascade until the slowest component leaves the final stage). A disadvantage of the method is the periodicity of the process. We plan to perform experimental studies and further improvement of extraction processes of separation and purification (development of the theory of continuous processes), which can be used to develop promising technologies for producing pure and special-purity substances, in particular, in the production of rare-earth metals.
REFERENCES
Ito, Y. and Bowman, R.L., J. Chromatogr. Sci., 1973, vol. 11, no. 6, pp. 284–291. https://doi.org/10.1093/chromsci/11.6.284
Kostanyan, A.E. and Voshkin, A.A., J. Chromatogr., A, 2009, vol. 1216, no. 45, pp. 7761–7766. https://doi.org/10.1016/j.chroma.2009.09.007
Kostanyan, A.E., Voshkin, A.A., and Kodin, N.V., Theor. Found. Chem. Eng., 2010, vol. 45, no. 5, pp. 779–785. https://doi.org/10.1134/S0040579511050095
Kostanyan, A.E., Voshkin, A.A., and Kodin, N.V., J. Chromatogr., A, 2011, vol. 1218, no. 36, pp. 6135–6143. https://doi.org/10.1016/j.chroma.2010.12.103
Kostanyan, A.A., Voshkin, A.A., and Belova, V.V., Molecules, 2020, vol. 25, art. no. 6020. https://doi.org/10.3390/molecules25246020
Conway, W.D., J. Chromatogr. A, 2011, vol. 1218, pp. 6015–6023. https://doi.org/10.1016/j.chroma.2011.03.056
Ignatova, S. and Sutherland, I., The 8th International Conference on Counter-Current Chromatography Held at Brunel University, London: UK, July 23–25, 2014. J. Chromatogr. A, 2015, vol. 1425, pp. 1–7. https://doi.org/10.1016/j.chroma.2015.10.096
Friesen, J.B., McAlpine, J.B., Chen, S.-N., and Pauli, G.F., J. Nat. Prod., 2015, vol. 78, pp. 1765–1796. https://doi.org/10.1021/np501065h
Sun, W., Jin, Y., Wang, C., Zhao, S., Wang, X., Luo, M., Yan, J., and Tong, S., J. Chromatogr., A, 2020, vol. 1617, art. no. 460834. https://doi.org/10.1016/j.chroma.2019.460834
Morley, R. and Minceva, M., J. Chromatogr., A, 2019, vol. 1594, pp. 140–148. https://doi.org/10.1016/j.chroma.2019.02.020
Dubuis, A., Masle, A.L., Chahen, L., Destandau, E., and Charon, N., J. Chromatogr., A, 2019, vol. 1597, pp. 159–166. https://doi.org/10.1016/j.chroma.2019.03.031
Chami, M.C., Bouju, E., Lequemener, C., de Vaumas, R., Hadji-Minaglou, F., Fernandez, X., and Michel, T., J. Chromatogr., A, 2018, vol. 1580, pp. 126–133. https://doi.org/10.1016/j.chroma.2018.10.044
Conway, W.D., Countercurrent Chromatography: Apparatus, Theory and Applications, New York: VCH, 1990.
Kostanyan, A.E. and Voshkin, A.A., Theor. Found. Chem. Eng., 2011, vol. 45, no. 1, pp. 68–74. https://doi.org/10.1134/S0040579510061028
Kostanyan, A.E. and Erastov, A.A., J. Chromatogr., A, 2018, vol. 1572, pp. 212–216. https://doi.org/10.1016/j.chroma.2018.08.039
Kostanyan, A.E., J. Chromatogr., A, 2015, vol. 1423, pp. 71–78. https://doi.org/10.1016/j.chroma.2015.10.052
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation under a state assignment for the Kurnakov Institute of General and Inorganic Chemistry, RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Kostanyan, A.E., Ivanov, V.K. & Voshkin, A.A. Theoretical Analysis of Periodic Processes of Extraction-Chromatographic Separation in a Closed Cascade of Apparatuses. Dokl Chem 499, 171–175 (2021). https://doi.org/10.1134/S0012500821080012
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012500821080012