Abstract
The extraction of lanthanide nitrates from 0.5 M NaNO3 solutions in 1 : 1 : 1 ternary aqueous organic systems using mixtures of trioctylammonium dialkyl phosphate and dicyclohexylammonium caprylate as extractants was studied. The extractability of lanthanides was shown to increase in the series La < Pr < Nd, Sm, Eu < Gd < Tb < Dy < Ho < Er < Tm, in which the atomic number of the metal increases. The selectivity of extraction in the ternary system was observed during the extraction of heavy lanthanides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rare-earth metals (REMs) are generally extracted and separated by extraction methods. Effective extractants of REMs are organophosphorus acids, for example, di-2-ethylhexylphosphoric acid (D2EHPA) [1, 2]. A disadvantage of the use of organic acids is the necessity of using relatively concentrated solutions of inorganic acids for re-extraction. An alternative are binary extractants, which, unlike cation-exchange extractants, allow easy re-extraction of metals from the organic phase in the absence of salting-out agents. These systems are characterized by insignificant influence of aqueous phase acidity on the interphase salt distribution and also by the possibility of obtaining large numbers of binary extractants with various properties by using combinations of various organic anions and cations [3–8].
The extraction separation of liquid mixtures using a combination of liquid extraction, liquid-liquid chromatography, and free liquid membrane techniques is being developed at the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences [9–28]. An important role in the development of these processes is played by the choice of extraction system. Unlike liquid extraction, in liquid–liquid extraction used mainly for separation and purification of biological products, environmentally safe multicomponent aqueous-organic two-phase systems are used. Earlier, we obtained data on the interphase distribution of lanthanide salts in ternary aqueous-organic systems with binary extractants based on D2EHPA and trioctylmethylammonium [29] depending on the compositions of the aqueous and organic phases. The goal of this study was to investigate the 1 : 1 : 1 ternary systems including the hydrophilic (water) and hydrophobic (hexane) components, as well as isopropanol, which shows both hydrophilic and hydrophobic properties. For REM extraction, extractants of various compositions were added to the organic phase (hexane), including binary extractants based on amines and mixtures thereof.
EXPERIMENTAL
The starting solutions of lanthanum and gadolinium nitrates were prepared by dissolving the La(NO3)3 ⋅ 6H2O and Gd(NO3)3 ⋅ 5H2O samples (“kh.ch.” (reagent) grade) in distilled water. The starting solutions of Pr, Nd, Sm, Eu, Tb, Dy, Ho, Er, and Tm nitrates were prepared by dissolving the metal oxide samples (“kh.ch.” (reagent) grade) in concentrated HNO3 followed by repeated evaporation of solutions with water on a water bath to remove excess acid.
To prepare the binary extractants and mixtures of extractants, the following starting reagents were used: trioctylamine (Fluka), dicyclohexylamine (Fisher Biotech), di(2-ethylhexyl)phosphoric acid (Merck), Cyanex 272 (Cytec), and caprylic acid. The solvents used were hexane (“ch.d.a.” (analytical) grade) and isopropanol (“kh.ch.” (reagent) grade). The binary extractants based on ternary (R3N) and secondary (R2NH) amines were prepared by dissolving equimolar amounts of trioctylamine or dicyclohexylamine and the corresponding organic acid (HA) in hexane.
The method for investigating the interphase distribution of REM nitrates in 1 : 1 : 1 multicomponent extraction systems was as follows. Aqueous solutions of REM nitrates in 0.5 M NaNO3 and solutions with different concentrations of extractant (mixture of extractants) in hexane and isopropanol were taken in equal volumes.
The phases were mixed at 20°C in test tubes with ground-in stoppers for 15 min, which was enough for setting constant lanthanide distribution coefficients. As the introduction of the third component with hydrophilic and hydrophobic properties in 1 : 1 : 1 systems leads to the aqueous phase containing organic substances, which can contain water, the volumes of the upper (organic) and lower (water) phases were determined after separation.
The lanthanide concentration in the starting solutions and aqueous phases after extraction was determined by trilonometric titration with xylenol orange. The lanthanide concentration in organic phases was determined from the differences between the concentrations in the starting solution and aqueous phase after extraction including the changes in the volumes of the aqueous and organic phases. The lanthanide distribution coefficients in the multicomponent system were calculated by the equation
where Cst and Caq are the metal concentrations in the starting solution and aqueous phase after extraction, respectively; Vaq and Vоrg are the volumes of the aqueous and organic phases after extraction. In some cases, quantitative re-extraction of metals from the organic phase with a 0.5 M nitric acid solution followed by determination of the lanthanide content in re-extracts was performed.
RESULTS AND DISCUSSION
The distribution of REM nitrates from 0.5 M NaNO3 solutions with trioctylammonium di(2-ethylhexyl) phosphate in a 1 : 1 : 1 system was studied at different concentrations of the binary extractant. According to Fig. 1, lanthanide extractability increases in the series La < Nd < Eu < Dy < Er, which corresponds to the increasing atomic number of the metals and correlates with their extractability in the starting extraction system with D2EHPA [2]. Note that at high lanthanide concentrations in the organic phase (over 0.01 M) in these systems, slightly soluble compounds LnA3 form, as in extraction systems [7].
Carboxylic acids and their salts with organic amines were found to be effective solvating additives, which increase the solubility of lanthanide di(2-ethylhexyl) phosphates [3, 8]. Therefore, we studied various extraction mixtures of trioctylamine (TOA), dicyclohexylamine (DCHA), caprylic acid (CA), D2EHPA, and Cyanex 272 using the extraction of europium nitrate from 0.5 M NaNO3 solutions in a 1 : 1 : 1 ternary system (Table 1). Precipitation was observed after extraction in all the systems except the system with a mixture of two binary extractants: trioctylammonium dialkylphosphate (R3NHA) and dicyclohexylammonium caprylate (R2NH ⋅ HA) at a ratio of 2 : 1 (the total concentration of binary extractants was 0.33 M); europium was quantitatively extracted in the organic phase (Table 1).
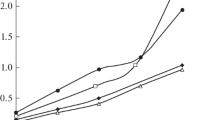
The mixture of trioctylammonium dialkyl phosphate and dicyclohexylammonium caprylate was chosen as an extractant for use in the 1 : 1 : 1 ternary system. Figure 2 shows the data on the extraction of lanthanum, europium, and erbium nitrates from 0.5 M NaNO3 solutions in a ternary system with a mixture of R3NHA and R2NH ⋅ HA (2 : 1) depending on the total concentration of the binary extractants.
Dependence of the extraction of lanthanide nitrates in the 1 : 1 : 1 system in the presence of mixtures of R3NHA and R2NH ⋅ HA depending on the total concentration of extractants. The 1 : 1 : 1 system: Ln(NO3)3 in 0.5 M NaNO3–R3NHA + R2NH ⋅ HA (2 : 1) in hexane–isopropanol (CLn(st) = 0.018 M; Vaq : Vorg = 5 : 4).
The results showed that the metal distribution coefficients increase with the total concentration of binary extractants, and that the lanthanide extractability increases in the series La < Eu < Er. The calculated lanthanide separation coefficients in this system (Table 2) showed that the separation of metals improves with increasing concentration of binary extractants in the mixture.
It was of interest to study the effects of the R3NHA to R2NH ⋅ HA ratio in the mixture on the distribution of lanthanide nitrates. For this purpose, the extraction of lanthanum nitrate from the 0.5 M NaNO3 solutions with isomolar mixtures of R3NHA and R2NH ⋅ HA in the 1 : 1 : 1 systems depending on the mole fraction of dicyclohexylammonium caprylate was studied (Fig. 3). According to the experimental data (Fig. 3), an increase in the proportion of the dicyclohexylammonium caprylate addition in the mixture of binary extractants leads to an increase in the lanthanum distribution coefficients; i.e., by varying the R3NHA to R2NH ⋅ HA ratio in the mixture, it is possible to regulate the interphase distribution of lanthanides. Earlier, a study of the extraction of lanthanum salts with trioctylammonium and dioctylammonium dialkylphosphates revealed lower extraction ability of the binary extractant based on secondary amine [7]. In this case, caprylate dicyclohexylammonium is a more efficient extractant. This may be due to the presence of bulky cyclic radicals in the dicyclohexylamine molecule and structural hindrances in the formation of the R2NH ⋅ HA binary extractant compared to R3NHA, which leads to increased lanthanum distribution coefficients (Fig. 3).
Extraction of lanthanum nitrate from 0.5 M NaNO3 solutions with isomolar mixtures of R3NHA and R2NH ⋅ HA in the 1 : 1 : 1 system depending on the mole fraction of dicyclohexylammonium caprylate (\({{C}_{{{{{\text{R}}}_{{\text{2}}}}{\text{NHHA}}}}}\) + \({{C}_{{{{{\text{R}}}_{{\text{3}}}}{\text{NHA}}\,{\text{Ln(st)}}}}}\) = 0.018 M; Vaq : Vorg = 5 : 4).
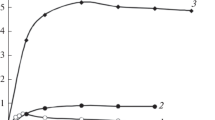
Figure 4 presents data on the distribution of REM nitrates from the 0.5 M NaNO3 solution in 1 : 1 : 1 systems in the presence of mixtures of R3NHA and R2NH ⋅ HA (2 : 1) depending on the serial number of lanthanide. It follows that the extractability of metals increases in the series La < Pr < Nd, Sm, Eu < Gd < Tb < Dy < Ho < Er < Tm in accordance with the increasing atomic number of lanthanides. This extractability series of REMs correlates with their extractability in the starting extraction system with D2EHPA [2]. The results (Fig. 4) showed that extraction selectivity in this case is observed mainly for extraction of heavy lanthanides (from dysprosium to thulium); this can be used, for example, for separating heavy lanthanides from light ones. The extractants have rather high capacity in this 1 : 1 : 1 multicomponent system. For example, during the extraction of erbium and thulium (Fig. 4), the ratio of the initial total concentration of binary extractants to the metal concentration in the organic phase is close to 2. The separation coefficients of lanthanides from the group of light metals differ slightly (curve 2, Fig. 4); therefore, the extraction of nitrates of these metals in the 1 : 1 : 1 system using mixtures of R3NHA and R2NH ⋅ HA of different compositions was studied. According to the experimental data of Fig. 5, the lanthanide distribution coefficients significantly increase at increased mole fraction of R2NH ⋅ HA in the mixture of binary extractants, but the extraction selectivity remains low. Thus, mixtures of binary extractants with mole fractions of dicyclohexylammonium caprylate of over 0.6 can be used for total extraction of REMs from nitrate solutions.
(1) Distribution and (2) separation coefficients of lanthanides during extraction from 0.5 M NaNO3 solutions in the 1 : 1 : 1 system in the presence of mixtures of R3NHA and R2NH ⋅ HA depending on the serial number of lanthanide. The 1 : 1 : 1 system: Ln(NO3)3 in 0.5 M NaNO3–R3NHA + R2NH ⋅ HA (2 : 1) in hexane–isopropanol = 0.0375 M; CLn(st) = 0.018 M; Vaq : Vоrg = 5 : 4.
Distribution coefficients of lanthanides during extraction from 0.5 M NaNO3 solutions in the 1 : 1 : 1 system in the presence of mixtures of R3NHA and R2NH ⋅ HA depending on the serial number of lanthanide. The 1 : 1 : 1 system: Ln(NO3)3 in 0.5 M NaNO3– R3NHA + R2NH ⋅ HA (2 : 1) in hexane–isopropanol (\({{C}_{{{{{\text{R}}}_{{\text{2}}}}{\text{NHHA}}}}}\) + \({{C}_{{{{{\text{R}}}_{{\text{3}}}}{\text{NHA}}}}}\) = 0.0375 M; CLn(st) = 0.018 M; Vaq : Vorg = 5 : 4). R3NHA : R2NH ⋅ HA = (1) 2 : 1; (2) 1 : 1; and (3) 1 : 2.
CONCLUSIONS
Data on the interphase distribution of lanthanide nitrates from 0.5 M NaNO3 solutions in 1 : 1 : 1 ternary aqueous organic systems using trioctylammonium di(2-ethylhexyl) phosphate and mixtures of trioctylammonium di(2-ethylhexyl) phosphate with dicyclohexylammonium caprylate as extractants were obtained. In contrast to trioctylammonium dialkyl phosphate systems, in ternary aqueous organic systems with mixtures of binary extractants based on tertiary and secondary amines, the solubility of extraction products is improved. The extractability of lanthanides in systems with mixtures of R3NHA and R2NH ⋅ HA increases in the series La < Pr < Nd, Sm, Eu < Gd < Tb < Dy < Ho < Er < Tm in accordance with the increasing atomic number of the metal and correlates with their extractability in the starting extraction system with D2EHPA. It was shown that the extraction and separation of REMs can be affected by varying the composition of the mixture of binary extractants and their total concentration. Extraction selectivity was observed mainly during the extraction of heavy lanthanides, which can be used, for example, for separating heavy lanthanides from light ones.
REFERENCES
Minagawa, Y. and Yamaguchi, K., Relative extraction rates of rare earth ions from weakly acidic solution of binary mixture of rare earth chlorides by di-(2-ethyl-hexyl) phosphoric acid/kerosine, Hydrometallurgy, 1990, vol. 24, no. 3, pp. 333–350. https://doi.org/10.1016/0304-386X(90)90097-L
Sato, T., Liquid-liquid extraction of rare-earth elements from aqueous acid solutions by acid organophosphorus compounds, Hydrometallurgy, 1989, vol. 22, nos. 1–2, pp. 121–140. https://doi.org/10.1016/0304-386X(89)90045-5
Kalyakin, S.N., Kuz’min, V.I., and Mulagaleeva, M.A., Binary extraction of lanthanide(III) chlorides using carboxylates and dialkylphosphates of secondary and tertiary amines, Hydrometallurgy, 2015, vol. 151, pp. 116–121. https://doi.org/10.1016/j.hydromet.2014.11.013
Egorova, N.S., Belova, V.V., Voshkin, A.A., Zhilov, V.I., and Khol’kin, A.I., Extraction of lanthanide chlorides by binary extractants based on phosphinic acid derivatives, Russ. J. Inorg. Chem., 2005, vol. 50, no. 11, pp. 1781–1784.
Belova, V.V., Voshkin, A.A., Kholkin, A.I., and Payrtman, A.K., Solvent extraction of some lanthanides from chloride and nitrate solutions by binary extractants, Hydrometallurgy, 2009, vol. 97, nos. 3–4, pp. 198–203. https://doi.org/10.1016/j.hydromet.2009.03.004
Zakhodyaeva, Yu.A., Belova, V.V., Egorova, N.S., and Khol’kin, A.I., Extraction of rare earth metal salts using methyltrioctylammonium dialkyl phosphate and dialkyl phosphinate, Khim. Tekhnol., 2015, vol. 16, no. 1, pp. 23–29.
Kalyakin, S.N., Kuz’min, V.I., and Mulagaleeva, M.A., The application of binary extractants based on di-(2-ethyl-hexyl) phosphoric acid to the separation of lanthanides, Tsvetn. Met., 2011, no. 3, pp. 51–54.
Kalyakin, S.N., Kuzmin, V.I., and Mulagaleeva, M.A., Binary extraction of lanthanides (III) nitrates with carboxylates and dialkylphosphates of secondary and tertiary amines, Theor. Found. Chem. Eng., 2016, vol. 50, pp. 878–883. https://doi.org/10.1134/S0040579516050080
Kostanyan, A.E., Voshkin, A.A., and Kodin, N.V., Controlled-cycle pulsed liquid–liquid chromatography. A modified version of Craig’s counter-current distribution, J. Chromatogr. A, 2011, vol. 1218, no. 36, pp. 6135–6143. https://doi.org/10.1016/j.chroma.2010.12.103
Kostanyan, A.E., On influence of sample loading conditions on peak shape and separation efficiency in preparative isocratic counter-current chromatography, J. Chromatogr. A, 2012, vol. 1254, pp. 71–77. https://doi.org/10.1016/j.chroma.2012.07.036
Kostanyan, A.E., On the description of liquid–liquid chromatography processes with a free stationary phase, Khim. Tekhnol., 2004, vol. 5, no. 8, pp. 39–42.
Kostanyan, A.E., Ignatova, S., Sutherland, I.A., Hewitson, P., Zakhodjaeva, Y.A., and Erastov, A.A., Steady-state and non-steady state operation of counter-current chromatography devices, J. Chromatogr. A, 2013, vol. 1314, pp. 94–105. https://doi.org/10.1016/j.chroma.2013.08.100
Kostanyan, A.E., Erastov, A.A., and Shishilov, O.N., Multiple dual mode counter-current chromatography with variable duration of alternating phase elution steps, J. Chromatogr. A, 2014, vol. 1347, pp. 87–95. https://doi.org/10.1016/j.chroma.2014.04.064
Kostanyan, A.E., Modeling of closed-loop recycling liquid–liquid chromatography: Analytical solutions and model analysis, J. Chromatogr. A, 2015, vol. 1406, pp. 156–164. https://doi.org/10.1016/j.chroma.2015.06.010
Kostanyan, A.E. and Erastov, A.A., Steady state preparative multiple dual mode counter-current chromatography: Productivity and selectivity. Theory and experimental verification, J. Chromatogr. A, 2015, vol. 1406, pp. 118–128. https://doi.org/10.1016/j.chroma.2015.05.074
Kostanyan, A.E., Erastov, A.A., and Shishilov, O.N., Separation of liquid mixtures by dynamic countercurrent cyclic extraction, Theor. Found. Chem. Eng., 2015, vol. 49, no. 4, pp. 560–566. https://doi.org/10.1134/S0040579515040119
Kostanyan, A.E., Analysis of the three-step cyclic process of countercurrent extraction, Theor. Found. Chem. Eng., 2015, vol. 49, no. 2, pp. 183–190. https://doi.org/10.1134/S0040579515020050
Friesen, J.B., Ahmed, S., and Pauli, G.F., Qualitative and quantitative evaluation of solvent systems for countercurrent separation, J. Chromatogr. A, 2015, vol. 1377, pp. 55–63. https://doi.org/10.1016/j.chroma.2014.11.085
Ito, Y., Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography, J. Chromatogr. A, 2005, vol. 1065, pp. 145–168. https://doi.org/10.1016/j.chroma.2004.12.044
Hewitson, P., Sutherland, I., Kostanyan, A.E., Voshkin, A.A., and Ignatova, S., Intermittent counter-current extraction—Equilibrium cell model, scaling and an improved bobbin design, J. Chromatogr. A, 2013, vol. 1303, pp. 18–27. https://doi.org/10.1016/j.chroma.2013.06.023
Kostanyan, A., Martynova, M., Erastov, A., and Belova, V., Simultaneous concentration and separation of target compounds from multicomponent mixtures by closed-loop recycling countercurrent chromatography, J. Chromatogr. A, 2018, vol. 1560, pp. 26–34. https://doi.org/10.1016/j.chroma.2018.05.032
Kostanyan, A.E. and Shishilov, O.N., An easy-to-use calculating machine to simulate steady state and non-steady-state preparative separations by multiple dual mode counter-current chromatography with semi-continuous loading of feed mixtures, J. Chromatogr. A, 2018, vol. 1552, pp. 92–98. https://doi.org/10.1016/j.chroma.2018.04.010
Kostanyan, A.E., On the application of liquid-membrane principle in a system of mixing-settling extractors, Theor. Found. Chem. Eng., 2013, vol. 47, no. 4, pp. 495–498. https://doi.org/10.1134/S004057951304009X
Kostanyan, A.E. and Belova, V.V., Copper extraction from sulfuric acid solutions in a three-phase extractor, Khim. Tekhnol., 2005, vol. 6, no. 4, pp. 12–14.
Kostanyan, A.E. and Belova, V.V., Pseudoliquid membranes with natural circulation of a continuous membrane phase, Khim. Tekhnol., 2004, vol. 5, no. 5, pp. 25–30.
Belova, V.V., Kostanyan, A.E., Zakhodyaeva, Yu.A., Kholkin, A.I., and Logutenko, O.A., On the application of bulk-supported liquid membrane techniques in hydrometallurgy, Hydrometallurgy, 2014, vol. 150, pp. 144–152. https://doi.org/10.1016/j.hydromet.2014.10.011
Belova, V.V., Free supported liquid membranes, Theor. Found. Chem. Eng., 2016, vol. 50, no. 4, pp. 642–647. https://doi.org/10.1134/S0040579516040059
Belova, V.V. and Zakhodyaeva, Yu.A., Analysis of the efficiency of liquid membranes in extraction processes, Russ. J. Inorg. Chem., 2014, vol. 59, pp. 766–772. https://doi.org/10.1134/S003602361407002X
Belova, V.V. and Martynova, M.M., Interphase distribution of lanthanide salts in multicomponent aqueous–organic two-phase systems, Theor. Found. Chem. Eng., 2019, vol. 53, pp. 921–924. https://doi.org/10.1134/S0040579518050056
Funding
This study was partly performed under the government contract in the field of fundamental research and financially supported by the Russian Foundation for Basic Research (project no. 17-03-00263).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Belova, V.V., Tsareva, Y.V. Interphase Distribution of Lanthanide Nitrates in Aqueous Organic Two-Phase Systems with Amine and Organic Acid Salts. Theor Found Chem Eng 54, 769–774 (2020). https://doi.org/10.1134/S0040579520040053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579520040053